Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12365
Peer-review started: August 10, 2022
First decision: September 5, 2022
Revised: September 26, 2022
Accepted: October 24, 2022
Article in press: October 24, 2022
Published online: November 26, 2022
Processing time: 105 Days and 0.6 Hours
Multiple myeloma (MM) complicated with extramedullary disease (EMD) has a poor prognosis and is a limiting factor in the treatment of MM, and no standard treatment is recommended in international guidelines. Few studies have reported MM with periorbital EMD.
In this paper, the clinical characteristics and survival of seven patients with multiple myeloma and orbital are described and analyzed. The common ocular symptoms were blurred vision, proptosis and/or eye movement disorders, IgG type MM may be a risk factor for orbital involvement. Of them, six patients were treated with bortezomib-based regimens. The median overall survival (OS) and progression free survival for the entire cohort were 48 and 33 mo, respectively, which was much worse than the OS reported for MM patients without orbital EMD.
Orbital MM may have significantly shortened survival for the entire cohort, so multidisciplinary collaboration is emphasized and recommended in the diagnosis and treatment of these difficult cases.
Core Tip: Orbital extramedullary disease (EMD) complicated with Multiple myeloma (MM) is rare. Herein, the clinical characteristics of seven patients from December 2007 to November 2019 were analyzed. IgG type may be a risk factor for orbital EMD. Unilateral disease is more common. General ocular symptoms are blurred vision, proptosis or eye movement disorders. In addition to a biopsy of the lesion, positron emission tomography/computed tomography (CT) or magnetic resonance imaging is more sensitive and useful than CT for the diagnosis and evaluation. Current standard treatment approaches and survival have not improved, therefore, multidisciplinary management of individualized targeted chemotherapy basis is recommended for this aggressive myeloma sub-entity.
- Citation: Hu WL, Song JY, Li X, Pei XJ, Zhang JJ, Shen M, Tang R, Pan ZY, Huang ZX. Clinical features and prognosis of multiple myeloma and orbital extramedullary disease: Seven cases report and review of literature. World J Clin Cases 2022; 10(33): 12365-12374
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12365
Multiple myeloma (MM) is a malignant plasmacytoma with clonal plasmacytosis in the bone marrow or extramedullary plasmacytoma, and the presence of monoclonal immunoglobulins (M protein) in the blood and/or urine. The median age for diagnosis MM is 70 years and the incidence of MM is currently on the rise[1]. The application of new drugs has improved the survival of MM patients, but MM is still an incurable disease[1,2].
Extramedullary disease (EMD) is characterized by subclones that proliferate independently of the bone marrow microenvironment, escape apoptosis, and are resistant to therapy. Adhesion molecules, epigenetic and immune changes, may play an important role in the molecular pathogenic mechanisms underlying EMD. Abnormalities in the tumor microenvironment, such as BRAF-MEK, RAS pathway and P53 gene deletion, account for 10%-20% of MM, and may be involved in pathological processes, and then the effective personalized therapy may have implications for the treatment of such diseases[2-6]. The incidence of diagnosis to recurrence in bone-related EMD (bEMD) and soft tissues related EMD (sEMD) is 7%-34% and 3.4%-10%, respectively[2-6].
Herein, seven patients with MM and orbital EMD patients were reported from our single center, and their clinical features and survival were analyzed.
Seven patients with MM and orbital EMD were enrolled from the inpatient departments of our hospital between 1 December 2007 and 30 November 2019. All of the patients met the diagnostic criteria both for MM and orbital EMD. There were 3 males and 4 females, with a median age of 41.4 (33-54) years. Information and chief complaints of seven patients are shown in Tables 1 and 2, including periorbital blurred vision, proptosis or eye movement disorders, and so on.
| Items | Data |
| Age (yr) | 41.4 |
| Gender: M/F (cases) | 3/4 |
| Type of MM: IgGk/k (cases, %) | 5 (71.4)/2 (28.6) |
| Stage of ISS: I/II/III (cases) | 3 (42.9)/2 (28.6)/2 (28.6) |
| High-risk cytogenetics in FISH: 1q21+ (cases, %) | 2 (28.6) |
| Features of EMD (cases) | 2 (28.6) |
| Spinal cord/thyroid/breast/armpit soft tissue/ovary/pleural effusion/brain | 2/2/2/2/2/1/1/0 |
| ≥ 2 sites (cases, %) | 6 (85.7) |
| ≥ 5 cm diameter (cases, %) | 2 (28.6) |
| PC (%) | 2.5 |
| Calcium > 2.75 (mmol/L, C) | 0 |
| Creatinine > 176.8 μmol/L (mmol/L, R) (cases, %) | 1 (14.3) |
| Hemoglobin < 100 (g/L, A) (cases, %) | 1 (14.3) |
| Bone lesion (B) (cases, %) | 7 (100) |
| Albumin < 35 g/L (cases, %) | 0 |
| β2-MG ≥ 5.5 mg/L (cases, %) | 1 (14.3) |
| LDH > 240 U/L (cases, %) | 1 (14.3) |
| Case | Gender/age (yr) | Chief complains | Ocular symptoms | Sites of orbital Lesions and Size | Operation treatment | Pathological diagnosis | Extraocular involvement |
| 1 | Male/44 | Enlarged left cheekbone mass for 8 mo | Left eyelid drooping, blurred vision three months later | Left eyelid drooping, blurred vision | Endoscopic tumor resection via nasal sella approach in March 2019 | Pasmacytoma of sellar region and clivus, infiltrating bone and submucosal | Left cheekbone mass 3 cm × 3 cm and mid-sternal mass 6 cm × 4 cm |
| 2 | Female/41 | Left eye socket swollen, lightly proptosis for more than one year | Left eye socket swollen, slightly proptosis and/or eye movement disorders | Left tear gland, mass size was unknown | The thyroid mass and left orbital lacrimal gland mass in May 2018 and April 2019 | left lacrimal gland plasmacytoma | Skull bone destruction and thyroid mass |
| 3 | Male/54 | Diagnosed with MM because of lung infection and elevated globulin in September 2011 | Blurred vision and proptosis and/or eye movement disorders in the right eye 2 yr later | The right orbital muscle and the left medial and lateral rectus muscles in orbital CT, mass size was unknown | No | No | Skull, ribs, lumbar skin, and mediastinum. The right ninth rib and L5 paravertebral multiple soft tissues, the largest mass reached 4.9 cm × 4.4 cm. |
| 4 | Male/42 | Diagnosed with MM because of bone pain in December 2007 | Blurred vision in left eye more than 3 yr later | Retina in left eye by head and eye MRI, mass in left retro-ocular choroid, size was 0.3 cm × 0.2 cm | Topical laser treatment | No | Cervical, thoracic and sacral vertebral bone lesion |
| 5 | Female/39 | Pain in the arch of the eyebrow and blurred vision in February 2010 | Pain in the arch of the eyebrow and blurred vision | left orbit mass size was 2.8 cm × 2.6 cm in PET-CT | Left orbital mass, bilateral uterine appendage and left breast mass excision in February and September 2010, respectively | Left orbital plasmacytoma | Bone lesion of thoracic vertebra ,Left breast, and ovarian mass, diagnosed with MM 8 mo later, left breast mass size was 12 cm × 10 cm in chest CT |
| 6 | Female/37 | Diagnosed with MM because of bone pain and increased creatinine in 2011 | Blurred vision in both eyes more than 4 yr later | Optic canal lesions in both eyes by head and eye MRI, the soft tissue mass were 1.5 cm × 0.8 cm and 0.9 cm × 0.3 cm in the right and left part | No | No | Sacral wing, sphenoid, clivus bone and pituitary |
| 7 | Female/33 | Diagnosed with MM because of Bone Pain and Lumbar Mass Biopsy in July 2014 | Discomfort and proptosis and/or eye movement disorders in the right eye more than 4 yr later | Right orbital inner and outer wall and musculoskeletal space mass, the right orbital mass was about 1.5 cm in diameter | Lumbar fracture internal fixation and tumor resection, right orbital tumor resection in July 2014 and March 2019, respectively | Right orbital plasmacytoma | Multiple bone destruction throughout the body |
The basic clinical characteristics and the ocular clinical manifestations of patients, such as ocular symptoms, the location of lesion, mass size, and extraocular manifestations, are shown in Tables 1 and 2, respectively.
Case 2 underwent resection of the thyroid mass and left orbital lacrimal gland mass in April 2018 and May 2019, respectively. Case 5 received left orbital mass, bilateral uterine appendage and left breast mass excision in February and September 2010, respectively. Case 7 underwent lumbar fracture internal fixation and tumor resection, right orbital tumor resection in July 2014 and March 2019, respectively.
Case 1 had a history of hypertension for more than 10 years. Case 2 suffered from hepatitis B at birth with long-term oral entecavir treatment. Since April 2019, she suffered from hypothyroidism and has been taking euthyrox for a long time. Case 3 was diagnosed as a hepatitis B carrier in 2010. There was nothing apparent in the past history of the other cases.
There were no specific tumor etiologies based on personal and family histories.
In case 1, bony masses of 3 cm × 3 cm and 6 cm × 4 cm were palpable on the left frontal bone and the left side of the sternum body, respectively, with a hard texture and poor mobility. In Case 3, a 4 cm × 4 cm mass was seen on the top of the head, with a hard texture and poor mobility. Case 4 was seen with strabismus of the left eye. In Case 5, a mass of about 3 cm in diameter was palpable in the left breast, firm, well-defined, and nontender. In Case 7, the right palpebral fissure was closed, and a 3 cm-long surgical suture incision was seen on the lower edge of the right eyebrow arch. No positive physical findings were found in other patients.
Immunofixation electrophoresis in 5 and 2 patients showed positive for IgG kappa and kappa light chain, respectively. Biochemical tests for elevated creatinine were seen in case 3, and hemoglobin less than 10 g/L and elevated lactate dehydrogenase were seen in case 7. Serum calcium levels were normal in all 7 patients.
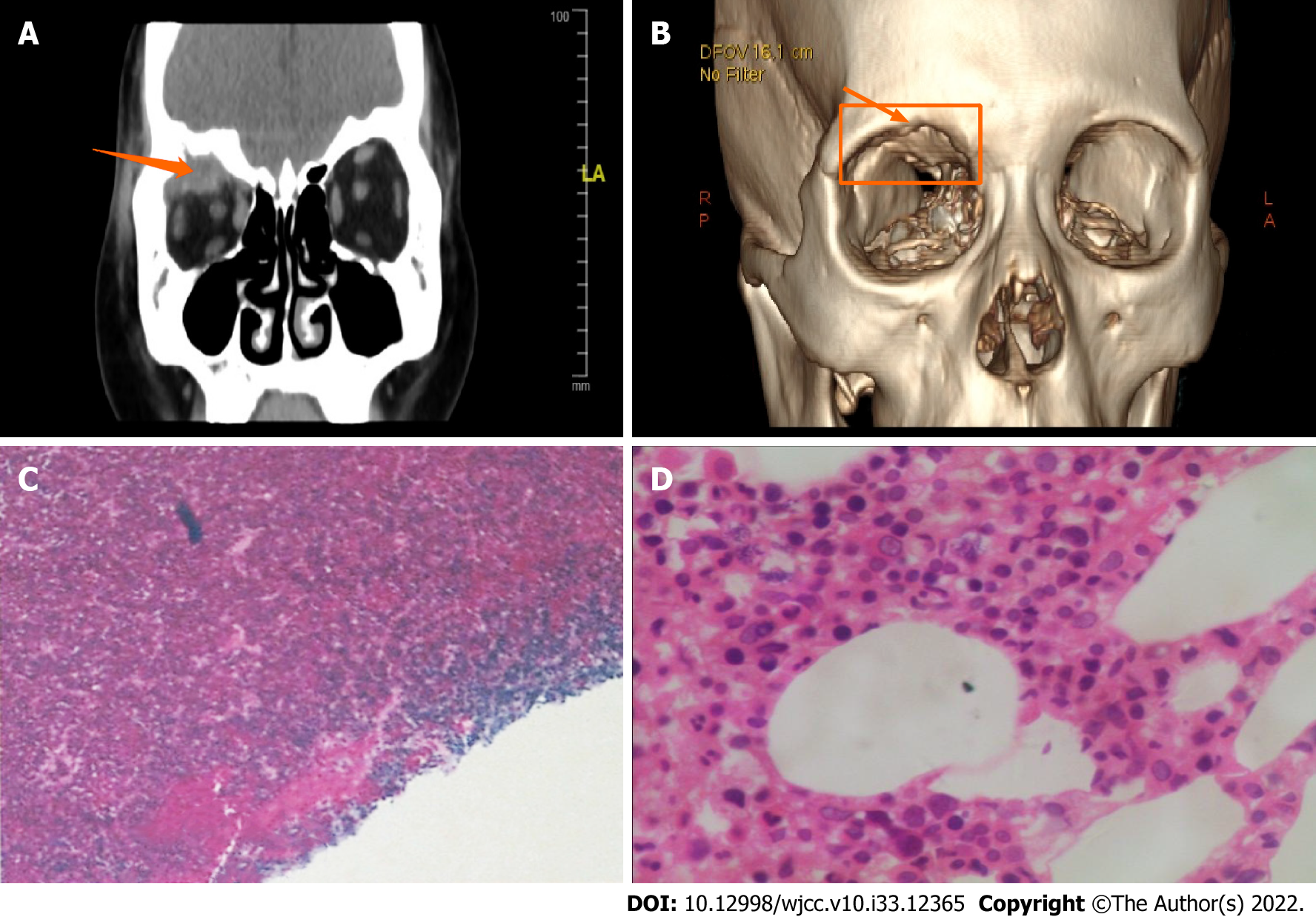
Bone marrow examination including bone marrow biopsy showed that the abnormal plasma cells in this group of patients were about 2.5%. Most of the patients were diagnosed with MM because of the local presence of biopsy-proven plasmacytoma; the latter belonged to peripheral EMD or extraocular EMD (Tables 1 and 2). Pathologic evaluation findings of biopsy specimens, the immunohistochemical characteristics were as follows: CD38 (+), CD138 (+ or -), Kappa (+), or CD56(+),and CD5, CD19, and CD20 were not expressed. The pathologic biopsy HE-stained smear is displayed in Figure 1.
All patients had bone destruction showed in all radiographic images including in computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography (PET)-CT or X-ray, of them, many patients had EMD masses, 6 patients had EMD at more than 2 locations, and the EMD was located in the spinal cord, thyroid gland, breast, armpit soft tissue, etc. There were 2 patients with EMD greater than 5 cm in maximum diameter.
Seven patients with MM and orbital EMD were followed up, the median follow-up time was 48 mo (range 24-120 mo). Three patients (14.3%) were ISS stages I and two patients (28.6%) were stage Ⅱ and III. There were five cases with IgG-κ type (71.4%) and two cases with κ-light chain type (28.6%). Among four patients who underwent FISH examination, two patients (28.6%) were positive for 1q21 amplification. The clinical data is shown in Table 1.
EMD was confirmed in all MM patients by radiologic examination and/or pathologic evaluation of biopsy specimens and immunohistochemistry. EMD involved orbital tissues in all patients, their diameter in two patients (28.6%) was ≥ 5 cm. In addition, six patients (85.7%) had EMD at sites other than the orbit. With the exception of orbital EMD, five patients (71.4%) had bEMD and two patients (28.6%) had sEMD. The sites of bEMD included the skull and ribs three patients each (42.9%), lumbar spine two cases (28.6%), sternum one patient (14.3%), acromial end and paralar sacrum one patient (14.3%). Non-orbital sEMD was detected in the thyroid gland, breast, and ovary in one patient each (14.3%). No cerebral parenchymal MM involvement was demonstrated in any patients.
Two patients (28.6%) had ocular symptoms as the presenting complaint, the other five patients (71.5%) presented with orbital EMD during the MM disease process. Two patients developed MM and EMD at the same time, while five patients developed MM and EMD sequentially. The interval between MM and orbital EMD spanned 0-52 mo, and it was with an average interval of 25 mo.
The two patients with ocular symptoms as the chief complaint were confirmed to have orbital plasmacytoma based on surgical and pathologic findings. Of the seven patients, five patients underwent ophthalmic surgery, and four of them were diagnosed with a plasmacytoma based on pathologic evaluation of biopsy specimens. Radiologic imaging findings of orbital EMD were available for all patients. Orbital EMD was shown to involve the superior orbital muscle intervertebral space, internal and external rectus muscles, lacrimal gland, optical canal, retina, and choroid plexus. The pathologic evaluation and immunohistochemistry findings are displayed in Figure 1.
Five patients (71.4%) had blurred vision and three patients (42.9%) had exophthalmos and/or ocular movement disorders. Other symptoms included eye discomfort, blepharoptosis, orbital swelling, and superciliary arch pain (one patient each, 14.3%). The clinical manifestations due to orbital EMD were usually related to the site of the lesion; the characteristics are shown in Table 2 and Figure 1.
Of the four patients diagnosed with plasmacytoma based on ophthalmic surgery, pathologic evaluation and the immunohistochemical findings (Figure 1).
The diagnostic criteria for MM conformed to the 2014 International Myeloma Working Group, and the International Staging System (ISS)[1] was used for the clinical staging of MM. Orbital EMD was diagnosed by imaging and/or pathologic evaluation of biopsy specimen, at least one tissue specimen exhibited as a clonal plasma cell infiltration or plasma cell tumor based on pathologic evaluation of a biopsy specimen and immunohistochemistry. The following exclusion criteria were applied in this study: non-periorbital EMD; plasma cell leukemia; and amyloidosis.
Based on the above systemic or ocular clinical manifestations, pathological and immunohistochemical results or radiographic findings of EMD, the seven patients were all diagnosed with MM combined with orbital EMD, and routine differential diagnosis was made to exclude other tumors.
The treatments were divided into induced remission, consolidation and maintenance therapy. Induction therapy was based on bortezomib (B or V), cyclophosphamide (C) or ifosfamide (IFO), adriamycin (A) or epirubicin (EPI) or liposome doxorubicin (PLD or D), dexamethasone (Dex or D), and lenalidomide (R) or thalidomide (T) for chemotherapy regimens, then received with maintenance therapy of thalidomide or lenalidomide. Generally, for every 1-2 courses of induced chemotherapy, serum or urine M protein was tested to determine the efficacy of the treatment, and it usually takes 6-9 cycles to carry out induced remission. Patient response was evaluated by M protein and CT or MRI based on the sites of extramedullary invasion[7].
Seven patients (100%) received chemotherapy, five patients (71.4%) underwent surgical resection of orbital tumors, and two patients (28.6%) received surgery, local radiotherapy, and combined chemotherapy synchronously.
Among the seven patients, six (85.7%) were treated with bortezomib-based chemotherapy and one (14.3%) with non-bortezomib-based chemotherapy. Six (85.7%) and one (14.3%) patients achieved a complete response (CR) and partial response (PR), respectively. However, one patient (14.3%) progressed rapidly within 6 mo after a CR was demonstrated. The treatment options and responses are shown in Table 3. During the treatment, the patient had good tolerance, and adverse reactions such as peripheral neuropathy, infection, and blood cell reduction were controllable.
| Case | Regimens | Treatment options | Response |
| 1 | VRD, ECHOP and lenalidomide maintenance therapy | Chemotherapy after surgery | PR |
| 2 | BAD and lenalidomide maintenance therapy | Chemotherapy and radiotherapy after surgery | PD soon after CR, then reached PR after undergoing Car-T therapy in another hospital |
| 3 | T-CAD, BAD and thalidomide maintenance therapy | Chemotherapy | CR |
| 4 | T-CAD and thalidomide maintenance therapy | Chemotherapy after surgery | CR |
| 5 | T-CAD, BAD, B-CEVAD and thalidomide maintenance therapy | Chemotherapy and radiotherapy after surgery | CR |
| 6 | T-CAD ,BAD and thalidomide maintenance therapy | Chemotherapy | CR |
| 7 | CAD,R-DECP, BED and lenalidomide maintenance therapy | Chemotherapy after surgery | CR |
The study was approved by the Ethics Committee of Beijing Chao-yang Hospital, Capital Medical University (Beijing, China) with detailed follow-up information obtained. All aspects of the study were conducted in accordance with the principles of the Declaration of Helsinki. Informed consent from patients was obtained to publish this paper.
Follow-up telephone contact was made with all patients who did not keep follow-up appointments or telephone interviews in the previous year. Death certificates were requested when needed.
SPSS 22.0 software was used for statistical description and analysis of relevant data as follows. Overall survival (OS) refer to the time from diagnosis to death or missed follow-up, and progression free survival (PFS) refer to the time from chemotherapy to relapse, progression, death of any cause or the last follow-up evaluation. The Kaplan Meier method was used to calculate OS and PFS functions, 5-year overall survival rate and the survival curves were drawn. The survival times of patients treated with different regimens were compared using the log-rank method (P < 0.05 was statistically significant).
All patients in this group were followed closely, and the last follow-up was May 2020. Three patients survived and four died. Two, one and one patient died of MM, multi-sites EMD and a severe pulmonary infection, respectively.
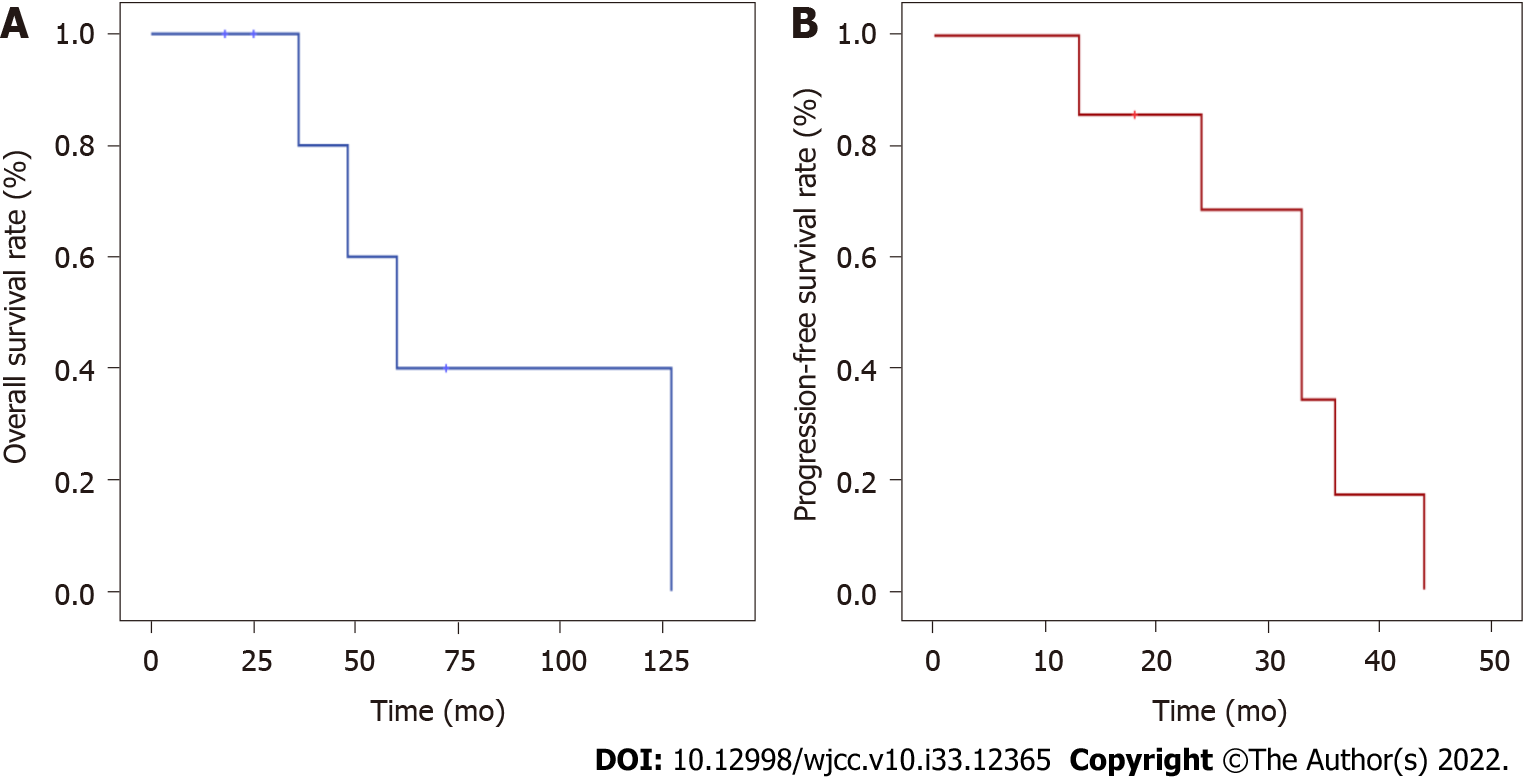
The log-rank method was used to compare OS of chemotherapy alone, surgery with chemotherapy, and surgery combined with chemotherapy and radiotherapy; there were no significant differences in treatment response (P = 0.176). Survival analysis showed that the OS ranged from 18 to 127 mo, with a median OS of 48 mo. In addition, PFS ranged from 18 to 44 mo, with a median PFS of 33 mo, and the 5-year OS rate was 40%. The survival curves are shown in Figure 2.
The clinical data of 737 patients with MM treated at our hospital in the most recent 12 years were reviewed in the present study. Among them, 186 patients were recognized to diagnose as MM with EMD, the incidence rate was 25.24%, which is similar to that reported in previous studies[1-6]. Orbital EMD accounted for 0.95% of MM patients during the same period and 3.76% of all patients with EMD. In a review of 2480 orbital lesions, most orbital tumors were benign, of which 317 (13%) were lymphoproliferative lesions and only 1% were plasmacytomas[8]. Therefore, MM complicated by orbital EMD is relatively rare.
The EMD diameter of two patients in this study was > 5 cm; however, how the focal size affects survival is controversial. Montefusco et al[9] concluded that EMD size is not correlated with PFS, but a significant difference exists with respect to OS. In 7 patients, the mean bone marrow plasma cell count 2.5%. Five of 7 (71.43%) patients had the IgG-k type of MM, the average IgG level was 9.52 g/L, and the 24-h urine κ level was 0.012 g/L in IgG k-type patients after developing eye symptoms. When EMD occurs, the number of naive plasma cells infiltrating tissues increased and the number of plasma cells in bone marrow decreased. EMD is related to an abnormality of adhesion molecules, and due to the decreased ability of the bone marrow microenvironment to control tumor cell invasion in the late stage of the disease, M protein secretion decreases due to plasmacytoblastization[5-6,10]. It has been suggested that there is an association between the MM subtype and the possibility of orbital infiltration, and IgG is more common according to the literature[11,12]. The IgG type of MM may be a risk factor for orbital involvement[13], which is in agreement with our results.
Orbital involvement is most common in patients with active MM disease, invasion of the orbit may be one of the extramedullary lesions, or may be the first manifestation of disease recurrence[14,15]. Of seven patients in this study, two patients presented with ocular involvement as the primary symptom (28.6%), and the remaining 5 patients developed periocular EMD after the diagnosis of MM was established (71.4%). MM complicated by orbital EMD, proptosis, and/or limited movement and other ocular symptoms can be treated as the first symptom, followed by ophthalmic surgery to remove the periorbital mass. The pathologic diagnosis of plasmacytoma or solitary plasmacytoma or MM can also be secondary to the course of MM. According to reports in the literature, orbital plasmacytoma mainly originates from the superior temporal quadrant of the orbit, and usually presents as progressive, painless swelling and protrusion of the eyeball, combined with blurred vision and retinal compression signs[11,16-18]. In addition, decreased visual acuity, edema, and diplopia are also common[11-12], most of which present as unilateral lesions[11,16]. Intraorbital disease tends to follow a more aggressive course compared with extraorbital plasmacytoma[11,17].
Within the entire group of patients, 71.4% had symptoms of blurred vision and 57.1% had EMD involving the orbital supraorbital muscle, extravertebral space, internal and external rectus muscles, lacrimal gland, optic canal area, retina, and choroid plexus. Among orbital lesions, intraocular involvement is less frequent than orbital involvement, and optic nerve involvement is rare, including direct infiltration, compression, or hyperviscosity syndrome caused by excess monoclonal immunoglobulin[6,16,19-22]. Optic neuropathy is further assessed with a fundus examination, CT or MRI, and laboratory tests to determine the cause of visual impairment.
There is no consensus on treatment approaches for patients with MM and EMD; however, a combination of EMD directed chemotherapy-based comprehensive treatment including novel agents which can increase local concentration through tissue may have the greatest efficacy[5]. In the present study, although no brain parenchyma involvement was detected, and six of seven patients (85.7%) had soft tissue involvement in > 2 sites, including the spinal cord, thyroid gland, breast, and axillary lesions. Six of seven of the patients were initially treated with bortezomib-based chemotherapy, and some patients also used bortezomib combined with liposomal doxorubicin or lenalidomide, etc. Three of seven patients received concurrent local radiotherapy combined with chemotherapy. With the exception of one patient who achieved a PR, the remaining six patients achieved a CR. Survival analysis showed that the median OS was 48 mo, which is similar to the reported survival[5,11], and significantly lower than the 5-7 years reported in the literature for MM patients without EMD[1], Thus, orbital EMD may be a negative factor for patient survival. Among the seven patients, two were 1q21-positive based on FISH amplification, that is also a poor prognostic factor in MM, with only 24 mo of survival time[2]. It is possible that EMD is an independent poor prognostic factor[23], which is significantly associated with decreased OS. CD38 monoclonal antibody (mAb) in combination with chemotherapy regimens, such as lenalidomide, pomalidomide, KRD (carfilzomib combined with RD) or DECP, and autologous stem cell transplantation (ASCT), including intrathecal injection, are currently used to increase drug concentration in tissues to eradicate the myeloma clone infiltration in extramedullary tissue[21,23]. The effect is limited, and patients often experience more aggressive disease later[9,24-26], with devastating visual impairment, and the main cause of the patient death is relapse or disease progression[5], presenting a more aggressive disease process, as in the current study.
Based on lymphoma-like regimens, such as PACE, DCEP, or 4 or more CD38 monoclonal antibody (mAb) drugs combined with VRD or KRD induction chemotherapy, followed by a long course of single or double ASCT, or a new therapy combined with Car-T, despite the unsatisfactory response to BCMA-based CAR-T therapy, only a limited time of remission can be maintained[27-29], as in case 2 in the current study. Although the ongoing lipid metabolomics studies by our group have not found specific tumor microenvironment abnormalities in MM patients with EMD[30], with the gradual clarification of the definition of EMD and the accumulation of evidence from multi-omics studies targeting high-risk MM patients, well-designed clinical trials of precision therapy will gradually overcome the poor prognosis of EMD in MM[29]. Due to the small number of cases and the long timespan of this study, there are inevitably many deficiencies. (1) The number of myeloma cells in the bone marrow is limited, and more importantly, there is a lack of abnormal biological data from the bone marrow or EMD tumor local parts, which may miss the design approaches of personalized targeted therapy; and (2) the application of appropriate therapies for high-risk MM, such as ASCT and CD38 mAb are also insufficient. So it is warranted of genome-directed personalized clinical trials with a large number of cases.
MM combined with orbital EMD is a rare clinical entity. The most common ocular symptoms are blurred vision, proptosis and/or ocular movement disorders. Although the response rate of bortezomib-based chemotherapy is high, survival was significantly shortened. Therefore, multidisciplinary management by hematologists and ophthalmic radiation oncologists is recommended (Timeline shown in Supplementary Table 1).
(1) MM with orbital EMD is rare, IgG type MM may be a risk factor for orbital involvement, and unilateral disease is more common; (2) General ocular symptoms are blurred vision, proptosis or eye movement disorders, and eye pain is not apparent. Orbital MM is an aggressive lesion that may cause catastrophic visual impairment. In addition to a biopsy of the lesion, PET/CT or MRI is more sensitive and useful than CT for the diagnosis and evaluation of orbital MM; and (3) nowadays, more precision, individualized therapy for these patients has not improved, and survival is inadequate.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reda R, Italy; Solimando AG, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Palumbo A, Rajkumar SV, San Miguel JF, Larocca A, Niesvizky R, Morgan G, Landgren O, Hajek R, Einsele H, Anderson KC, Dimopoulos MA, Richardson PG, Cavo M, Spencer A, Stewart AK, Shimizu K, Lonial S, Sonneveld P, Durie BG, Moreau P, Orlowski RZ. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Da Vià MC, Solimando AG, Garitano-Trojaola A, Barrio S, Munawar U, Strifler S, Haertle L, Rhodes N, Teufel E, Vogt C, Lapa C, Beilhack A, Rasche L, Einsele H, Kortüm KM. CIC Mutation as a Molecular Mechanism of Acquired Resistance to Combined BRAF-MEK Inhibition in Extramedullary Multiple Myeloma with Central Nervous System Involvement. Oncologist. 2020;25:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Rosiñol L, Beksac M, Zamagni E, Van de Donk NWCJ, Anderson KC, Badros A, Caers J, Cavo M, Dimopoulos MA, Dispenzieri A, Einsele H, Engelhardt M, Fernández de Larrea C, Gahrton G, Gay F, Hájek R, Hungria V, Jurczyszyn A, Kröger N, Kyle RA, Leal da Costa F, Leleu X, Lentzsch S, Mateos MV, Merlini G, Mohty M, Moreau P, Rasche L, Reece D, Sezer O, Sonneveld P, Usmani SZ, Vanderkerken K, Vesole DH, Waage A, Zweegman S, Richardson PG, Bladé J. Expert review on soft-tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br J Haematol. 2021;194:496-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Bansal R, Rakshit S, Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021;11:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 6. | Bhutani M, Foureau DM, Atrash S, Voorhees PM, Usmani SZ. Extramedullary multiple myeloma. Leukemia. 2020;34:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Yu Y, Huang Z, Hu W, Li X, Shen M, Zhang J, Tang R, Chen S, Chen W. Clinical Analysis of Cardiac Involvement in 53 Patients With Multiple Myeloma Coexistent With Light Chain Amyloidosis. Clin Lymphoma Myeloma Leuk. 2020;20:519-525.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Bonavolontà G, Strianese D, Grassi P, Comune C, Tranfa F, Uccello G, Iuliano A. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthalmic Plast Reconstr Surg. 2013;29:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Montefusco V, Gay F, Spada S, De Paoli L, Di Raimondo F, Ribolla R, Musolino C, Patriarca F, Musto P, Galieni P, Ballanti S, Nozzoli C, Cascavilla N, Ben-Yehuda D, Nagler A, Hajek R, Offidani M, Liberati AM, Sonneveld P, Cavo M, Corradini P, Boccadoro M. Outcome of paraosseous extra-medullary disease in newly diagnosed multiple myeloma patients treated with new drugs. Haematologica. 2020;105:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Das R, Strowig T, Verma R, Koduru S, Hafemann A, Hopf S, Kocoglu MH, Borsotti C, Zhang L, Branagan A, Eynon E, Manz MG, Flavell RA, Dhodapkar MV. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat Med. 2016;22:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Burkat CN, Van Buren JJ, Lucarelli MJ. Characteristics of orbital multiple myeloma: a case report and literature review. Surv Ophthalmol. 2009;54:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Tai E, Sim SK, Haron J, Wan Hitam WH. Orbital multiple myeloma: a diagnostic challenge. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Thoumazet F, Donnio A, Ayeboua L, Brebion A, Diedhou A, Merle H. Orbital and muscle involvement in multiple myeloma. Can J Ophthalmol. 2006;41:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Barmas-Alamdari D, Sodhi GS, Shenouda TA. Bilateral Proptosis in a Case of Recurring Multiple Myeloma: Uncommon Orbital Presentation of Plasmacytoma. Int Med Case Rep J. 2020;13:297-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mani M, Kasturi N, Sravya R, Kaliaperumal S, Gochhait D. Orbital plasmacytoma as the presenting feature in multiple myeloma. Eur J Ophthalmol. 2021;31:NP1-NP4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Chin KJ, Kempin S, Milman T, Finger PT. Ocular manifestations of multiple myeloma: three cases and a review of the literature. Optometry. 2011;82:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Uceda-Montañés A, Blanco G, Saornil MA, Gonzalez C, Sarasa JL, Cuevas J. Extramedullary plasmacytoma of the orbit. Acta Ophthalmol Scand. 2000;78:601-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Matos A, Goulart A, Ribeiro A, Freitas R, Monteiro C, Martins P. Orbital Plasmacytoma, An Uncommon Presentation of Advanced Multiple Myeloma. Eur J Case Rep Intern Med. 2020;7:001149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Sandor KP, Micieli JA, Peragallo JH. Optic nerve head plasmacytoma as a manifestation of multiple myeloma. Taiwan J Ophthalmol. 2021;11:97-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Kwaan HC, Bongu A. The hyperviscosity syndromes. Semin Thromb Hemost. 1999;25:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Adkins JW, Shields JA, Shields CL, Eagle RC Jr, Flanagan JC, Campanella PC. Plasmacytoma of the eye and orbit. Int Ophthalmol. 20:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Jurczyszyn A, Grzasko N, Gozzetti A, Czepiel J, Cerase A, Hungria V, Crusoe E, Silva Dias AL, Vij R, Fiala MA, Caers J, Rasche L, Nooka AK, Lonial S, Vesole DH, Philip S, Gangatharan S, Druzd-Sitek A, Walewski J, Corso A, Cocito F, Vekemans MC, Atilla E, Beksac M, Leleu X, Davila J, Badros A, Aneja E, Abildgaard N, Kastritis E, Fantl D, Schutz N, Pika T, Butrym A, Olszewska-Szopa M, Usnarska-Zubkiewicz L, Usmani SZ, Nahi H, Chim CS, Shustik C, Madry K, Lentzsch S, Swiderska A, Helbig G, Guzicka-Kazimierczak R, Lendvai N, Waage A, Andersen KT, Murakami H, Zweegman S, Castillo JJ. Central nervous system involvement by multiple myeloma: A multi-institutional retrospective study of 172 patients in daily clinical practice. Am J Hematol. 2016;91:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Deng S, Xu Y, An G, Sui W, Zou D, Zhao Y, Qi J, Li F, Hao M, Qiu L. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single-center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Tathineni P, Cancarevic I, Malik BH. Uncommon Presentations of Multiple Myeloma. Cureus. 2020;12:e8400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Kuo HH, Shen EP. Hyperviscosity retinopathy as the initial presentation of aggressive multiple myeloma. Tzu Chi Med J. 2020;32:401-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Wang SSY, Lee MB, George A, Wang SB, Blackwell J, Moran S, Francis IC. Five cases of orbital extramedullary plasmacytoma: diagnosis and management of an aggressive malignancy. Orbit. 2019;38:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Zamagni E, Barbato S, Cavo M. How I treat high-risk multiple myeloma. Blood. 2022;139:2889-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Leypoldt LB, Besemer B, Asemissen AM, Hänel M, Blau IW, Görner M, Ko YD, Reinhardt HC, Staib P, Mann C, Lutz R, Munder M, Graeven U, Peceny R, Salwender H, Jauch A, Zago M, Benner A, Tichy D, Bokemeyer C, Goldschmidt H, Weisel KC. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT trial. Leukemia. 2022;36:885-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Bladé J, Beksac M, Caers J, Jurczyszyn A, von Lilienfeld-Toal M, Moreau P, Rasche L, Rosiñol L, Usmani SZ, Zamagni E, Richardson P. Extramedullary disease in multiple myeloma: a systematic literature review. Blood Cancer J. 2022;12:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 30. | Wei Y, Wang J, Chen F, Li X, Zhang J, Shen M, Tang R, Huang Z. Serum Abnormal Metabolites for Evaluating Therapeutic Response and Prognosis of Patients With Multiple Myeloma. Front Oncol. 2022;12:808290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |