Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12319
Peer-review started: July 11, 2022
First decision: September 25, 2022
Revised: October 10, 2022
Accepted: November 2, 2022
Article in press: November 2, 2022
Published online: November 26, 2022
Processing time: 134 Days and 18 Hours
Acute intermittent porphyria (AIP) is a rare autosomal dominant porphyrin metabolic disease caused by a mutation in the hydroxymethylbilane synthase
A 22-year-old Chinese woman developed severe abdominal pain, lumbago, sinus tachycardia, epileptic seizure, hypertension, and weakness in lower limbs in March, 2018. Biochemical examinations indicated hypohepatia and hyponatremia. Her last menstrual period was 45 d prior to admission, and she was unaware of the pregnancy, which was confirmed by a pregnancy test after admission. Sunlight exposure of her urine sample for 1 h turned it from yellow to wine red. Urinary porphyrin test result was positive. Based on these clinical manifestations, AIP was diagnosed. After increasing her daily glucose intake (250–300 g/d), abdominal pain was partially relieved. Three days after hospitalization, sponta
We identified a novel HMBS gene mutation in a Chinese patient with AIP and confirmed its pathogenicity.
Core Tip: The possible pathogenic loci in a woman with acute intermittent porphyria were identified by direct sequencing, which revealed a novel heterozygous splicing mutation (c.648_651+1delCCAGG) in exon 10 of hydroxymethylbilane synthase gene. Aberrant splicing, which led to the production of a truncated protein, was confirmed through an in vitro minigene assay and bioinformatics analysis.
- Citation: Zhou YQ, Wang XQ, Jiang J, Huang SL, Dai ZJ, Kong QQ. Novel hydroxymethylbilane synthase gene mutation identified and confirmed in a woman with acute intermittent porphyria: A case report. World J Clin Cases 2022; 10(33): 12319-12327
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12319.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12319
Acute intermittent porphyria (AIP) is a rare metabolic disease, and its incidence is difficult to accurately calculate. Reports vary between different countries and regions, the incidence of symptomatic AIP is 5.9 per million in Europe, 6.3 per million in Spain, 7.6 in France and 10-15 in Switzerland[1]. Available reports have demonstrated that AIP has various clinical manifestations. Typical clinical features during an acute attack of AIP, also known as overt AIP, include abdominal pain, constipation, nausea, vomiting, tachycardia, hypertension, muscle weakness, respiratory failure, epilepsy, and hyponatremia. Cases with posterior reversible encephalopathy syndrome have also been reported recently[1-3]. AIP attacks can be stimulated through several factors including sex hormone changes (estrogen and progesterone) involved in menstruation, pregnancy and childbirth, starvation, emotional fluctuations, excessive fatigue, and alcohol[4,5]. Drugs and bacterial infections also play an important role in the growth and development of various diseases including porphyria, which has been confirmed in recently published articles.[6]. Patients with latent AIP present with no or only mild clinical symptoms, and their biochemical tests may be normal[4,5]. Diagnosis of AIP is mainly dependent on clinical symptoms, biochemical examinations, and molecular analyses[7]. The detection of previously identified heterozygous hydroxymethylbilane synthase (HMBS) gene mutations through DNA analysis is the gold standard for diagnosis[7-11]. We report a case of a 22-year-old female diagnosed with AIP who was found to have a novel heterozygous mutation (c.648_651+1delCCAGG) in HMBS gene, which was also identified in her relatives. These findings could help the proband and related variant carriers to avoid life-threatening acute episodes. This case contributes to the existing knowledge on AIP and its causative genetic mutations.
A Chinese woman presented to our hospital because of severe abdominal pain and lumbago.
A 22-year-old Chinese woman developed severe abdominal pain and lumbago without obvious inducement 1 wk ago, the symptoms worsened accompanied by epileptic seizure and weakness in lower limbs for 3 d.
In February, 2017, she gave birth to a baby after an unremarkable pregnancy. In July, 2017, she began to experience abdominal pain, back pain, and lumbago 5–10 d before her menstrual period. The pain was not severe and was relieved during menstruation.
Neither the patient nor her family members had any remarkable medical history.
Her blood pressure was 160/100 mmHg and her heart rate was 110 bpm. Abdominal examination was normal with no tenderness or rebound pain elicited. Muscle tone in the extremities was normal, the muscle strength of both upper limbs was at level 5, and muscle strength in both lower limbs was at level 3 (Medical Research Council Scale for Muscle Strength is described in Table 1).
| Function of the muscle | Grade |
| No movement is observed | 0 |
| Only a trace or flicker of movement is seen or felt in the muscle or fasciculations are observed in the muscle | 1 |
| Muscle can move only if the resistance of gravity is removed | 2 |
| Muscle strength is further reduced such that the joint can be moved only against gravity with the examiner’s resistance completely removed | 3 |
| Muscle strength is reduced but muscle contraction can still move joint against resistance | 4 |
| Muscle contracts normally against full resistance | 5 |
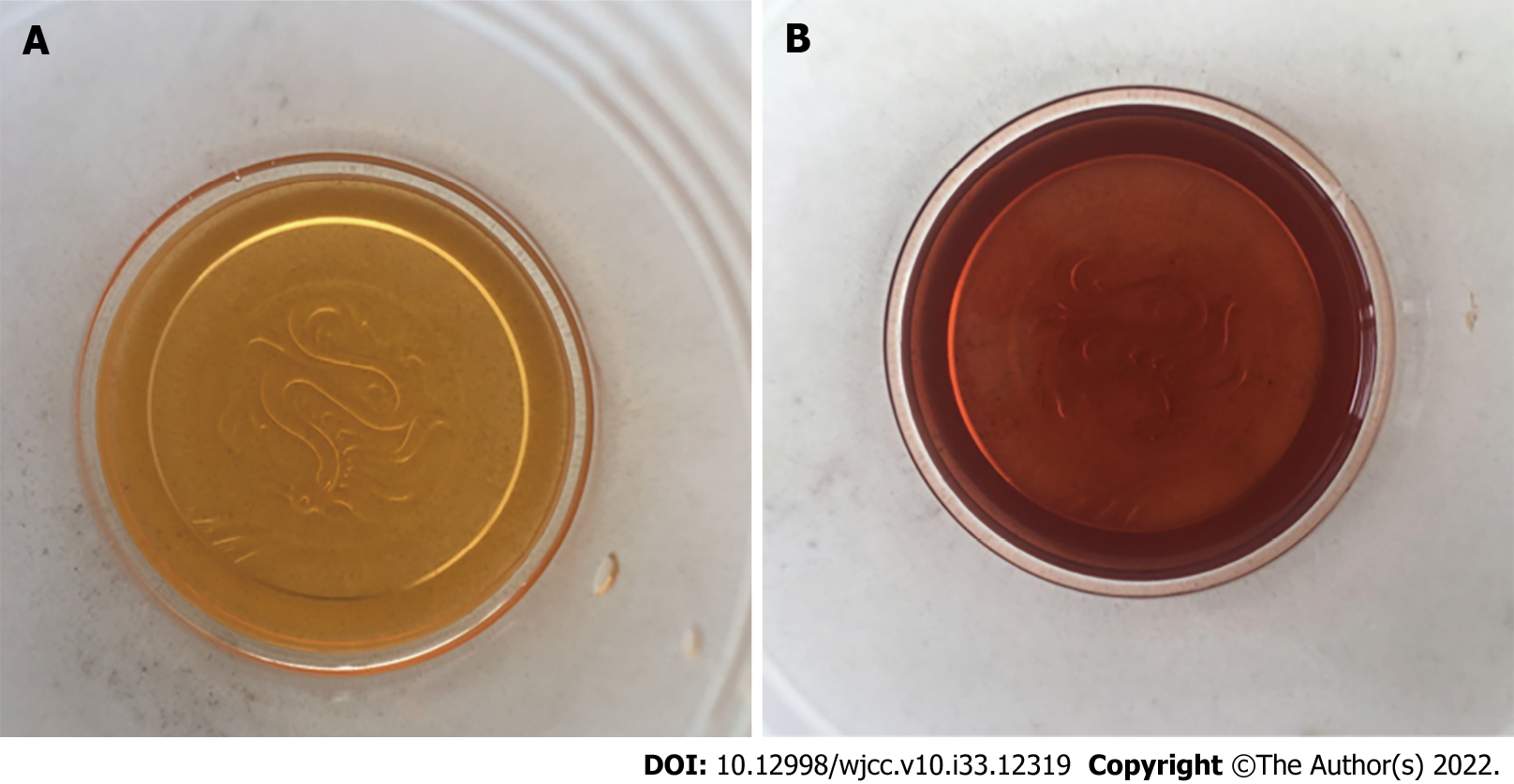
Routine test results, such as kidney function, myocardial enzymes, and thyroid function, were normal. Serum chemistry findings were as follows: Hyponatremia (Na: 119–128 mmol/L; normal: 135–146 mmol/L), and hypohepatia (alanine aminotransferase: 272 IU/L, normal: 7–40 IU/L, aspartate aminotransferase: 72 IU/L, normal: 13–35 IU/L). When the patient’s urine sample was exposed to sunlight for 1 h, it turned from yellow to wine red (Figure 1). A qualitative test of urinary porphyrin was positive.
No radiological investigations were performed because she was pregnant and was diagnosed with AIP soon after admission.
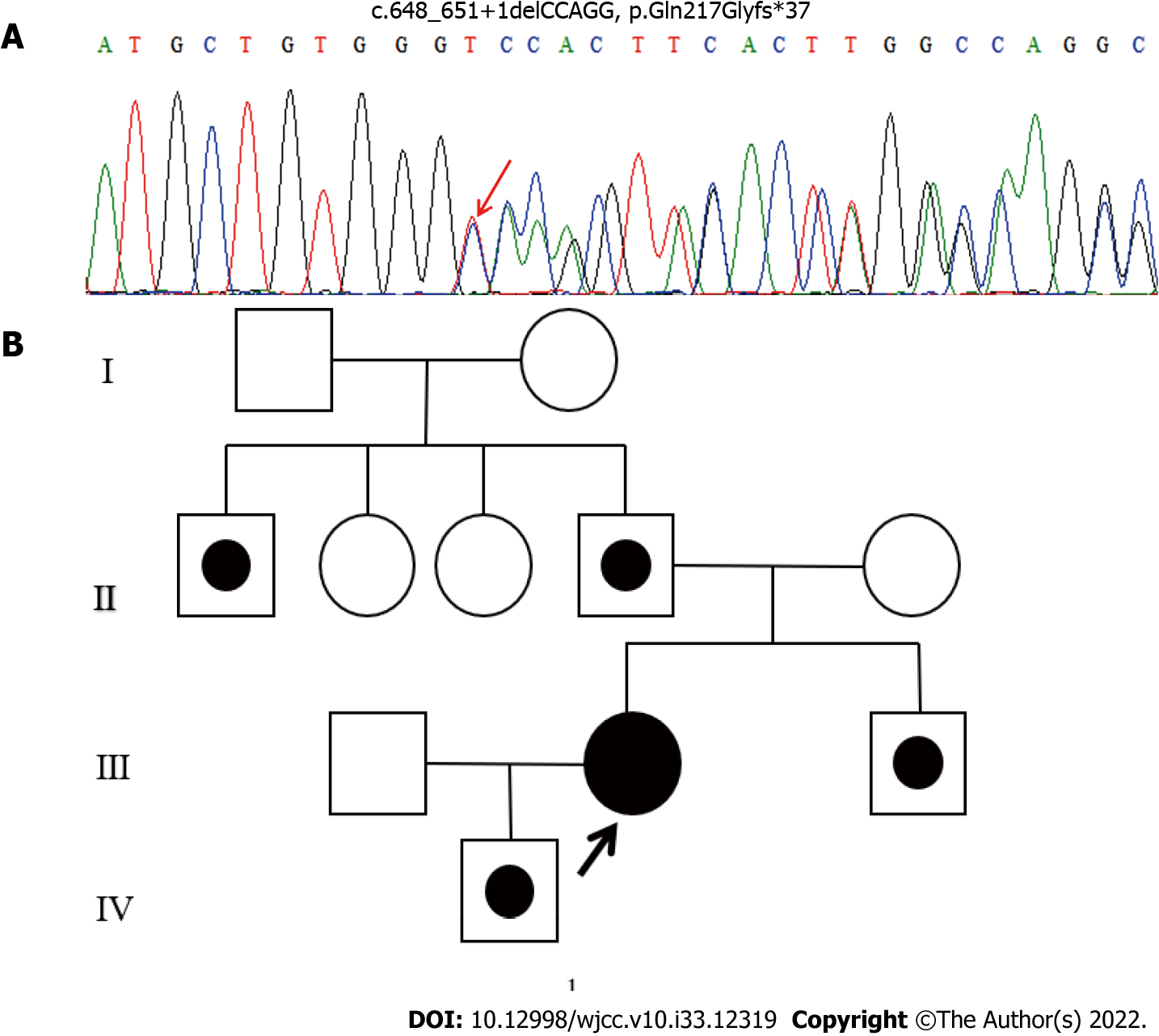
The study was approved by the ethics committee of Dongguan Hospital of Traditional Chinese Medicine [Ethical number: (2022), No. 15]. Genomic DNA was extracted from peripheral blood samples using QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) and sent to the Macro-micro-test Company (Beijing, China). The HMBS (NM_000190) gene was analyzed by direct sequencing using a 3730XL DNA Analyzer (Applied Biosystems, Foster City, California). The sequence exhibited a heterozygous mutation (c.648_651+1delCCAGG) in exon 10 of HMBS gene of the proband (Figure 2A). In addition to the proband, four other family members were identified as carriers of the mutation: Her father (II.4), paternal uncle (II.1), younger brother (III.3), and son (IV.1) (Figure 2B). Her mother and two aunts tested negative for the mutant allele. All family members denied consanguinity, and no abnormalities were identified on physical or laboratory examinations.
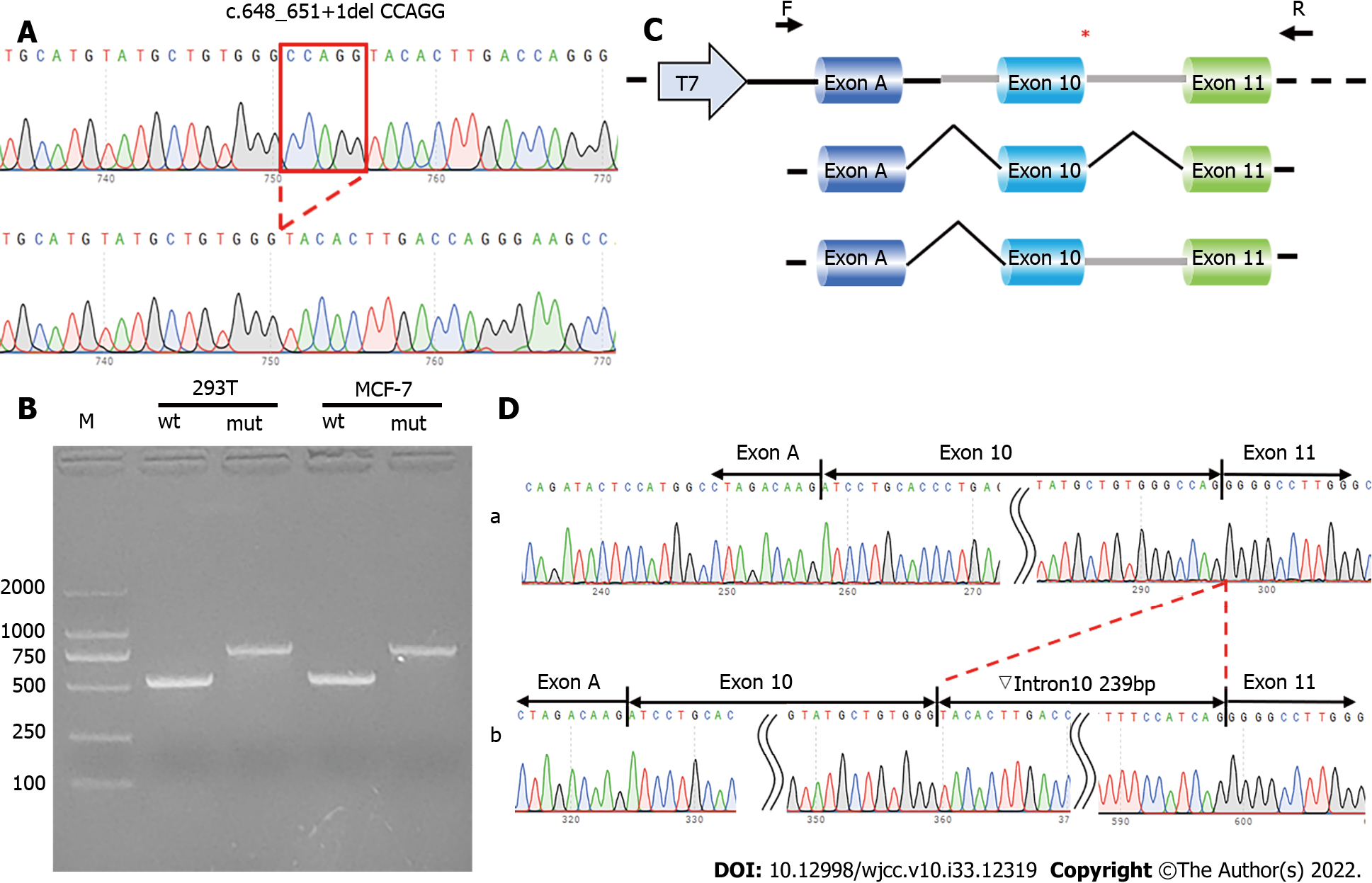
To verify the functional effect of the c.648_651+1delCCAGG mutation in HMBS gene on pre-mRNA splicing, a minigene assay was carried out to evaluate the effect of the mutation on mRNA splicing (Figure 3). Wild-type and mutant minigenes were inserted into a pcMINI-C vector. The construction strategy inserted part of intron 9 (251 bp), exon 10 (39 bp), intron 10 (240 bp), and exon 11 (120 bp) of HMBS gene, in addition to exon A-intron A of MSC [exon A-intron A-intron 9 (251 bp)-exon 10 (39 bp)-intron 10 (240 bp)-and exon 11 (120 bp)] (Figure 3A and C). A total of four recombinant vectors were transfected into HeLa and 293T cells. After 48 h, four samples were collected, total RNA was extracted and reverse-transcribed into cDNA, and the cDNA was amplified using reverse transcription primers. Polymerase Chain Reaction (PCR) products were used to observe whether the exon A-exon 10-exon 11 transcript had a splicing pathogenic variant. Recombinant vectors showed that both wild-type and mutant minigenes were successfully inserted into the corresponding vectors (Figure 3A). Reverse Transcription PCR for the PcMINI-C-HMBS-wt/mut minigene showed that, in both cell lines, the wild-type minigene had a band of the expected size (553 bp; Band a), while the mutant minigene had a larger band (Band b). When both were sequenced, Band a was a normally spliced band with the same splicing pattern as exon A-exon 10 (39 bp)-exon 11 (120 bp), while Band b retained the complete intron 10 sequence. Its splice pattern was exon A-exon 10 (39 bp)-▽intron 10 (239 bp)-exon 11 (120 bp) (Figure 3B and D). The mutation led to an overall retention of intron 10, turning this into a coding region. The minigene expression in vitro confirmed that the variant c.648_651+1delCCAGG could lead to aberrant mRNA splicing.
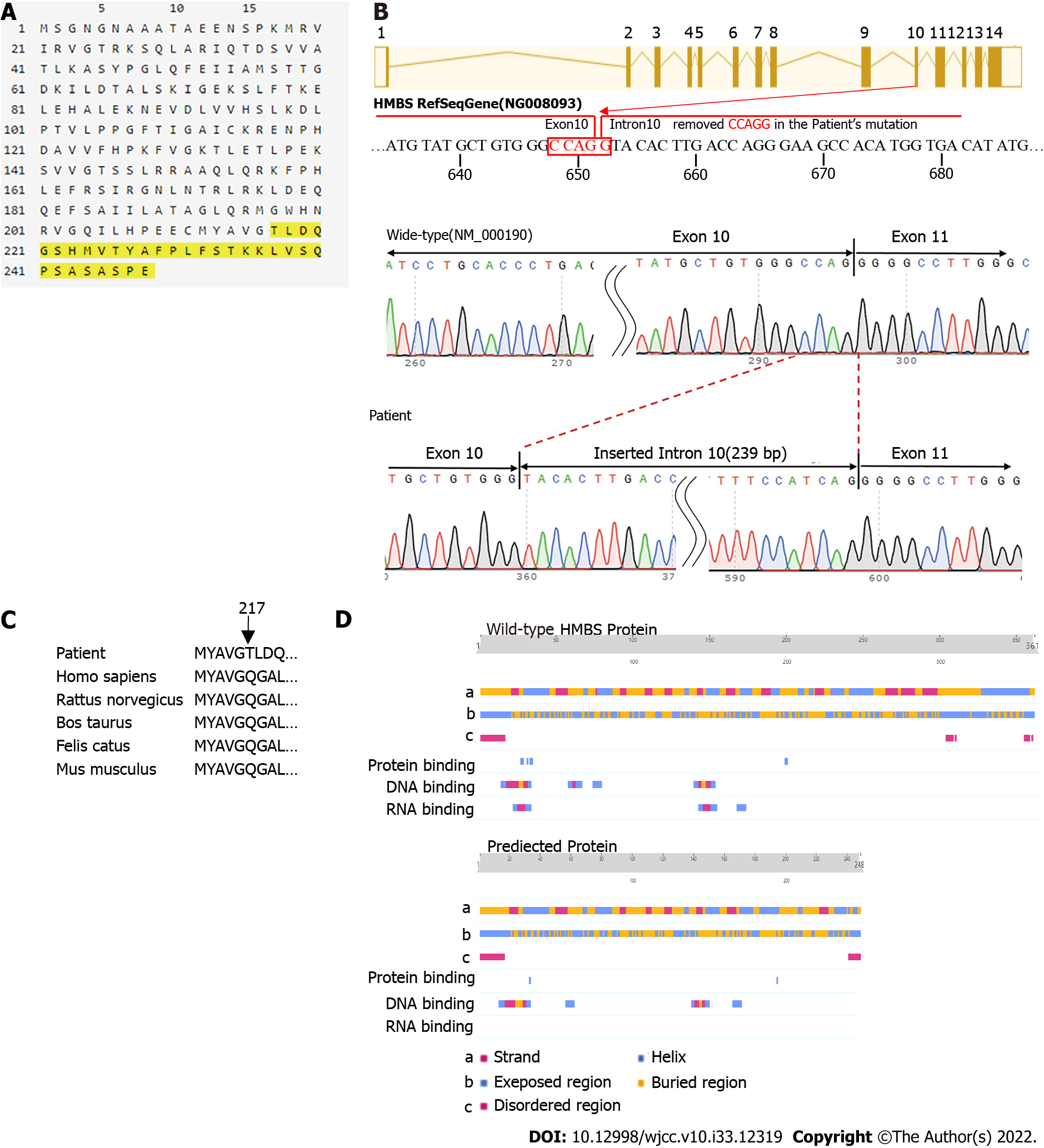
The genotype of the proband and her family members was identified according to the sequencing results yielded by SeqMan software (Lasergene Genomics, DNASTAR, Inc., Madison, Wisconsin). The mutation identified in these individuals was not in the Human Gene Mutation Database (HGMD, http://www.hgmd.org), Exome Aggregation Consortium (http://exac.broadinstitute.org), or the 1000 Genomes Project (http://www.1000genomes.org). We used ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder) to predict the reading frame of the amino acid sequence and PredictProtein (https://www.predictprotein.org) to analyze whether the secondary structure and function of proteins were altered by the mutation. ORFfinder demonstrated that the mutation site affects the normal translation of the protein. The c.648_651+1delCCAGG mutation caused the original donor recognition site of exon 10 to be destroyed. When the original donor recognition site was destroyed, the original splicing site might not have been spliced, resulting in retention of intron 10. Because the stranded intron 10 contains a stop codon, mRNA is terminated prematurely during translation, resulting in a protein variant with a length of 248 amino acids (Figure 4A and B). In comparison with the amino acid sequence of wild-type HMBS, the Q217T mutation of the new mutant occurred at site 217, and the translation was terminated after synthesis of 31 amino acids at the mutant site (Figure 4A and C). These findings are consistent with the results of the minigene assay.
We conducted further analysis using PredictProtein and found that the secondary structure of the protein also changed as follows: (1) The partially folded and helical regions of the truncated protein sequence were changed; (2) The exposed area of the surface was partially changed; (3) The disordered area at position 350 was missing; and (4) The RNA and protein binding regions had changed (Figure 4D).
The patient was ultimately diagnosed with AIP.
The administration of high glucose (250–300 g/d) partially relieved the patient’s abdominal pain. Three days after hospitalization, the patient spontaneously aborted. This was followed by complete resolution of symptoms.
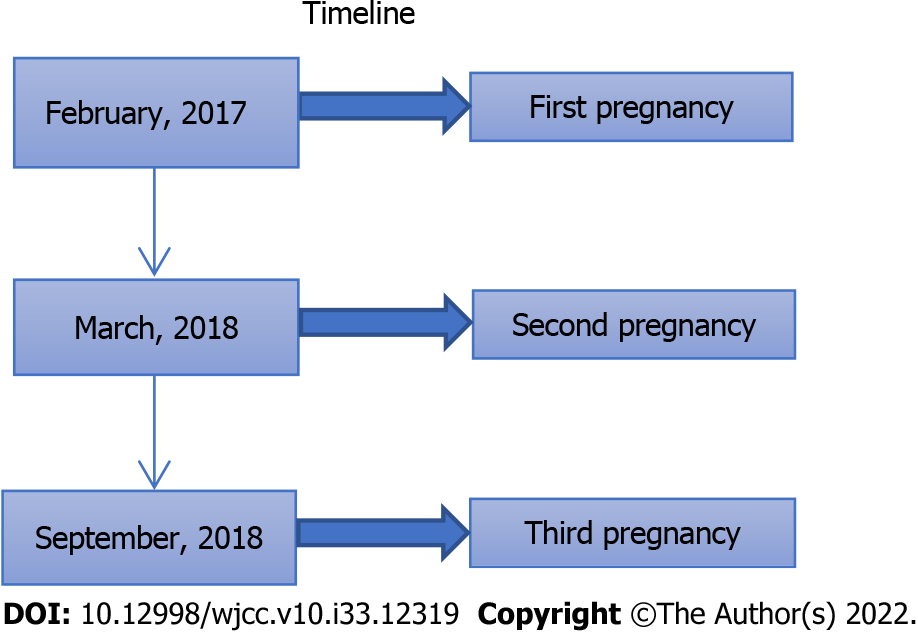
We advised the proband and the variant carriers to avoid triggers of acute attacks. In September, 2018, the patient had an unplanned pregnancy. Symptoms, such as abdominal pain and weakness, recurred. Considering her illness and at her request, we performed an abortion on the patient. All her symptoms resolved after the termination of the pregnancy. She was advised to increase sugar intake and to use contraception. She was asymptomatic on follow-up. A timeline of her three pregnancies is shown in Figure 5.
AIP is a rare autosomal dominant metabolic disease caused by mutations in the gene that encodes HMBS[7,8,12]. The HMBS gene is located on chromosome 11q23.3 and includes 15 exons and two different promoters. To date, 504 mutations have been identified, of which 203 are point mutations, 107 are deletions mutations, 102 are splicing mutations, 48 are insertion mutations, and 44 are other types of mutations (HGMD, http://www.hgmd.cf.ac.uk/ac/index.php)[13]. A study from France has shown that the prevalence of symptomatic AIP is approximately 7.6 per million inhabitants, while pathogenetic mutations in the HMBS gene are observed in 1/1782[1,14]. This means that the penetrance of AIP is very low, being observed in only approximately 1% of the general population and 20%-50% of families with AIP[1,14]. In principle, AIP is an autosomal dominant metabolic disease, with no sex predilection[15]. However, female mutation carriers are more likely to experience acute attacks after puberty, which are attributed to shifting levels of estrogen and progesterone related to the menstrual cycle, while male mutation carriers occasionally have mild or no clinical symptoms[16]. In our study, the female variant carrier (proband) suffered pregnancy-induced acute attacks, which could be diagnosed based on clinical symptoms and biochemical examinations. Of the male variant carriers, two experienced irregular abdominal pain and constipation (her father and uncle), while two others (her younger brother and son) experienced no clinical symptoms. The observed penetrance of the disease and the different clinical manifestations between men and women were consistent with that of previous clinical reports[1,4-6].
Increased estrogen and progesterone during pregnancy can precipitate acute attacks of porphyria, and the pregnancy outcomes of women with AIP can include uneventful pregnancy, normal delivery, spontaneous abortion and termination of the fetus as a result of not only the pregnancy but also the high incidence of associated hyperemesis gravidarum and drug use[5,7]. This partly explains why manifestation of the mutation did not interfere in the patient’s first pregnancy.
A novel splicing pathogenic variant (c.648_651+1delCCAGG) was identified in the family using Sanger sequencing. This mutation occurs in the junction region of exon 10 and intron 10, thus depriving the junction region of the GU-AG splice site[17] and preventing the intron cleavase enzyme from recognizing the splicing site; therefore, the whole of intron 10 is retained in the mature mRNA when the pre-mRNA is spliced. However, because the retained intron sequence contains a stop codon, it causes early termination of translation, potentially producing a shortened protein. ORFfinder predicted that the shortened protein sequence contains 248 amino acids. Compared with the wild-type protein sequence of 361 amino acids, the truncated protein sequence shows changes in the structure and function of the enzyme due to the deletion of 113 amino acids. The in vitro minigene expression also confirmed that the variant could lead to retention of intron 10 and aberrant mRNA splicing.
We predicted and analyzed the secondary structure of the truncated protein using PredictProtein and found that the beta-fold and alpha-helix sites in the shortened protein changed; furthermore, the DNA, RNA, and protein binding sites were altered. In particular, the lack of RNA binding sites may affect its function, because the interaction between protein and RNA plays an important role in many biological activities, such as post-transcriptional gene regulation and regulation of gene expression[18-20]. In addition, the mutant generated a new disordered region at position 247 but missed the disordered regions at positions 310 and 361 of the wild-type. Such disordered regions of proteins are also involved in gene signal transduction and regulation[21,22]. Therefore, the new mutant may cause disease due to loss of a functional region.
Fifty percent of missense mutations identified at the DNA and RNA levels have been confirmed to disrupt mRNA splicing[23]. So far, more than 500 HMBS gene variants that cause AIP have been verified in HGMD (http://www.hgmd.cf.ac.uk/ac/index.php), of which 108 Led to splicing. We analyzed the pathogenicity of the c.648_651+1delCCAGG mutation using a bioinformatics method and in vitro minigene assay. To further explore the molecular pathogenesis of the mutation, we intend to detect the influence of the mutation on enzyme activity. We aim to conduct further research to determine whether the mutation can lead to nonsense-mediated mRNA decay as well as detect the presence of truncated proteins, degradation of truncated proteins, and interaction of downstream proteins in vivo. Furthermore, we intend to demonstrate the pathogenicity of gene mutation from multiple perspectives.
We identified a novel splicing mutation (c.648_651+1delCCAGG) in the HMBS gene of a Chinese woman with AIP and verified the pathogenicity of the variant through bioinformatics methods and an in vitro minigene assay. We provided the mutation carriers a clearer picture of their condition and provided recommendations on how to prevent potentially life-threatening acute episodes. Our discovery has also expanded the known spectrum of pathogenic HMBS gene mutations.
The authors thank the family members for providing blood samples, cooperating with treatment, and agreeing to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ata F, Qatar; Giacomelli L, Italy S-Editor: Wei ZH L-Editor: A P-Editor: Wei ZH
| 1. | Ma L, Tian Y, Peng C, Zhang Y, Zhang S. Recent advances in the epidemiology and genetics of acute intermittent porphyria. Intractable Rare Dis Res. 2020;9:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Wylie K, Testai FD. Neurological Manifestations of Acute Porphyrias. Curr Neurol Neurosci Rep. 2022;22:355-362. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Simon NG, Herkes GK. The neurologic manifestations of the acute porphyrias. J Clin Neurosci. 2011;18:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Marsden JT, Rees DC. A retrospective analysis of outcome of pregnancy in patients with acute porphyria. J Inherit Metab Dis. 2010;33:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Wang B, Rudnick S, Cengia B, Bonkovsky HL. Acute Hepatic Porphyrias: Review and Recent Progress. Hepatol Commun. 2019;3:193-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Ma Y, Teng Q, Zhang Y, Zhang S. Acute intermittent porphyria: focus on possible mechanisms of acute and chronic manifestations. Intractable Rare Dis Res. 2020;9:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Fu Y, Jia J, Yue L, Yang R, Guo Y, Ni X, Shi T. Systematically Analyzing the Pathogenic Variations for Acute Intermittent Porphyria. Front Pharmacol. 2019;10:1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Hu Y, Li W, Kang N, Ma L, Teng Q, Mo G, Wu J, Wang X, Bi R, Zhang S. Identification and molecular analysis of 17 novel variants of hydroxymethylbilane synthase in Chinese patients with acute intermittent porphyria. Clin Genet. 2022;101:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Morán-Jiménez MJ, Borrero-Corte MJ, Jara-Rubio F, García-Pastor I, Díaz-Díaz S, Castelbón-Fernandez FJ, Enríquez-de-Salamanca R, Méndez M. Molecular Analysis of 55 Spanish Patients with Acute Intermittent Porphyria. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Loskove Y, Yasuda M, Chen B, Nazarenko I, Cody N, Desnick RJ. Acute hepatic porphyrias: Identification of 46 hydroxymethylbilane synthase, 11 coproporphyrinogen oxidase, and 20 protoporphyrinogen oxidase novel mutations. Mol Genet Metab. 2019;128:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Ren Y, Xu LX, Liu YF, Xiang CY, Gao F, Wang Y, Bai T, Yin JH, Zhao YL, Yang J. A novel 55-basepair deletion of hydroxymethylbilane synthase gene found in a Chinese patient with acute intermittent porphyria and her family: A case report. Medicine (Baltimore). 2018;97:e12295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Llewellyn DH, Scobie GA, Urquhart AJ, Whatley SD, Roberts AG, Harrison PR, Elder GH. Acute intermittent porphyria caused by defective splicing of porphobilinogen deaminase RNA: a synonymous codon mutation at -22 bp from the 5' splice site causes skipping of exon 3. J Med Genet. 1996;33:437-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Yasuda M, Chen B, Desnick RJ. Recent advances on porphyria genetics: Inheritance, penetrance & molecular heterogeneity, including new modifying/causative genes. Mol Genet Metab. 2019;128:320-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Chen B, Solis-Villa C, Hakenberg J, Qiao W, Srinivasan RR, Yasuda M, Balwani M, Doheny D, Peter I, Chen R, Desnick RJ. Acute Intermittent Porphyria: Predicted Pathogenicity of HMBS Variants Indicates Extremely Low Penetrance of the Autosomal Dominant Disease. Hum Mutat. 2016;37:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Kauppinen R, von und zu Fraunberg M. Molecular and biochemical studies of acute intermittent porphyria in 196 patients and their families. Clin Chem. 2002;48:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Vassiliou D, Lempessi C, Harper P, Sardh E. Challenges in the management of acute intermittent porphyria with recurrent attacks during pregnancy: A case report. Clin Case Rep. 2020;8:2483-2487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Maeda N, Horie Y, Sasaki Y, Ueta E, Adachi K, Nanba E, Kawasaki H, Kudo Y, Kondo M. A splicing mutation in the hydroxymethylbilane synthase gene in a Japanese family with acute intermittent porphyria. Clin Biochem. 1999;32:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Nechay M, Kleiner RE. High-throughput approaches to profile RNA-protein interactions. Curr Opin Chem Biol. 2020;54:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Corley M, Burns MC, Yeo GW. How RNA-Binding Proteins Interact with RNA: Molecules and Mechanisms. Mol Cell. 2020;78:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 20. | Graindorge A, Pinheiro I, Nawrocka A, Mallory AC, Tsvetkov P, Gil N, Carolis C, Buchholz F, Ulitsky I, Heard E, Taipale M, Shkumatava A. In-cell identification and measurement of RNA-protein interactions. Nat Commun. 2019;10:5317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Uversky VN. Functional roles of transiently and intrinsically disordered regions within proteins. FEBS J. 2015;282:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 22. | Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1761] [Article Influence: 176.1] [Reference Citation Analysis (0)] |
| 23. | López-Bigas N, Audit B, Ouzounis C, Parra G, Guigó R. Are splicing mutations the most frequent cause of hereditary disease? FEBS Lett. 2005;579:1900-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 282] [Article Influence: 14.1] [Reference Citation Analysis (0)] |