Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12430
Peer-review started: September 10, 2022
First decision: September 26, 2022
Revised: September 28, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: November 26, 2022
Processing time: 73 Days and 19.9 Hours
Phlegmonous gastritis (PG) is a rare bacterial infection of the gastric submucosa and is related to septicemia, direct gastric mucosal injury, or the direct influence of infection or inflammation in neighboring organs. Here, we present a patient who had spontaneous biloma caused by choledocholithiasis and then PG resulting from bile leakage after biloma drainage.
A 79-year-old man with a medical history of hypertension had persistent diffuse abdominal pain for 4 d. Physical examination showed stable vital signs, icteric sclera, diffuse abdominal tenderness, and muscle guarding. Laboratory tests showed hyperbilirubinemia and bandemia. Contrast computed tomography (CT) of the abdomen showed a dilated common bile duct and left subphrenic abscess. Left subphrenic abscess drainage revealed bilious fluid, and infected biloma was confirmed. Repeated abdominal CT for persistent epigastralgia after drainage showed gastric wall thickening. Esophagogastroduodenoscopy (EGD) showed an edematous, hyperemic gastric mucosa with poor distensibility. The gastric mucosal culture yielded Enterococcus faecalis. PG was diagnosed based on imaging, EGD findings, and gastric mucosal culture. The patient recovered successfully with antibiotic treatment.
PG should be considered in patients with intraabdominal infection, especially from infected organs adjacent to the stomach.
Core Tip: We report a case of spontaneous biloma caused by choledocholithiasis followed by phlegmonous gastritis (PG) resulting from biloma rupture after biloma drainage. Additionally, we analyzed 44 PG cases reported from 2012 to 2022. The etiology of PG is mainly direct microbial invasion from gastric mucosa injury or hematogenous/lymphogenous spread and the most important risk factor for PG is an immunocompromised state. In our case, the patient was immunocompetent and PG was caused by bile leakage after biloma drainage rather than the direct influence of infected biloma.
- Citation: Yang KC, Kuo HY, Kang JW. Phlegmonous gastritis after biloma drainage: A case report and review of the literature. World J Clin Cases 2022; 10(33): 12430-12439
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12430.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12430
Phlegmonous gastritis (PG) is a rare bacterial infection of the gastric submucosa and is related to septicemia, direct gastric mucosal injury, or direct influence of infection or inflammation in neighboring organs. It is fatal if not diagnosed and treated immediately. An immunocompromised state associated with malignancy, chemotherapy-induced neutropenia, acquired immunodeficiency syndrome, alcoholism, and immunosuppressive drugs is considered the main risk factor[1,2].
Here, we present a patient with spontaneous biloma caused by choledocholithiasis followed by PG induced by bile leakage after biloma drainage.
A 79-year-old male complained of persistent diffuse abdominal pain for 4 d.
Initially, the abdominal pain occurred abruptly after eating a big meal, 4 d prior to admission. The initial abdominal pain was mainly located in the right upper quarter abdominal area and then migrated to the whole abdomen. Additionally, the patient experienced nausea, vomiting, constipation, and fever. Recurrent abdominal pain was noted for the 4 d as well. Sonography-guided percutaneous catheter drainage of the left subphrenic abscess, as shown by contrast computed tomography (CT) of the abdomen, was performed. The bilirubin level was 76.0 mg/dL and volume of abscess drainage was around 600 mL. The abscess culture yielded Enterococcus faecalis and Enterobacter cloacae complex. The blood culture yielded no pathogen isolates. Biloma was confirmed. However, the patient still complained of epigastric pain after drainage.
The patient had a medical history of hypertension and had taken an antihypertensive drug regularly.
The patient’s personal and family histories were unremarkable.
Initial vital signs were a temperature of 37.8 °C, heart rate of 126 beats/min, blood pressure of 163/93 mmHg, and respiratory rate of 32 breaths/min. There was no apparent loss of consciousness. Physical examination showed icteric sclera, abdominal fullness, diffuse tenderness, and muscle guarding. Follow-up vital signs before repeat abdominal CT for persistent abdominal pain were a temperature of 37.5 °C, heart rate of 116 beats/min, blood pressure of 162/84 mmHg, and respiratory rate of 20 breaths/min. Severe muscle guarding and diffuse tenderness were observed.
Abnormal laboratory findings included hyperbilirubinemia (total bilirubin: 3.0 mg/dL; reference range: ≤ 1.2 mg/dL), mildly elevated alkaline phosphatase (138 U/L; reference range: 40-129 U/L), hyponatremia (sodium: 127 mmol/L; reference range: 136-145 mmol/L), impaired renal function (creatinine: 1.59 mg/dL; reference range: 0.70-1.20 mg/dL), white blood cell count of 3200/μL (reference range: 3400-9500/μL), and 8% band form of white blood cells (reference range: 0.0%-4.2%).
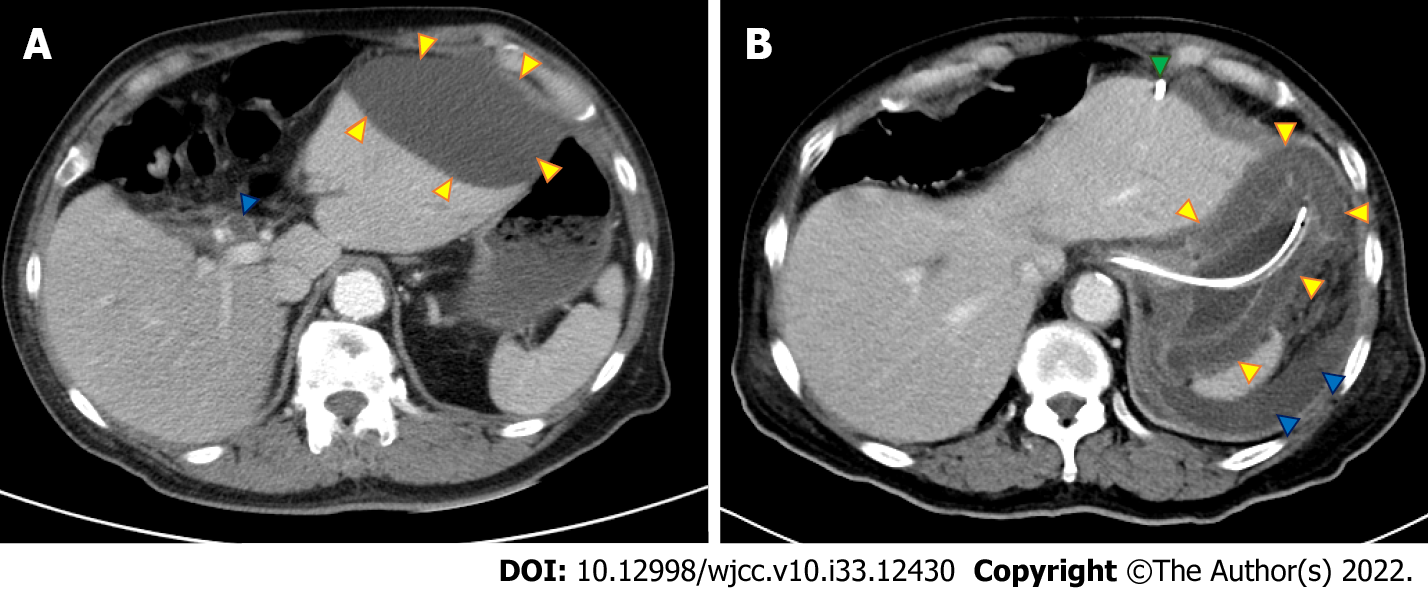
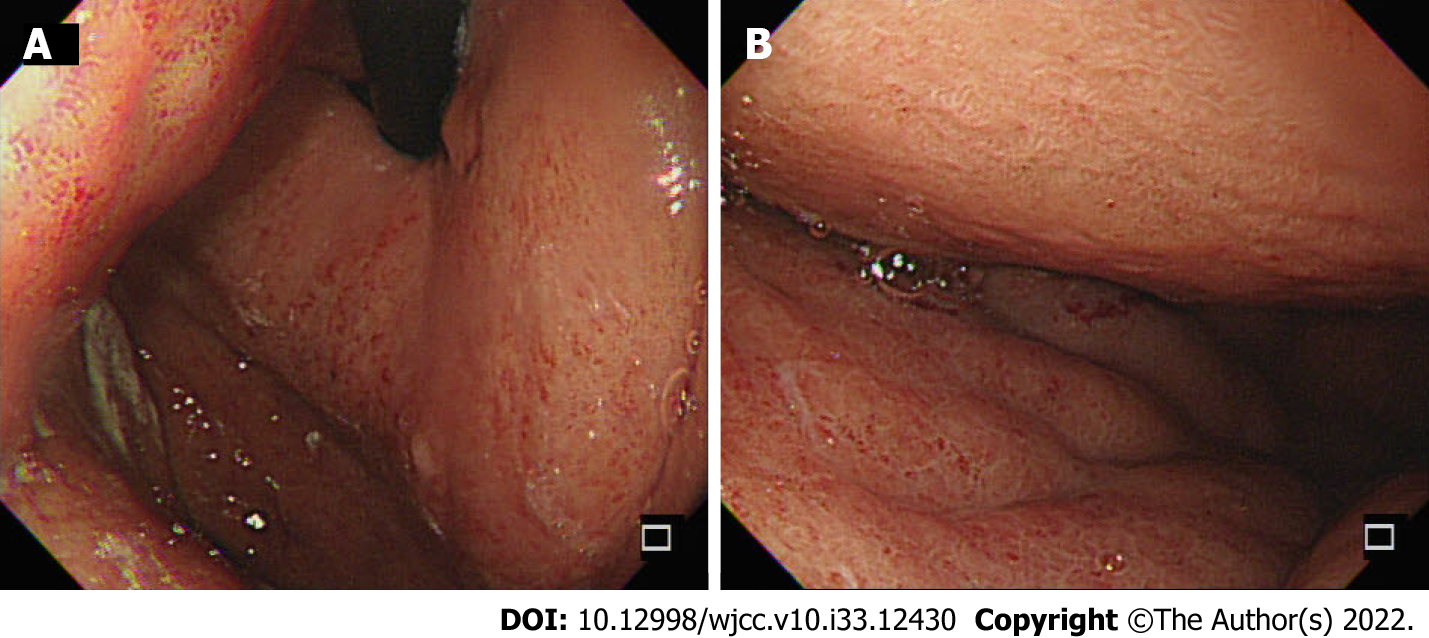
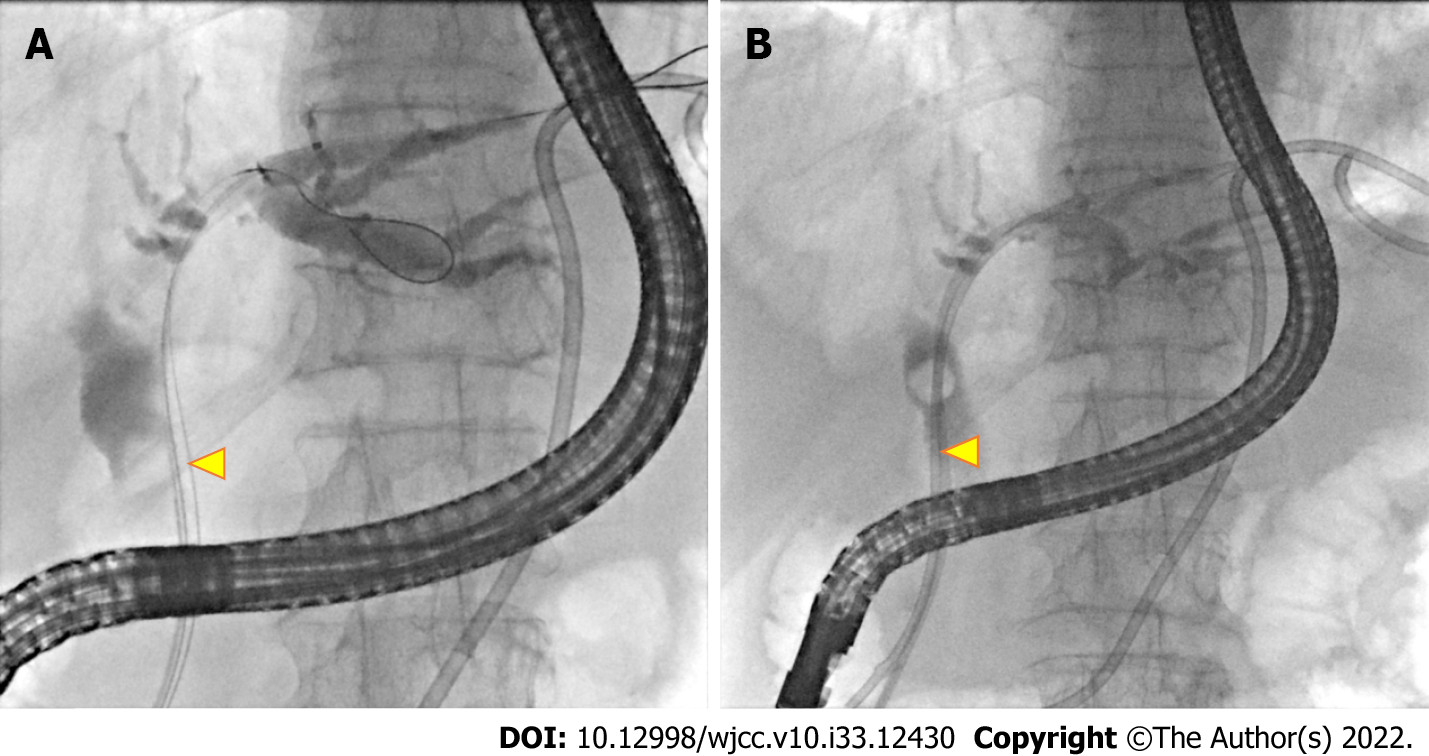
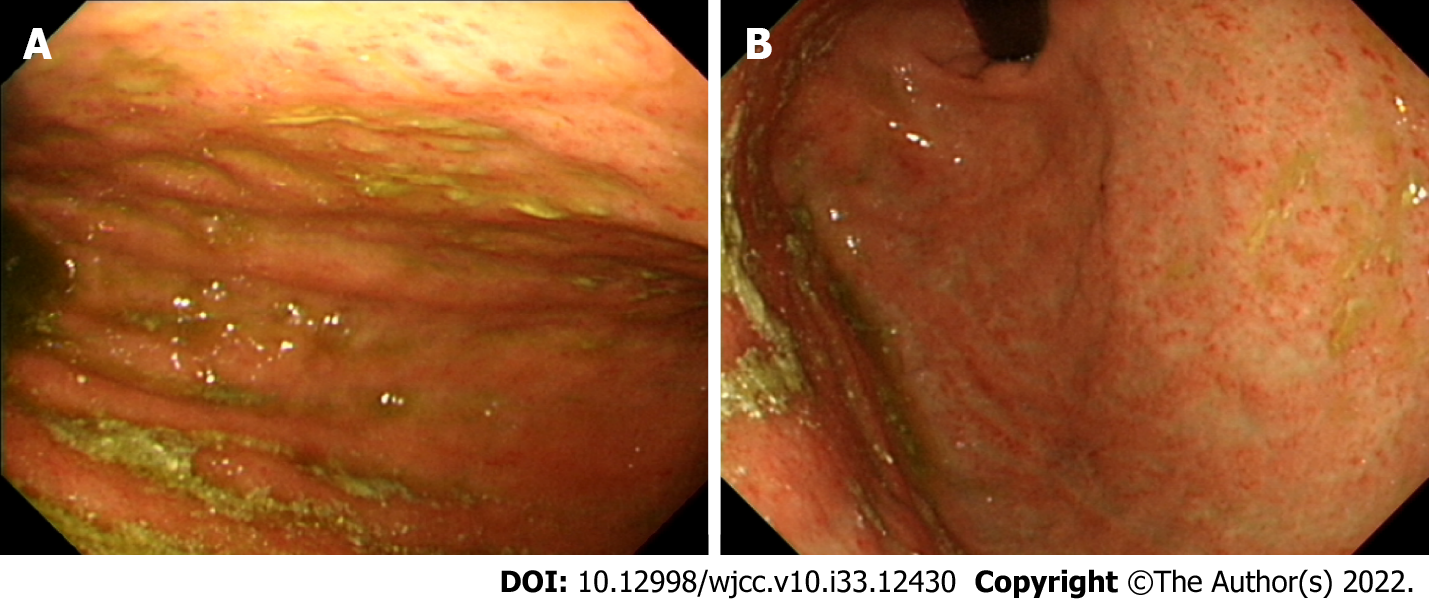
Plain abdominal radiography showed ileus and contrast abdominal CT showed a dilated common bile duct (CBD) and left subphrenic abscess (Figure 1A). Repeat contrast CT of the abdomen was performed for persistent abdominal pain after biloma drainage and showed bile leakage and gastric wall thickening (Figure 1B). Esophagogastroduodenoscopy (EGD) showed an edematous, hyperemic gastric mucosa with poor distensibility (Figure 2). Endoscopic retrograde cholangiopancreatography after EGD showed a dilated CBD with one filling defect of about 10 mm in size (Figure 3A).
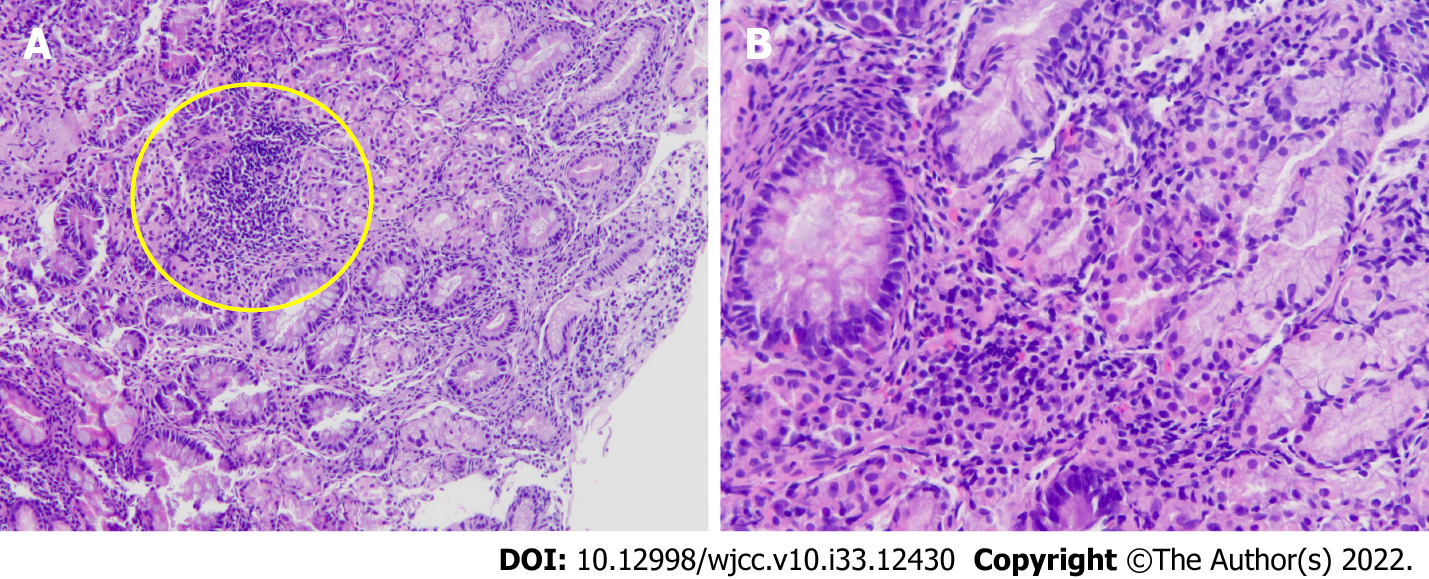
The gastric mucosal culture yielded Enterococcus faecalis and the biopsy showed that the gastric submucosa and mucosa were infiltrated by clusters of lymphocytes, neutrophils, and plasma cells (Figure 4). According to initial CT and endoscopic retrograde cholangiopancreatography, the etiology of the initial abdominal pain with fever was a CBD stone with cholangitis and spontaneous biloma. However, according to serial CT images and gastric mucosal culture, the persistent pain after biloma drainage was caused by PG. The etiology of PG was bile leakage after biloma drainage.
PG resulting from bile leakage after biloma drainage.
The patient underwent conservative therapy for PG including parenteral nutrition, biloma drainage, an initial broad-spectrum antibiotic (cefepime 2 g, twice daily), and then a definitive antibiotic of ampicillin 2 g, 6 times a day for 10 d. Endoscopic retrograde biliary drainage was performed for internal biloma drainage (Figure 3B). Neither endoscopic sphincterotomy nor stone extraction was performed due to coagulopathy.
The abdominal pain was relieved after antibiotic treatment, and follow-up EGD 1 mo later showed a normal gastric mucosa with improved distensibility of the stomach (Figure 5). Follow-up abdominal CT 2 mo later showed that the biloma was almost resolved, and the biloma drainage was removed before discharge. The patient is currently being followed as an outpatient.
PG is an infrequent bacterial infection of the gastric submucosa. It was first described in 1862 by Cruveilhier[3] and an average of one case report per year has appeared over the last 60 years[4]. We searched for papers indexed in the PubMed database using the keyword “phlegmonous gastritis”. In recent decades, from 2012 to 2022, 44 cases of PG have been reported in the English-language literature, which are summarized in Table 1[1,4-42]. Our literature review showed that PG affects all age groups (age range: 7 to 89 years old) and is highly common in the decades of 40s to 70s, with a male-to-female ratio of about 2:1. According to our findings of the literature review, half of the cases (22/44) were in an immunocompromised state, such as alcoholism, diabetes mellitus, acquired immunodeficiency syndrome, chronic hepatitis B, neutropenia after chemotherapy, and treatment with immunosuppressant drugs. Thus, an immunocompromised state appears to be a main risk factor for PG. Other risk factors for PG are prior endoscopic procedure (including for mucosal resection, submucosal dissection, hemostasis, ultrasonography with fine needle aspiration, and mucosal biopsy; 5/44), recent upper airway infection (3/44), malignancy (8/44), and prior gastrectomy or esophagectomy (3/44).
| Ref. | Year of publication | Age in yr | Sex | Risk factors | Type | Symptom | Diagnosis | Microorganism | Treatment | Result |
| Saito et al[5] | 2012 | 55 | F | ALL | Diffuse | Septic shock | CT + EGD | Bacillus species | ATB | Discharge |
| Itonaga et al[6] | 2012 | 70 | F | EUS-FNA | Diffuse | EP | CT + EGD | Streptococcus spp. | ATB | Discharge |
| Fan et al[7] | 2013 | 65 | M | Splenectomy + esophagectomy | Diffuse | EP, fever | CT + EGD | Staphylococcus aureus | ATB | Discharge |
| Liu et al[8] | 2013 | 84 | M | Nil | Diffuse | EP | CT + EGD | Nil | Gastrectomy | Discharge |
| Yu et al[9] | 2013 | 52 | M | Liver cirrhosis, HBV, hepatectomy | Diffuse | EP, palpitation, dyspnea | EL + intraoperative EGD | Klebsiella pneumoniae | ATB | Discharge |
| Nair et al[10] | 2013 | 72 | M | Nil | Diffuse | EP, fever, N/V | CT + EGD | Nil | ATB | Discharge |
| Alonso et al[11] | 2013 | 55 | M | Nil | Localized | EP, fever, N/V, diarrhea | CT | Streptococcus pyogenes | ATB, endoscopic drainage | Discharge |
| Sahnan et al[12] | 2013 | 56 | F | GAVE s/p APC | Diffuse | EP, palpitation | EL | Streptococcus | Total gastrectomy | Death |
| Cortes-Barenque et al[13] | 2014 | 35 | M | Nil | Diffuse | EP, melena, hematemesis | CT + EGD | Group A streptococcus | ATB | Discharge |
| Rada-Palomino et al[14] | 2014 | 62 | M | HIV | Diffuse | EP, N/V, hematemesis, diarrhea | CT + EGD | Streptococcus pyogenes | ATB | Discharge |
| Min et al[15] | 2014 | 51 | F | Nil | Diffuse | EP, vomiting, palpitation | CT + EL | Streptococcus pyogenes | Total gastrectomy + ATB | Discharge |
| Morimoto et al[16] | 2014 | 77 | M | DM, GU | Diffuse | N/V, palpitation | CT | Group A streptococcus | ATB | Death |
| Nomura et al[17] | 2015 | 80 | F | SMA syndrome | Diffuse | EP, N/V | CT + EGD | Enterococcus faecium | Total gastrectomy + ATB | Discharge |
| Flor-de-Lima et al[18] | 2015 | 7 | M | Acute tonsillitis | Diffuse | EP, N/V | CT + EGD | Streptococcus pneumoniae | ATB | Discharge |
| Kato et al[19] | 2015 | 64 | M | Chronic pancreatitis, DM, subtotal gastrectomy | Diffuse | EP, N/V | CT + EGD | Peptostreptococcus spp. | ATB | Discharge |
| Matsumoto et al[20] | 2015 | 74 | M | MF, MM | Diffuse | EP, N/V | CT + EGD | Bacillus thuringiensis | ATB | Death |
| Kim et al[21] | 2016 | 74 | M | DM, alcoholic liver cirrhosis, HCC, GC | Diffuse | EP, N/V, palpitation | CT + EGD | Nil | ATB | Discharge |
| Kim et al[22] | 2017 | 51 | M | AS s/p infliximab | Diffuse | N/V | CT + EGD | Nil | ATB | Discharge |
| Hagiwara et al[23] | 2018 | 65 | M | ESCC | Localized | EP, fever | CT + EGD | Streptococcus viridans | ATB + total gastrectomy | Discharge |
| Ishioka et al[24] | 2018 | 84 | F | Dementia | Diffuse | Hematemesis | CT + EGD | Proteus mirabilis, α-Streptococcus | ATB | Discharge |
| Ishioka et al[24] | 2018 | 44 | M | DM | Diffuse | EP | CT + EGD | Staphylococci | ATB | Discharge |
| Ishioka et al[24] | 2018 | 64 | M | Brain tumor s/p chemotherapy | Diffuse | N/V, hematemesis | CT + EGD | Nil | ATB | Death |
| De Davide and Beaudoin[25] | 2018 | 42 | M | PA s/p infliximab | Diffuse | EP, N/V, fever | CT + EGD | Nil | ATB | Discharge |
| Yang et al[4] | 2018 | 47 | M | URI, alcoholism, GU | Diffuse | EP, N/V, fever | CT + EL | Group A streptococcus | Total gastrectomy + ATB | Discharge |
| Ramphal et al[26] | 2018 | 45 | M | Nil | Diffuse | EP, N/V, palpitation | CT + EL | Group A Streptococcus | Total gastrectomy + ATB | Discharge |
| Iqbal et al[1] | 2018 | 56 | F | AML | Diffuse | EP, fever | CT + EGD | Citrobacter freundii, Enterococcus faecalis, Bacillus cereus | ATB | Discharge |
| Matsuura et al[27] | 2018 | 76 | F | MDS, DM, GC s/p ESD | Diffuse | EP, fever | CT + EGD | Klebsiella pneumoniae, Pseudomonas aeruginosa | ATB | Discharge |
| Saeed et al[28] | 2019 | 59 | M | Morbid obesity s/p laparoscopic sleeve gastrectomy | Localized | EP, N/V, fatigue, chills | CT | Streptococcus sanguinis | ATB + CT-guided drainage | Discharge |
| Shi et al[29] | 2019 | 33 | M | ALL s/p chemotherapy | Diffuse | EP, hematemesis | CT | Stenotrophomonas maltophilia | ATB | Discharge |
| Yasuda et al[30] | 2020 | 74 | F | Had eaten raw Ayu fish | Localized | EP, N/V, diarrhea | CT + EGD | Aeromonas hydrophila | ATB | Discharge |
| Campos-Murguía et al[31] | 2019 | 37 | F | MG, thymoma s/p resection | Diffuse | EP, N/V, melena | CT + EGD | Streptococcus oralis | ATB + total gastrectomy | Discharge |
| Kuriyama et al[32] | 2020 | 70 | F | Gastric DLBCL s/p chemotherapy | Diffuse | EP, N/V, fever | CT + EGD | Pseudomonasaeruginosa | ATB | Discharge |
| Yakami et al[33] | 2021 | 32 | M | Alcoholism | Diffuse | EP, N/V, fever | CT + EGD | Nil | ATB | Discharge |
| Yakami et al[33] | 2021 | 33 | M | Alcoholism | Localized | EP | CT + EGD | Streptococcus viridans | ATB | Discharge |
| Yakami et al[33] | 2021 | 19 | M | Nil | Localized | EP, N/V, fever | CT + EGD | Pseudomonas aeruginosa, Streptococcus viridans | ATB | Discharge |
| Taniguchi et al[34] | 2021 | 21 | M | URI | Diffuse | EP | CT + EGD | Streptococcus constellatus/milleri | ATB | Discharge |
| DeCino et al[35] | 2021 | 47 | M | DM | Diffuse | EP, N/V, fever | CT + EGD + EUS | Group A streptococcus | ATB | Discharge |
| Elisabeth et al[36] | 2021 | 70 | F | Nil | Diffuse | EP, N/V, fever, diarrhea | CT + EGD + EL | Streptococcus pyogenes | ATB | Discharge |
| Modares and Tabari[37] | 2021 | 67 | M | DM, s/p gastric mucosal biopsy | Diffuse | EP, N/V, fever | CT + EGD + EL | Group A Streptococci | ATB + total gastrectomy | Discharge |
| Takase et al[38] | 2021 | 89 | F | DM, CKD | Diffuse | EP, N/V | CT | Nil | ATB | Discharge |
| Saito et al[39] | 2021 | 70 | F | ALL s/p chemotherapy | Diffuse | Septic shock | CT + EGD | Bacillus cereus | ATB | Discharge |
| Wang et al[40] | 2021 | 22 | M | Eating contaminated food | Diffuse | EP, N/V, fever, hematemesis | CT + EGD | Enterococcus cecorum | ATB | Discharge |
| Durdella et al[41] | 2022 | 44 | F | Nil | Localized | EP, N/V | CT | Nil | ATB | Discharge |
| Yu et al[42] | 2022 | 72 | F | Gastric adenoma s/p ESD | Localized | No | CT | Nil | Distal gastrectomy | Discharge |
PG type is classified as diffuse or localized, according to the lesion range[4]. The diffuse type involves the complete stomach, and represents most cases[43]. In contrast, the localized type is most commonly restricted to the antrum, with rare cases involving the cardia or pylorus. Gastric wall abscess is a localized form of PG[43]. In our literature view, only 6 localized type PG cases were identified. The etiology of PG can be classified into primary, secondary, or idiopathic[17]. Primary PG represents a direct microbial invasion from gastric mucosa injury, which is caused by trauma, malignancy, peptic ulcer, or endoscopic interventions. Secondary PG represents a hematogenous/lymphogenous spread or direct influence of infection or inflammation in neighboring organs such as infection due to upper airway infection, pancreatitis, or cholecystitis. Idiopathic PG represents an unknown cause with absence of a primary lesion. In our case, the PG was secondary.
The most common clinical presentation of PG is severe and acute epigastric pain accompanied by fever, vomiting, palpitation, melena, and hematemesis. The symptoms of PG mainly occur within 24 h, although they can develop over several days. It is important to differentiate PG from other acute abdomen etiologies such as acute pancreatitis, cholecystitis, and bowel perforation. Diagnostic modalities for PG include EGD, abdominal CT, and endoscopic ultrasonography (EUS). EGD findings show an edematous mucosa with fibrinopurulent exudates and superficial ulcerations, loss of rugae, and poor distensibility; however, these features are nonspecific to PG. Several differential diagnoses need to be considered, like acute gastric mucosal lesion, scirrhous gastric cancer, gastric syphilis, corrosive gastritis, malignant lymphoma of the stomach, gastrointestinal stromal tumor, and anisakiasis[16,35]. Each of these diseases is diagnosed according to the collective findings from EGD imaging examination along with patient data on clinical pattern, medical history, and culture test results. CT findings include obvious thickening of the gastric wall, and low-intensity areas within the gastric wall[17]. Of note, EUS has not been routinely recommended but is an excellent tool for detecting and tracking thickening of the gastric wall and degree of inflammation[16]. Standard forceps biopsy may not be diagnostic because it does not obtain sufficient submucosal tissue, which is the typically involved layer in PG.
In our review, the most common microorganism was Streptococcus spp. (56%, 19/34), followed by Enterococcus spp. (10%, 3/34). This result was in line with the 2014 bibliographic review by Rada-Palomino et al[14].
PG has a high mortality rate; the key to successful treatment is early diagnosis and therapy. Before invention with antibiotics, the mortality rate was 83%-92%[14]. Kim et al[2] reviewed 36 cases of PG between 1973 and 2003. The mortality rates for surgical intervention and conservative treatment with antibiotics were 20% and 50%, respectively. Recently, Rada-Palomino et al[14] reviewed 45 cases from 1980 to 2014 and found that the mortality rates for surgically and medically treated patients were 11% and 19%, respectively. In our review of cases reported from 2012 to 2022, the mortality rate was 12% (1/8) for surgery and 8% (3/36) for medical treatment. Through early diagnosis and appropriate antibiotic treatment, the mortality rate of PG has been gradually decreasing but is still high. Surgery should always be considered in refractory cases, which show clinical deterioration despite optimal medical management, and in the presence of complications, such as delayed perforation, abdominal compartment syndrome, bleeding, or stricture[9,15,17,22,37]. The surgery itself can be a partial or total gastrectomy, according to the range of inflammation[7]. In our case, conservation therapy with antibiotics alone was successful.
The recurrence rate of PG is low according to our literature review. Only one case, which was reported by Taniguchi et al[34], had recurrent PG at 5 d after discharge, and the causes considered most likely were a steroid treatment for allergy and a short-term course of antibiotics. To avoid recurrence, optimizing the process/timing of antibiotic cessation is important and should be determined by laboratory testing data and clinical pattern along with findings from follow-up imaging examinations (e.g., CT or EUS).
A biloma is defined as a collection of bile located outside the bile duct. The main causes of such are iatrogenic or traumatic injuries[44], with cases of spontaneous biloma being relatively uncommon. The most frequent cause of spontaneous biloma is choledocholithiasis[45], with the underlying mechanism hypothesized as an increase in intraductal pressure due to stone obstruction. Unfortunately, the clinical presentations of biloma are non-specific, including abdominal pain, fever, nausea, vomiting, and jaundice[46].
Nowadays, treatment for spontaneous biloma is nonsurgical, including antibiotics and percutaneous drainage via pigtail catheter. ERCP is a feasible alternative with additional benefit because it can not only decompress the biliary tract by endoscopic sphincterotomy and stent placement but also identify the location and severity of an active bile leakage. Surgery is reserved for patients who fail endoscopic stone extraction or present a persistent active leak.
This case report describes a rare case of spontaneous biloma caused by choledocholithiasis followed by bile leakage-induced PG after the biloma drainage. PG itself is an uncommon diagnosis due to abdominal pain, but should be considered in patients with intraabdominal infection, especially from an infected organ adjacent to the stomach. The key to successful treatment is early diagnosis and initiation of therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jovandaric M, Serbia; Li W, China; Sachdeva S, India S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Iqbal M, Saleem R, Ahmed S, Jani P, Alvarez S, Tun HW. Successful Antimicrobial Treatment of Phlegmonous Gastritis: A Case Report and Literature Review. Case Rep Hematol. 2018;2018:8274732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (2)] |
| 2. | Kim GY, Ward J, Henessey B, Peji J, Godell C, Desta H, Arlin S, Tzagournis J, Thomas F. Phlegmonous gastritis: case report and review. Gastrointest Endosc. 2005;61:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Cruveilhier J. Traite d'Anatomie Pathologique Generale, vol 4. Paris: Masson et Cie, 1862: 485. |
| 4. | Yang H, Yan Z, Chen J, Xie H, Wang H, Wang Q. Diagnosis and treatment of acute phlegmonous gastritis: A case report. Medicine (Baltimore). 2018;97:e0629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Saito M, Morioka M, Kanno H, Tanaka S. Acute phlegmonous gastritis with neutropenia. Intern Med. 2012;51:2987-2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Itonaga M, Ueda K, Ichinose M. Phlegmonous gastritis caused by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Dig Endosc. 2012;24:488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Fan JQ, Liu DR, Li C, Chen G. Phlegmonous gastritis after esophagectomy: a case report. World J Gastroenterol. 2013;19:1330-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Liu YJ, Siracuse JJ, Gage T, Hauser CJ. Phlegmonous gastritis presenting as portal venous pneumatosis. Surg Infect (Larchmt). 2013;14:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yu HH, Tsang S, Cheung TT, Lo CM. Surviving emphysematous gastritis after hepatectomy. Case Reports Hepatol. 2013;2013:106383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Nair R, Agrawal S, Nayal B. Spontaneous Resolution of Emphysematous Gastritis with Vaso-occlusive Disease-A Case Report. Malays J Med Sci. 2013;20:68-70. [PubMed] |
| 11. | Alonso JV, de la Fuente Carillo JJ, Gutierrez Solis MA, Vara Morate FJ, López Ruiz DJ. Gastric wall abscess presenting as thoracic pain: rare presentation of an old disease. Ann Gastroenterol. 2013;26:360-362. [PubMed] |
| 12. | Sahnan K, Davis BJ, Bagenal J, Cullen S, Appleton S. Acute gastric necrosis after routine oesophagogastroduodenoscopy with therapeutic argon plasma coagulation. Ann R Coll Surg Engl. 2013;95:e99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cortes-Barenque F, Salceda-Otero JC, Angulo-Molina D, Lozoya-González D. Acute phlegmonous gastritis. Rev Gastroenterol Mex. 2014;79:299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Rada-Palomino A, Muñoz-Duyos A, Pérez-Romero N, Vargas-Pierola H, Puértolas-Rico N, Ruiz-Campos L, Espinós-Pérez J, Veloso-Veloso E. Phlegmonous gastritis: A rare entity as a differential diagnostic of an acute abdomen. Description of a case and a bibliographic review. Rev Esp Enferm Dig. 2014;106:418-424. [PubMed] |
| 15. | Min SY, Kim YH, Park WS. Acute phlegmonous gastritis complicated by delayed perforation. World J Gastroenterol. 2014;20:3383-3387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Morimoto M, Tamura S, Hayakawa T, Yamanishi H, Nakamoto C, Nakamoto H, Ikebe T, Nakano Y, Fujimoto T. Phlegmonous gastritis associated with group A streptococcal toxic shock syndrome. Intern Med. 2014;53:2639-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Nomura K, Iizuka T, Yamashita S, Kuribayashi Y, Toba T, Yamada A, Furuhata T, Kikuchi D, Matsui A, Mitani T, Ogawa O, Hoteya S, Inoshita N, Kaise M. Phlegmonous gastritis secondary to superior mesenteric artery syndrome. SAGE Open Med Case Rep. 2015;3:2050313X15596651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Flor-de-Lima F, Gonçalves D, Marques R, Silva C, Lopes J, Silva R, Tavares M, Trindade E, Carneiro F, Amil-Dias J. Phlegmonous gastritis: a rare cause of abdominal pain. J Pediatr Gastroenterol Nutr. 2015;60:e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kato K, Tominaga K, Sugimori S, Nagami Y, Kamata N, Yamagami H, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Arakawa T. Successful Treatment of Early-Diagnosed Primary Phlegmonous Gastritis. Intern Med. 2015;54:2863-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Matsumoto H, Ogura H, Seki M, Ohnishi M, Shimazu T. Fulminant phlegmonitis of the esophagus, stomach, and duodenum due to Bacillus thuringiensis. World J Gastroenterol. 2015;21:3741-3745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kim KH, Kim CG, Kim YW, Moon H, Choi JE, Cho SJ, Lee JY, Choi IJ. Phlegmonous Gastritis with Early Gastric Cancer. J Gastric Cancer. 2016;16:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kim BY, Kim HS. Erratum: Phlegmonous gastritis in an ankylosing spondylitis patient treated with infliximab. Korean J Intern Med. 2017;32:1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Hagiwara N, Matsutani T, Umezawa H, Nakamizo M, Yokoshima K, Shinozuka E, Nomura T, Uchida E. Phlegmonous gastritis associated with advanced esophageal cancer. Clin J Gastroenterol. 2018;11:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Ishioka M, Watanabe N, Sawaguchi M, Fukuda S, Shiga H, Matsuhashi T, Jin M, Iijima K. Phlegmonous Gastritis: A Report of Three Cases with Clinical and Imaging Features. Intern Med. 2018;57:2185-2188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | De Davide L, Beaudoin A. A Case of Phlegmonous Gastritis in a Patient with Psoriatic Arthritis on Infliximab. Case Rep Gastrointest Med. 2018;2018:3624627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Ramphal W, Mus M, Nuytinck HKS, van Heerde MJ, Verduin CM, Gobardhan PD. Sepsis caused by acute phlegmonous gastritis based on a group A Streptococcus. J Surg Case Rep. 2018;2018:rjy188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Matsuura K, Hiramatsu S, Taketani R, Ishibashi K, Uraoka M, Baba S, Nakamura A, Takihara H, Ueda C, Inoue T. Medical Treatment of Postendoscopic Submucosal Dissection Phlegmonous Gastritis in an Elderly Diabetic Woman with Myelodysplastic Syndrome. Case Rep Gastrointest Med. 2018;2018:8046817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Saeed S, Alothman S, Saeed K, Ahmed L, Gray S. Phlegmonous Gastritis in a Bariatric Patient After Sleeve Gastrectomy. Cureus. 2019;11:e5898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Shi D, He J, Lv M, Liu R, Zhao T, Jiang Q. Phlegmonous gastritis in a patient with mixed-phenotype acute leukemia in the neutropenia phase during chemotherapy: A case report. Medicine (Baltimore). 2019;98:e17777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Yasuda T, Yagi N, Nakahata Y, Kurobe T, Yasuda Y, Omatsu T, Obora A, Kojima T. A case of phlegmonous gastritis with hepatic portal venous gas caused by Aeromonas hydrophila successfully treated with medication. Clin J Gastroenterol. 2020;13:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Campos-Murguía A, Marfil-Garza BA, León-Lara X, Jiménez Gutiérrez JM, Botello-Partida SL. A Patient With Good Syndrome Complicated With Phlegmonous Gastritis. ACG Case Rep J. 2019;6:e00246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Kuriyama K, Koyama Y, Tsuto K, Tokuhira N, Nagata H, Muramatsu A, Oshiro M, Hirakawa Y, Iwai T, Uchiyama H. Gastric lymphoma complicated by phlegmonous gastritis and Guillain-Barré syndrome: A case report. Medicine (Baltimore). 2020;99:e20030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Yakami Y, Yagyu T, Bando T. Phlegmonous gastritis: a case series. J Med Case Rep. 2021;15:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Taniguchi H, Aimi M, Matsushita H, Shimazaki G. A case of phlegmonous gastritis after acute pharyngitis. Clin J Gastroenterol. 2021;14:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | DeCino A, Gonzalez Martinez JL, Wright R. Phlegmonous Gastritis: A Case Report of Successful Early Antibiotic Treatment. Cureus. 2021;13:e13359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Elisabeth P, Cornelia M, Athinna S, Anastasia A, Apostolos A, George D. Phlegmonous Gastritis and Streptoccocal Toxic Shock Syndrome: An Almost Lethal Combination. Indian J Crit Care Med. 2021;25:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Modares M, Tabari M. Phlegmonous gastritis complicated by abdominal compartment syndrome: a case report. BMC Surg. 2021;21:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Takase R, Fukuda N, Sui O, Hagiya H. Emphysematous gastritis. Clin Case Rep. 2021;9:e05094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Saito M, Morioka M, Izumiyama K, Mori A, Ogasawara R, Kondo T, Miyajima T, Yokoyama E, Tanikawa S. Phlegmonous gastritis developed during chemotherapy for acute lymphocytic leukemia: A case report. World J Clin Cases. 2021;9:6493-6500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Wang J, Zhang T, Zhou X, Huang H, Wang M, Xie M. Combination of antibiotics, gastric lavage and nasojejunal feeding-an effective alternative for the management of acute phlegmonous gastritis: a case report. J Int Med Res. 2021;49:300060520985742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Durdella H, Everett S, Rose JA. Acute phlegmonous gastritis: A case report. J Am Coll Emerg Physicians Open. 2022;3:e12640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Yu SJ, Lee SH, Yoon JS, Lee HS, Jee SR. Gastric Wall Abscess after Endoscopic Submucosal Dissection. Clin Endosc. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Park CW, Kim A, Cha SW, Jung SH, Yang HW, Lee YJ, Lee HIe, Kim SH, Kim YH. A case of phlegmonous gastritis associated with marked gastric distension. Gut Liver. 2010;4:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Sachdeva S, Sonika U, Dalal A, Sethi SS, Kumar M. Spontaneous giant biloma resulting from multifocal left hepatic duct perforation. Indian J Gastroenterol. 2022;41:415-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Soni A, Arivarasan K, Sachdeva S, Kumar A, Sakhuja P, Puri AS. Education and Imaging. Hepatobiliary and Pancreatic: Spontaneous bilioma secondary to ampullary cancer: a rare presentation. J Gastroenterol Hepatol. 2016;31:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Arramón M, Sciarretta M, Correa GJ, Yantorno M, Redondo A, Baldoni F, Tufare F. Spontaneous Biloma Secondary to Choledocholithiasis. ACG Case Rep J. 2021;8:e00620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |