Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12388
Peer-review started: August 20, 2022
First decision: October 12, 2022
Revised: October 25, 2022
Accepted: November 2, 2022
Article in press: November 2, 2022
Published online: November 26, 2022
Processing time: 94 Days and 16.1 Hours
Neuronal intranuclear inclusion disease (NIID) is a rare neurological degenerative disorder with diverse manifestations and inadequate awareness. Only a few cases of NIID have been reported, and typical imaging findings can provide certain clues for the diagnosis of the disease. Furthermore, skin biopsy and genetic testing are important to confirm the diagnosis.
An 84-year-old man presented to the Neurology Department of our hospital complaining of a progressive course of cognitive impairment and unsteady gait for 2 years. The symptoms gradually progressed and affected his daily life. The patient was initially diagnosed with Parkinson’s disease and vascular dementia. The patient did not respond to conventional treatment, such as dopasehydrazine. Therefore, magnetic resonance imaging (MRI) was performed. Based on the imaging findings, we suspected an NIID diagnosis. During the 3-year follow-up in our hospital, his clinical symptoms gradually progressed, and imaging findings became more significant. A high signal intensity along the corticomedullary junction persisted on MRI. Gene testing and skin biopsy were recommended in our hospital; however, the patient refused these procedures. NIID was also considered when he went to a superior hospital in Shanghai. The patient eventually agreed to undergo gene testing. This revealed abnormal GGC repeat expansions in the NOTCH2NLC gene.
The clinical manifestations of NIID are diverse. Patients with clinical manifestations similar to Parkinson’s disease and dementia may have NIID.
Core Tip: Neuronal intranuclear inclusion disease (NIID) is a rare neurodegenerative disease that has gradually gained recognition in recent years. We report a patient with typical NIID imaging findings of high signal intensity along the corticomedullary junction on magnetic resonance imaging. The main symptoms of NIID in older adults are a progressive course of cognitive impairment and unsteady walking. Gene testing indicated that GGC repeats were slightly expanded in the NOTCH2NLC gene. NIID was therefore suspected. Unfortunately, he refused a skin biopsy. Currently, the patient is being followed-up regularly in our outpatient clinic. We will continue to monitor the evolution of this patient.
- Citation: Gao X, Shao ZD, Zhu L. Typical imaging manifestation of neuronal intranuclear inclusion disease in a man with unsteady gait: A case report. World J Clin Cases 2022; 10(33): 12388-12394
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12388.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12388
Neuronal intranuclear inclusion disease (NIID) is characterised by the formation of eosinophilic hyaline intranuclear inclusions in the nervous system; however, these inclusion bodies widely exist in other cells and systems, such as in adipocytes, renal tubules, and dermal cells. As a rare progressive neurodegenerative disease, the clinical manifestations vary. NIID may be familial or sporadic, juvenile-onset (even infantile-onset), or adult-onset. NIID mostly has a chronic course, but acute attacks of headaches[1], epilepsy[2], and stroke-like[3] onset have also been reported. Sone et al[4] divided adult-onset NIID into two groups. In the dementia-dominant group, dementia was the most prominent initial symptom followed by miosis, ataxia, and unconsciousness. In the limb weakness group, muscle weakness was the most common symptom, followed by sensory disturbances, miosis, bladder dysfunction, and dementia. However, these two groups are not separate entities. The variety of symptoms and age of onset make NIID difficult to diagnose. Here, we report a case of NIID.
An 84-year-old man was admitted to our hospital in March, 2018 because of slow reactions and gait instability for 2 years.
The patient gradually developed unsteady walking and lags in response since 2016. He was found wearing clothes inside out or backwards. He felt that his limbs were inflexible and his movement were obviously slow, especially in the right limb.
He had a history of type II diabetes mellitus with good glycaemic control.
He denied having any familially-inherited disease.
Physical examination revealed normal cranial nerve evaluation and grade V limb muscle strength; involuntary movements and ataxia were not detected.
Lumbar puncture examination revealed white blood cells < 1 × 106/L, red blood cells 1 × 106/L, glucose 5.2 mmol/L, protein 1123 mg/L, and chloride 121 mmol/L in the cerebrospinal fluid (CSF). The results of CSF ink staining, smear and fungus testing, and tumour cell screening were all negative. CSF pressure was normal. There was no improvement in gait and cognitive assessment after releasing 30 mL of CSF.
Skin biopsy and genetic testing were suggested to confirm the diagnosis; however, at first, the patient refused to undergo these procedures.
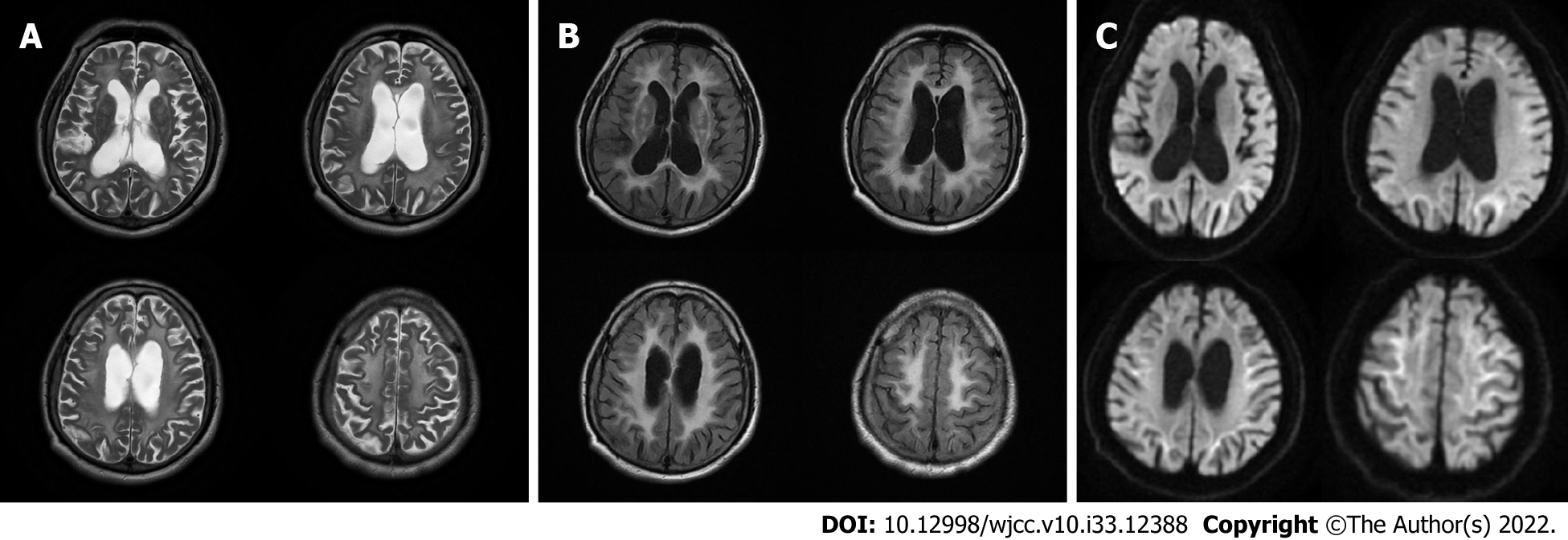
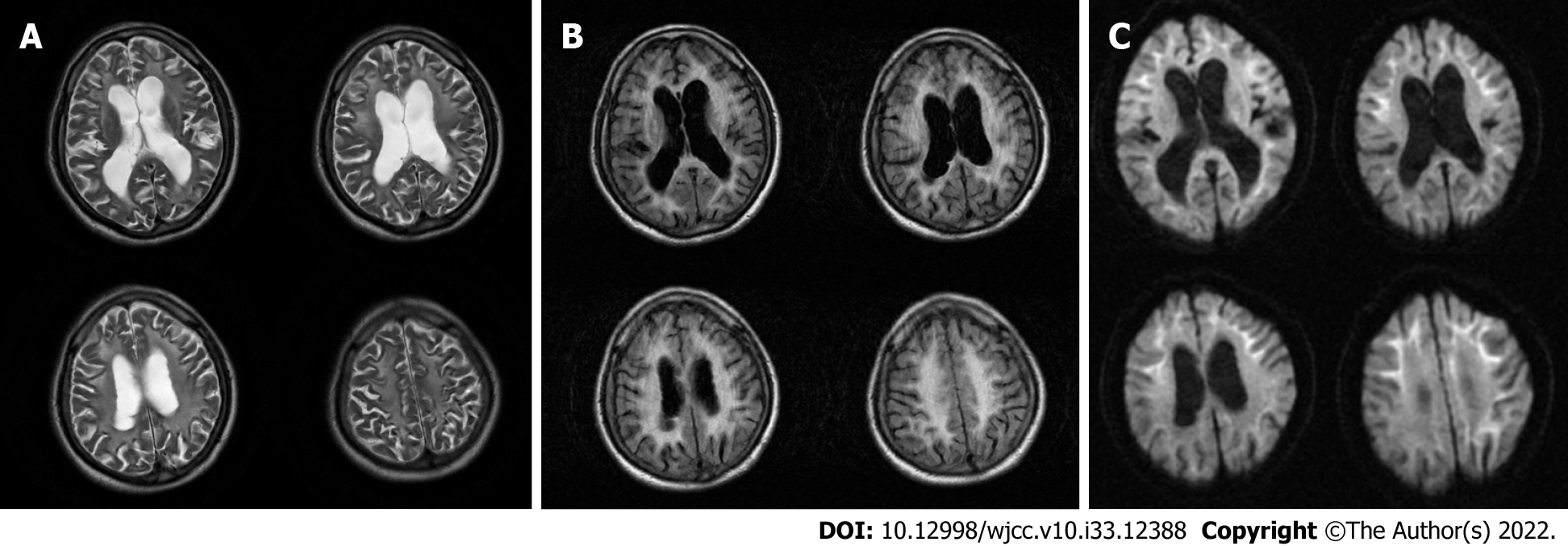
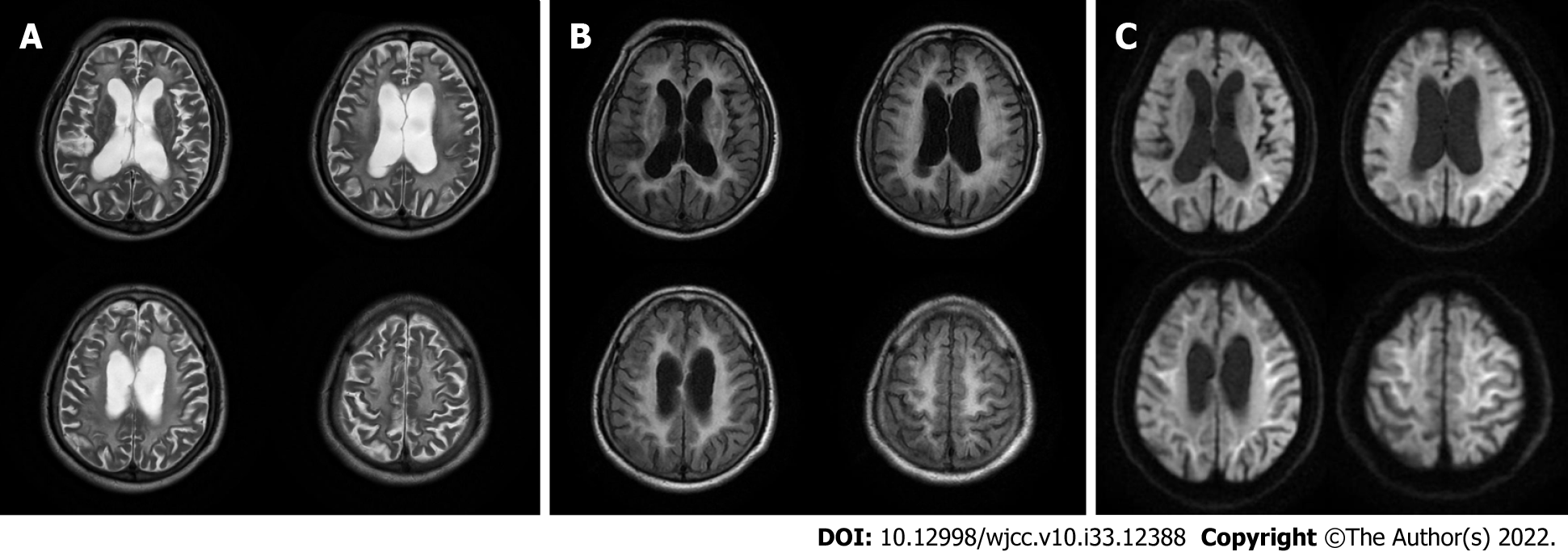
Brain magnetic resonance imaging (MRI) (Figures 1-3) revealed high signal intensity along the corticomedullary junction on diffusion-weighted imaging (DWI). During the 2-year follow-up, the patient showed progressive deterioration in motor retardation and cognitive impairment. In 2021, superior hospital cranial MRI showed multiple patchy foci of abnormal signal intensity in the white matter of the bilateral frontal and parietal lobes and periventricular area, high signal intensity on fluid-attenuated inversion recovery (FLAIR), high serrated marginal signal on DWI, and an enlarged ventricular system.
Lumbar puncture examination revealed white blood cells < 1 × 106/L, red blood cells 1 × 106/L, glucose 5.2 mmol/L, protein 1123 mg/L, and chloride 121 mmol/L in the cerebrospinal fluid (CSF). The results of CSF ink staining, smear and fungus testing, and tumour cell screening were all negative. CSF pressure was normal. There was no improvement in gait and cognitive assessment after releasing 30 mL of CSF.
Skin biopsy and genetic testing were suggested to confirm the diagnosis; however, at first, the patient refused to undergo these procedures.
With these findings, we considered the diagnosis of NIID.
The patient was administered aspirin, drugs for prevention of cerebrovascular disease and dopasehydrazine, and other anti-Parkinson's disease drugs. However, the therapeutic effect was poor.
Currently, the patient is being followed-up regularly in our outpatient clinic.
NIID is a heterogeneous disease. Auxiliary examination is useful for NIID diagnosis. Brain MRI plays an important role in the diagnosis of NIID, and some studies have suggested that MRI can be a prerequisite for skin biopsy and provide new diagnostic clues for NIID[5]. DWI findings of high signal intensity along the corticomedullary junction have been generally accepted as MRI features of NIID. High signal intensities in the paravermal area (the medial part of the cerebellar hemisphere right beside the vermis) and middle cerebellar peduncle, diffuse high signal intensities of the cerebral white matter on FLAIR images, and atrophy of the cerebellum have also been observed in patients with NIID. In addition, abnormal FLAIR signals of the cerebellum have been considered as characteristic MRI features of NIID[6]. It has been reported that high DWI signal intensities disappear during long-term radiological follow-up of patients with NIID[7]. Therefore, typical imaging findings and pathological features may be absent in some cases of NIID.
A study conducted in Japan identified a GGC repeat expansion in the 5’-UTR region of the NOTCH2NLC gene using long-read sequencing, elucidating the genetic cause of familial and sporadic NIID[8,9]. Boivin et al[10] found that GGC repeats are embedded in a small upstream open reading frame and are translated into a polyglycine protein, which forms intranuclear inclusions, and is toxic in cell and animal models. Similar clinical, histopathological, and genetic features were found in fragile X-associated tremor/ataxia syndrome (FXTAS) and NIID[10]. Because FXTAS resembles NIID, FMR1 gene mutation analysis should also be performed[7]. GGC repeat length is correlated with the clinical manifestations of NIID. Carriers of intermediate size (40-80) GGC repeats are susceptible to parkinsonism-dominant NIID[11], and cases with longer GGC repeats have a higher risk of dementia- and limb weakness-dominant NIID[8]. In a case study, intranuclear inclusions were prominently distributed in hippocampal neurones, indicating that NIID may be a complex pathological entity[12].
The clinical manifestations of NIID are highly heterogeneous and are easily misdiagnosed in clinical practice. For this patient, the following diseases needed to be considered and differentiated. First, the patient’s main clinical manifestations were gait instability and cognitive impairment; therefore, normal pressure hydrocephalus should be considered. However, Evans’ index was less than 0.3 and no disproportionately enlarged subarachnoid space hydrocephalus was found in this patient’s brain MRI. Further, we performed a fluid discharge test, but no improvement was seen in the patient’s clinical symptoms. Second, it has been found that the GGC repeat expansion in the 5’ untranslated region of the NOTCH2NLC gene is associated with many neurodegenerative diseases, such as NIID, Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease, adult leucoencephalopathy, essential tremor, and multiple system atrophy[13]. Therefore, the concept of NIID-related disorders has been proposed, which includes NIID and other diseases caused by the GGC repeat expansion of NOTCH2NLC[14]. These neurodegenerative diseases overlap, making diagnosis difficult, and further research on NOTCHNLC is required to reveal the pathological mechanisms of these diseases.
The patient’s characteristic imaging findings and genetic analysis facilitated the consideration of a NIID diagnosis; however, previous reports have indicated that GGC repeat expansion can also be observed in Alzheimer’s disease[14], Parkinson’s disease[14], adult leucoencephalopathy[15], essential tremor[16], and multiple system atrophy[17]. Skin biopsy is an important diagnostic clue that greatly increases the rate of antemortem diagnosis. Sone et al[4] suggested that a favourable skin biopsy region is 10 cm above the lateral malleolus. Intranuclear inclusions have been observed in neurons, fat cells, sweat duct epithelial cells, and other cell types. Nerve conduction studies of motor and/or sensory nerve damage and autonomic neuropathy are also indicative of NIID[4]. Unfortunately, the patient still refused skin biopsy despite our recommendation. Nevertheless, skin biopsy alone is not sufficient to distinguish NIID from other diseases, such as FXTAS[18].
NOTCH2NLC GGC repeat expansions were also found in some patients with sporadic Parkinson’s disease, in whom typical imaging and clinical features of NIID were not detected[19].
This study provides a further understanding of NIID through the follow-up of an elderly man with an unsteady gait. In conclusion, the diagnosis of NIID requires a comprehensive judgement of clinical manifestations, imaging findings, biopsy and genetic analysis. Although the patient refused to undergo skin biopsy to confirm the diagnosis, this case may further our understanding of NIID.
The thank Lei Zhu from Huainan First People’s Hospital for her support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Sade R, Turkey S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Xiao F, Tian X, Wang XF. Cerebral Atrophy and Leukoencephalopathy in a Young Man Presenting With Encephalitic Episodes. JAMA Neurol. 2018;75:1563-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Yamanaka H, Hashimoto S, Suenaga T. [Neuronal intranuclear inclusion disease with prolonged impaired consciousness and status epilepticus: a case report]. Rinsho Shinkeigaku. 2019;59:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Lin P, Jin H, Yi KC, He XS, Lin SF, Wu G, Zhang ZQ. A Case Report of Sporadic Adult Neuronal Intranuclear Inclusion Disease (NIID) With Stroke-Like Onset. Front Neurol. 2020;11:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Sone J, Mori K, Inagaki T, Katsumata R, Takagi S, Yokoi S, Araki K, Kato T, Nakamura T, Koike H, Takashima H, Hashiguchi A, Kohno Y, Kurashige T, Kuriyama M, Takiyama Y, Tsuchiya M, Kitagawa N, Kawamoto M, Yoshimura H, Suto Y, Nakayasu H, Uehara N, Sugiyama H, Takahashi M, Kokubun N, Konno T, Katsuno M, Tanaka F, Iwasaki Y, Yoshida M, Sobue G. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139:3170-3186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 5. | Sone J, Kitagawa N, Sugawara E, Iguchi M, Nakamura R, Koike H, Iwasaki Y, Yoshida M, Takahashi T, Chiba S, Katsuno M, Tanaka F, Sobue G. Neuronal intranuclear inclusion disease cases with leukoencephalopathy diagnosed via skin biopsy. J Neurol Neurosurg Psychiatry. 2014;85:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Sugiyama A, Sato N, Kimura Y, Maekawa T, Enokizono M, Saito Y, Takahashi Y, Matsuda H, Kuwabara S. MR Imaging Features of the Cerebellum in Adult-Onset Neuronal Intranuclear Inclusion Disease: 8 Cases. AJNR Am J Neuroradiol. 2017;38:2100-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Chen L, Wu L, Li S, Huang Q, Xiong J, Hong D, Zeng X. A long time radiological follow-up of neuronal intranuclear inclusion disease: Two case reports. Medicine (Baltimore). 2018;97:e13544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Sone J, Mitsuhashi S, Fujita A, Mizuguchi T, Hamanaka K, Mori K, Koike H, Hashiguchi A, Takashima H, Sugiyama H, Kohno Y, Takiyama Y, Maeda K, Doi H, Koyano S, Takeuchi H, Kawamoto M, Kohara N, Ando T, Ieda T, Kita Y, Kokubun N, Tsuboi Y, Katoh K, Kino Y, Katsuno M, Iwasaki Y, Yoshida M, Tanaka F, Suzuki IK, Frith MC, Matsumoto N, Sobue G. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 337] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 9. | Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, Novak AM, van den Bout A, Bishara A, Rosenkrantz JL, Lorig-Roach R, Field AR, Haeussler M, Russo L, Bhaduri A, Nowakowski TJ, Pollen AA, Dougherty ML, Nuttle X, Addor MC, Zwolinski S, Katzman S, Kriegstein A, Eichler EE, Salama SR, Jacobs FMJ, Haussler D. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell. 2018;173:1356-1369.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 10. | Boivin M, Deng J, Pfister V, Grandgirard E, Oulad-Abdelghani M, Morlet B, Ruffenach F, Negroni L, Koebel P, Jacob H, Riet F, Dijkstra AA, McFadden K, Clayton WA, Hong D, Miyahara H, Iwasaki Y, Sone J, Wang Z, Charlet-Berguerand N. Translation of GGC repeat expansions into a toxic polyglycine protein in NIID defines a novel class of human genetic disorders: The polyG diseases. Neuron. 2021;109:1825-1835.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Ma D, Tan YJ, Ng ASL, Ong HL, Sim W, Lim WK, Teo JX, Ng EYL, Lim EC, Lim EW, Chan LL, Tan LCS, Yi Z, Tan EK. Association of NOTCH2NLC Repeat Expansions With Parkinson Disease. JAMA Neurol. 2020;77:1559-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Cupidi C, Dijkstra AA, Melhem S, Vernooij MW, Severijnen LA, Hukema RK, Rozemuller AJM, Neumann M, van Swieten JC, Seelaar H. Refining the Spectrum of Neuronal Intranuclear Inclusion Disease: A Case Report. J Neuropathol Exp Neurol. 2019;78:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Cao L, Yan Y, Zhao G. NOTCH2NLC-related repeat expansion disorders: an expanding group of neurodegenerative disorders. Neurol Sci. 2021;42: 4055-4062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Tian Y, Wang JL, Huang W, Zeng S, Jiao B, Liu Z, Chen Z, Li Y, Wang Y, Min HX, Wang XJ, You Y, Zhang RX, Chen XY, Yi F, Zhou YF, Long HY, Zhou CJ, Hou X, Wang JP, Xie B, Liang F, Yang ZY, Sun QY, Allen EG, Shafik AM, Kong HE, Guo JF, Yan XX, Hu ZM, Xia K, Jiang H, Xu HW, Duan RH, Jin P, Tang BS, Shen L. Expansion of Human-Specific GGC Repeat in Neuronal Intranuclear Inclusion Disease-Related Disorders. Am J Hum Genet. 2019;105:166-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 15. | Okubo M, Doi H, Fukai R, Fujita A, Mitsuhashi S, Hashiguchi S, Kishida H, Ueda N, Morihara K, Ogasawara A, Kawamoto Y, Takahashi T, Takahashi K, Nakamura H, Kunii M, Tada M, Katsumoto A, Fukuda H, Mizuguchi T, Miyatake S, Miyake N, Suzuki J, Ito Y, Sone J, Sobue G, Takeuchi H, Matsumoto N, Tanaka F. GGC Repeat Expansion of NOTCH2NLC in Adult Patients with Leukoencephalopathy. Ann Neurol. 2019;86:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Sun QY, Xu Q, Tian Y, Hu ZM, Qin LX, Yang JX, Huang W, Xue J, Li JC, Zeng S, Wang Y, Min HX, Chen XY, Wang JP, Xie B, Liang F, Zhang HN, Wang CY, Lei LF, Yan XX, Xu HW, Duan RH, Xia K, Liu JY, Jiang H, Shen L, Guo JF, Tang BS. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain. 2020;143:222-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 17. | Fang P, Yu Y, Yao S, Chen S, Zhu M, Chen Y, Zou K, Wang L, Wang H, Xin L, Hong T, Hong D. Repeat expansion scanning of the NOTCH2NLC gene in patients with multiple system atrophy. Ann Clin Transl Neurol. 2020;7:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Toko M, Ohshita T, Kurashige T, Morino H, Kume K, Yamashita H, Sobue G, Iwasaki Y, Sone J, Kawakami H, Maruyama H. FXTAS is difficult to differentiate from neuronal intranuclear inclusion disease through skin biopsy: a case report. BMC Neurol. 2021;21:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Shi CH, Fan Y, Yang J, Yuan YP, Shen S, Liu F, Mao CY, Liu H, Zhang S, Hu ZW, Fan LY, Li MJ, Fan SH, Liu XJ, Xu YM. NOTCH2NLC Intermediate-Length Repeat Expansions Are Associated with Parkinson Disease. Ann Neurol. 2021;89:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |