Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12313
Peer-review started: June 21, 2022
First decision: August 1, 2022
Revised: August 24, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 26, 2022
Processing time: 155 Days and 6.5 Hours
Congenital esophageal stenosis (CES) is a rare malformation of the digestive tract. Endoscopic dilation and thoracotomy have been the main treatments for CES. However, there is no well-defined management protocol. Magnetic compression stricturoplasty (MCS) has been used in refractory esophageal stricture in children after esophageal atresia.
We describe the first case of MCS for CES in one female child patient. The child (aged 3 years and 1 mo) was admitted due to frequent vomiting and choking after eating complementary food since 7 mo old. Esophagography and gastroendoscopy showed that there was stenosis in the lower esophagus, suggesting a diagnosis of CES. The patient did not receive any treatment for esophageal stricture including surgery or endoscopic dilation procedures before MCS. MCS procedure was smoothly conducted without complications. At 24 mo after MCS, durable esophageal patency without dysphagia was achieved.
MCS may serve as an alternative and efficient method for patients with CES.
Core Tip: Magnetic compression stricturoplasty (MCS) is a relatively safe dredge method for patients with postoperative obstruction, stricture, or dehiscence of anastomosis. For the first time, we describe the application of MCS in treating congenital esophageal stenosis (CES) without using endoscopic bougienage and dilation treatment in a child patient. The data on diagnosis, treatment, and long-term follow-up of CES are analyzed. Additionally, key properties of MCS in treating segmental fibromuscular hypertrophy stricture following CES are discussed.
- Citation: Liu SQ, Lv Y, Luo RX. Endoscopic magnetic compression stricturoplasty for congenital esophageal stenosis: A case report. World J Clin Cases 2022; 10(33): 12313-12318
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12313.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12313
Congenital esophageal stenosis (CES) is a very rare clinical condition[1,2]. Membranous web (MW), segmental fibromuscular hypertrophy (FM), and ectopic tracheobronchial remnants (TBR) are histological subtypes of CES. They each have different treatment strategies[3]. Magnetic compression stricturoplasty (MCS) is a relatively safe dredge method for patients with postoperative obstruction, stricture, or dehiscence of anastomosis. This method has been successfully used for refractory esophageal stenosis and choledochojejunostomy[4,5]. For the first time, we report the use of MCS in treating CES without using endoscopic bougienage and dilation treatment in a female child patient. The data on the diagnosis, treatment, and long-term follow-up of CES are analyzed. Furthermore, key properties of MCS in treating FM stricture following CES are discussed.
A female child aged 3 years and 1 mo presented vomiting and choking for more than 2 years after eating complementary food.
The symptoms started 2 years and 6 mo ago with recurrent difficulty in eating solid food.
The child was born at 38 wk of gestation through vaginal delivery with a birth weight of 3.7 kg. Her Apgar score was 10 at 1 min, 10 at 5 min, and 10 at 10 min. Prenatal ultrasound showed polyhydramnios. On the subsequent 6 mo after birth, she received breast feeding and had normal growth. At 7 mo old, she had frequent vomiting and choking after eating complementary food and could only eat a full flow diet.
A family history of malignant tumors was denied.
The vital signs were as follows: Body temperature, 36.5 °C; blood pressure, 80/50 mmHg; heart rate, 100 beats per min; and, respiratory rate, 20 breaths per min. Physical examination of the chest and abdomen revealed no abnormalities.
Routine blood and urine test results were normal.
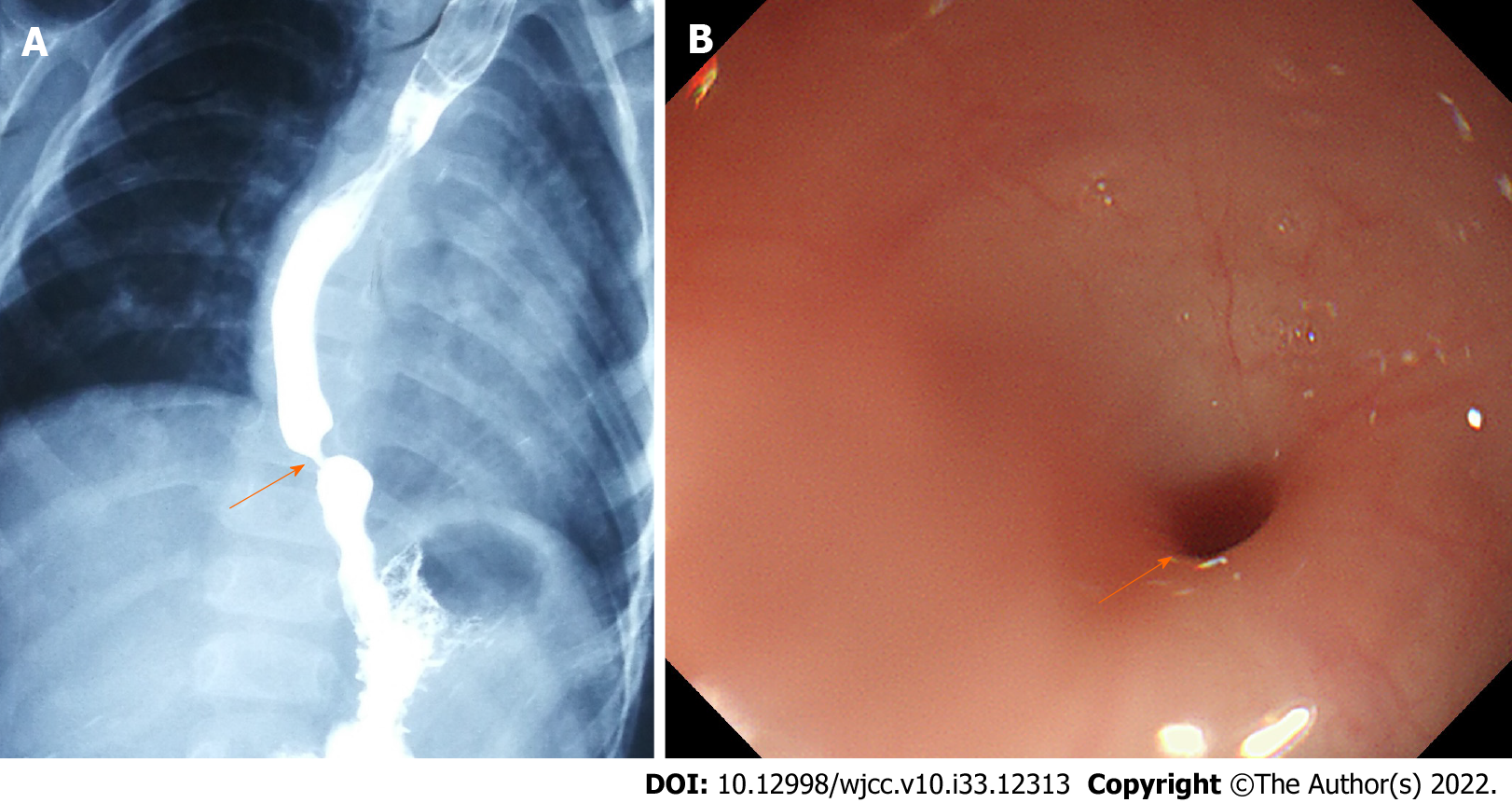
Esophagography and gastroendoscopy showed that there was stenosis in the lower esophagus (Figures 1A and 1B), suggesting a diagnosis of CES.
The patient was finally diagnosed as having CES.
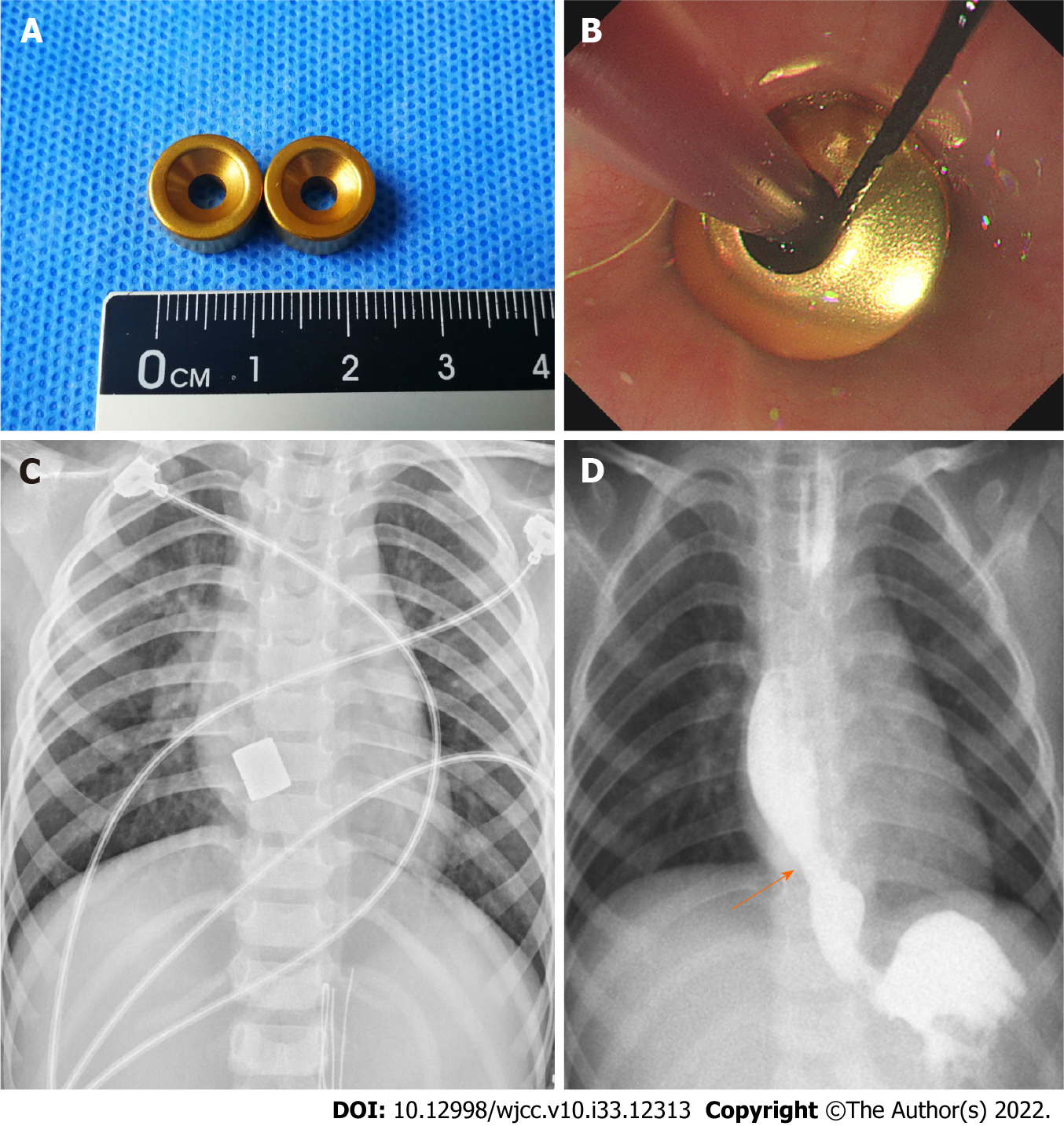
Segmental resection or balloon dilatation was recommended for this patient. However, because of the potential complications such as restenosis, these procedures were not performed. Following preoperative evaluation, the patient received an endoscopy-guided MCS of the esophagus. Magnetic rings were prepared with neodymium-iron-boron (Nd-Fe-B) alloy and the titanium nitride film of 10 μm thickness was coated on the rings (Figure 2A). The outer diameter was 11 mm, the height was 6 mm, and the magnetic strength was 3750 Gs.
A transoral approach was used for the placement of magnetic rings. A guide wire was placed through the esophageal stenosis under endoscopic guidance. The end of a gastric tube with a side hole was fixed on the guide wire. The daughter ring was fixed on the gastric tube. The guide wire was retracted through the mouth, so the end of the gastric tube with a side hole was retrograded through the stenosis segment and was retracted through the mouth. The placement of daughter ring was completed with the placement of the gastric tube. Next, another magnetic ring (mother ring) that had opposite polarity to the daughter ring reached the proximal esophageal lumen above the stenotic segment along the gastric tube under endoscopic guidance (Figure 2B). Intraoperative X-ray confirmed that the two magnets naturally attracted together and accurately compressed the proliferation tissue of stenosis (Figure 2C). Gradual MCS was conducted for 13 d to achieve esophageal stricturoplasty adequacy. During MCS, the patient was fed via the gastric tube. The magnets were moved by pulling the tube daily, starting within 1 wk after surgery. The apposition of magnets was confirmed by anteroposterior or lateral chest radiographs. Subsequently, under fluoroscopic guidance, the magnets were removed. On removal, a remarkably increased luminal diameter was observed by esophagogram (Figure 2D). A 9 mm anastomotic stoma was formed without esophageal perforation and other complications. Temporary stent placement and balloon dilatation were not needed after MCS.
Follow-up at 24 mo after MCS showed that there was no sign indicating esophageal stricture. The patient had normal development and was in good health, without dysphagia or reflux. On esophagram, there was no residual stricture.
CES is a rare developmental malformation of the digestive tract, often accompanied with multiple organ malformation and even chromosomal abnormality. Traditional treatment methods mainly include endoscopic esophageal dilatation or incision. CES histological diagnosis is difficult because it is difficult to identify TBR and FM characteristics without histological examination. Intraesophageal ultrasonography is helpful to distinguish tracheobronchial heterotopia[6,7]. For CES patients lacking a histological diagnosis, surgery may be suggested if there is persistent dysphagia after dilation for two series[2]. For severe esophageal stenosis, repeated esophageal dilatation[2] or even partial esophagectomy by thoracotomy or thoracoscopic minimally invasive surgery is required for stricturoplasty[8]. However, patients may still have anastomotic re-stricture after surgery. Thus, a more effective method for treating CES in children is needed.
MCS is a cavity organ anastomosis measure based on magnetic compression anastomosis (MCA), which utilizes the magnetic force between magnetic materials to induce ischemic necrosis and shedding of the compressed tissue and the re-healing of the adjacent tissue and mucosa. MCA has been used for minimally invasive cardiac bypass surgery[9,10] and for the treatment of biliary obstruction after liver transplantation[5,11-13]. In recent years, in addition to the application of MCA in gastrointestinal anastomosis[14-17], rectum atresia, and colorectal stricture in children[4,16,18,19], more clinical studies have been reported on its application in refractory stricture after esophageal atresia (EA). Zaritzky et al[18] showed that MCA achieved esophageal reanastomosis within 6 d in infants with EA who had primary or secondary anastomotic stenosis. Importantly, we report the first MCS case for CES in one female child.
On the basis of the compatibility of Nd-Fe-B permanent magnet alloy material in vivo and the application of magnetic anastomosis devices in the field of surgery, our team developed a magnetic anastomosis device suitable for the treatment of digestive tract malformations in children. The titanium oxide coating on Nd-Fe-B alloy magnetic rings could resist gastric acid or succus entericus corrosion. In this case, we used the digestive tract endoscopy and gastric fistula respectively to put the magnetic ring into the proximal and distal end of the esophageal stenosis site. The magnet magnetic field force was used for the minimally invasive treatment of CES between. The tissues at the stenotic site were continuously compressed by the magnet rings, causing ischemic necrosis and natural shedding of the compressed tissues and achieving esophageal patency. An ideal anastomosis between the mucosa and mucosa of esophageal wall was achieved. There were no complications such as esophageal perforation or fistula after operation. In particular, the child did not undergo endoscopic dilation or other treatment procedures before or after MCS. The child was followed for 24 mo and received a general diet. There was no dysphagia upon esophagram and clinical examination. Esophageal continuity was achieved after MCS for FM repair without short or long-term complications. MCS for MW has not been reported. Theoretically, MW has a thinner and weaker structure than FM, so it would be easier to treat by MCS. These findings suggest that MCS is a safe, effective, and alternative method for FM and MW type of CES patients. However, the long-term safety of MCS treatment in children is unclear. We are still following this special case to observe the long-term effects of MCS treatment in children.
In this case, MCS successfully established the patency of the esophageal stenosis. This report is the first to utilize MCS in CES. It provides a new reliable method for the treatment of CES. Additionally, attempting MCS does not exclude further esophageal dilatation or segmental resection. We believe that MCA may have good application prospects for children with esophageal stenosis diseases.
The authors would like to thank Dr. Hong-Bin Yang (Department of Digestive Diseases, The Xi’an Children’s Hospital) for performing the endoscopic procedures.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Asreah RH, Iraq; Kamran M, Pakistan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Bluestone CD, Kerry R, Sieber WK. Congenital esophageal stenosis. Laryngoscope. 1969;79:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Michaud L, Coutenier F, Podevin G, Bonnard A, Becmeur F, Khen-Dunlop N, Auber F, Maurel A, Gelas T, Dassonville M, Borderon C, Dabadie A, Weil D, Piolat C, Breton A, Djeddi D, Morali A, Bastiani F, Lamireau T, Gottrand F. Characteristics and management of congenital esophageal stenosis: findings from a multicenter study. Orphanet J Rare Dis. 2013;8:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Takamizawa S, Tsugawa C, Mouri N, Satoh S, Kanegawa K, Nishijima E, Muraji T. Congenital esophageal stenosis: Therapeutic strategy based on etiology. J Pediatr Surg. 2002;37:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Woo R, Wong CM, Trimble Z, Puapong D, Koehler S, Miller S, Johnson S. Magnetic Compression Stricturoplasty For Treatment of Refractory Esophageal Strictures in Children: Technique and Lessons Learned. Surg Innov. 2017;24:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Li Y, Zhang N, Lv Y; Scientific Committee of the Third International Conference of Magnetic Surgery*. Expert consensus on magnetic recanalization technique for biliary anastomotic strictures after liver transplantation. Hepatobiliary Surg Nutr. 2021;10:401-404. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Athanasiou T, Ashrafian H, Glenville B, Casula R. Coronary artery bypass with the use of a magnetic distal anastomotic device: surgical technique and preliminary experience. Heart Surg Forum. 2004;7:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Morbiducci U, Lemma M, Ponzini R, Boi A, Bondavalli L, Antona C, Montevecchi FM, Redaelli A. Does the Ventrica magnetic vascular positioner (MVP) for coronary artery bypass grafting significantly alter local fluid dynamics? Int J Artif Organs. 2007;30:628-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Umemura A, Sasaki A, Nitta H, Takahara T, Hasegawa Y, Wakabayashi G. Magnetic compression anastomosis for the stricture of the choledochocholedochostomy after ABO-incompatible living donor liver transplantation. Clin J Gastroenterol. 2014;7:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Ersoz G, Tekin F, Bozkaya H, Parildar M, Turan I, Karasu Z, Ozutemiz O, Tekesin O. Magnetic compression anastomosis for patients with a disconnected bile duct after living-donor related liver transplantation: a pilot study. Endoscopy. 2016;48:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Koksal AS, Eminler AT, Parlak E, Gurakar A. Management of biliary anastomotic strictures after liver transplantation. Transplant Rev (Orlando). 2017;31:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | van Hooft JE, Vleggaar FP, Le Moine O, Bizzotto A, Voermans RP, Costamagna G, Devière J, Siersema PD, Fockens P. Endoscopic magnetic gastroenteric anastomosis for palliation of malignant gastric outlet obstruction: a prospective multicenter study. Gastrointest Endosc. 2010;72:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Pichakron KO, Jelin EB, Hirose S, Curran PF, Jamshidi R, Stephenson JT, Fechter R, Strange M, Harrison MR. Magnamosis II: Magnetic compression anastomosis for minimally invasive gastrojejunostomy and jejunojejunostomy. J Am Coll Surg. 2011;212:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Dauser B, Winkler T, Loncsar G, Herbst F. Compression anastomosis revisited: prospective audit of short- and medium-term outcomes in 62 rectal anastomoses. World J Surg. 2011;35:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Machytka E, Bužga M, Zonca P, Lautz DB, Ryou M, Simonson DC, Thompson CC. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc. 2017;86:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Zaritzky M, Ben R, Zylberg GI, Yampolsky B. Magnetic compression anastomosis as a nonsurgical treatment for esophageal atresia. Pediatr Radiol. 2009;39:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Liu SQ, Lv Y, Fang Y, Luo RX, Zhao JR, Luo RG, Li YM, Zhang J, Zhang PF, Guo JZ, Li QH, Han MX. Magnetic compression for anastomosis in treating an infant born with long-gap oesophageal atresia: A case report. Medicine (Baltimore). 2020;99:e22472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Liu SQ, Li QF, Lv Y, Zhao JR, Luo RX, Zhang PF, Guo JZ, Zhang AP, Li QH. Magnetic compression anastomosis for rectal atresia following necrotizing enterocolitis: A case report. Medicine (Baltimore). 2020;99:e23613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Zaritzky M, Ben R, Johnston K. Magnetic gastrointestinal anastomosis in pediatric patients. J Pediatr Surg. 2014;49:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Liu S, Lv Y. Constant magnetic field in treating congenital esophageal and anorectal malformation: a review. World J Pediat Surg. 2020;3:e000130. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |