Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12305
Peer-review started: June 18, 2022
First decision: September 5, 2022
Revised: September 15, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 26, 2022
Processing time: 158 Days and 2.6 Hours
Locally advanced penile squamous cell carcinoma with unresectable inguinal lymph node metastasis has a poor prognosis, and surgical treatment alone offers limited benefits. Effective conversion therapy regimens are urgently needed.
We describe a locally advanced penile squamous cell carcinoma patient with bulky, fixed inguinal lymph node metastasis complicated with genital skin ulcers who underwent inguinal lymph node dissection and achieved a pathological complete response with conversion therapy comprising immunotherapy plus chemotherapy.
For unresectable locally advanced penile squamous cell carcinoma, neoadjuvant immunotherapy combined with chemotherapy is a potential treatment approach. Biomarkers of immunotherapy efficacy need to be explored, and clinical trials are needed to test these strategies.
Core Tip: Locally advanced penile squamous cell carcinoma with bilateral inguinal lymph node metastases and extra-nodal extension does not respond well to surgical treatment alone, and the prognosis is very poor. In this study, the patient successfully completed inguinal lymph node dissection after conversion therapy of immunotherapy plus chemotherapy. This is the first patient with human papillomavirus 16+ unresectable locally advanced penile cancer treated with immunotherapy plus chemotherapy who eventually achieved a pathologically complete response.
- Citation: Long XY, Zhang S, Tang LS, Li X, Liu JY. Conversion therapy for advanced penile cancer with tislelizumab combined with chemotherapy: A case report and review of literature. World J Clin Cases 2022; 10(33): 12305-12312
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12305.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12305
Penile carcinoma is a rare tumor in the male genitourinary system, and the main histopathological type is squamous cell carcinoma. The etiology of penile carcinoma is not entirely clear; however, there is a strong association with human papillomavirus (HPV)[1,2] and a chronic inflammatory state of the foreskin and glans[3,4]. According to previous studies, partial or total excision of the penis is the primary treatment for localized tumors[5]. Unfortunately, patients with penile squamous cell carcinoma (PSCC) are usually diagnosed in the advanced stage. For locally advanced PSCC patients with bulky, fixed, bilateral inguinal lymph node metastases and extra-nodal extension, surgery alone offers little benefit, and the prognosis is very poor[6-8]. The use of multiple strategies to reduce the pathological stage and even convert the case to operable can improve prognosis and increase the survival rate. Neoadjuvant chemotherapy is the usual treatment for this patient population[9], but multiple small cohort studies have demonstrated that nearly half of the patients do not benefit from neoadjuvant chemotherapy alone, highlighting the urgent need to find new and effective treatments[10,11].
In recent years, immune checkpoint inhibitors (ICIs) have become a hot topic in oncology treatment and the standard of care for cancers such as melanoma and lung cancer[12-16]. By inhibiting programmed cell death protein 1 (PD-1) or PD-1 ligand (PD-L1), ICIs block the inhibition of CD8+ effector T cells and reverse the suppressive tumor microenvironment, which may improve the prognosis of patients with disease resistant to other treatment modalities (e.g., chemotherapy/radiotherapy)[17]. For some unresectable tumors, neoadjuvant immunotherapy offers a potential solution by mediating tumor regression to the point of becoming operable or even cured[18,19]. Accumulating evidence in lung and breast cancer has demonstrated that neoadjuvant immunotherapy increases the rate of pathological response and improves overall survival[20,21]. However, immunotherapy combined with chemotherapy for patients with unresectable locally advanced PSCC has rarely been reported.
A 60-year-old male patient, who was found to have a subpreputial mass for 1 year, was admitted to the West China Hospital of Sichuan University for 3 mo after the diagnosis of unresectable penile cancer.
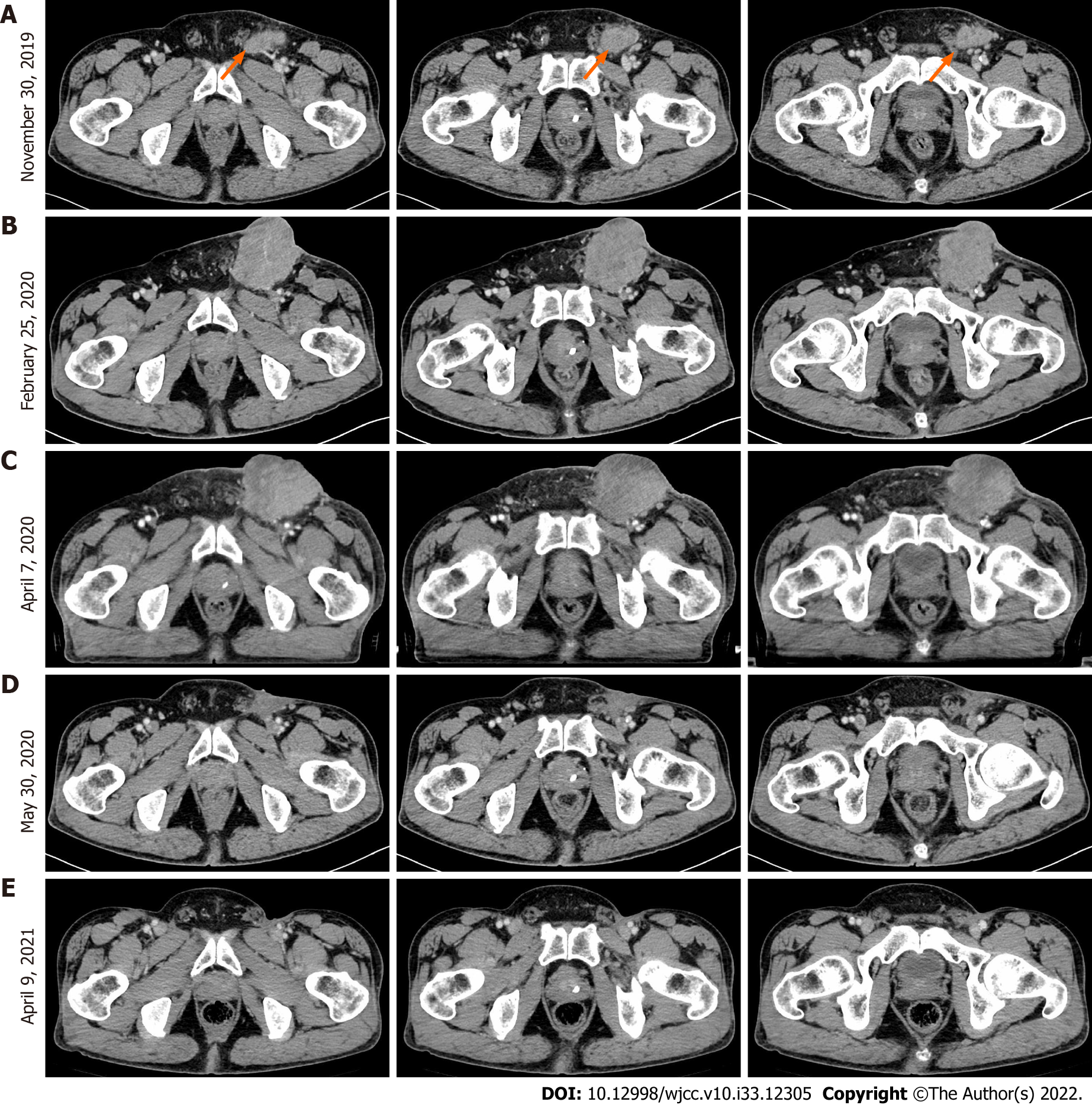
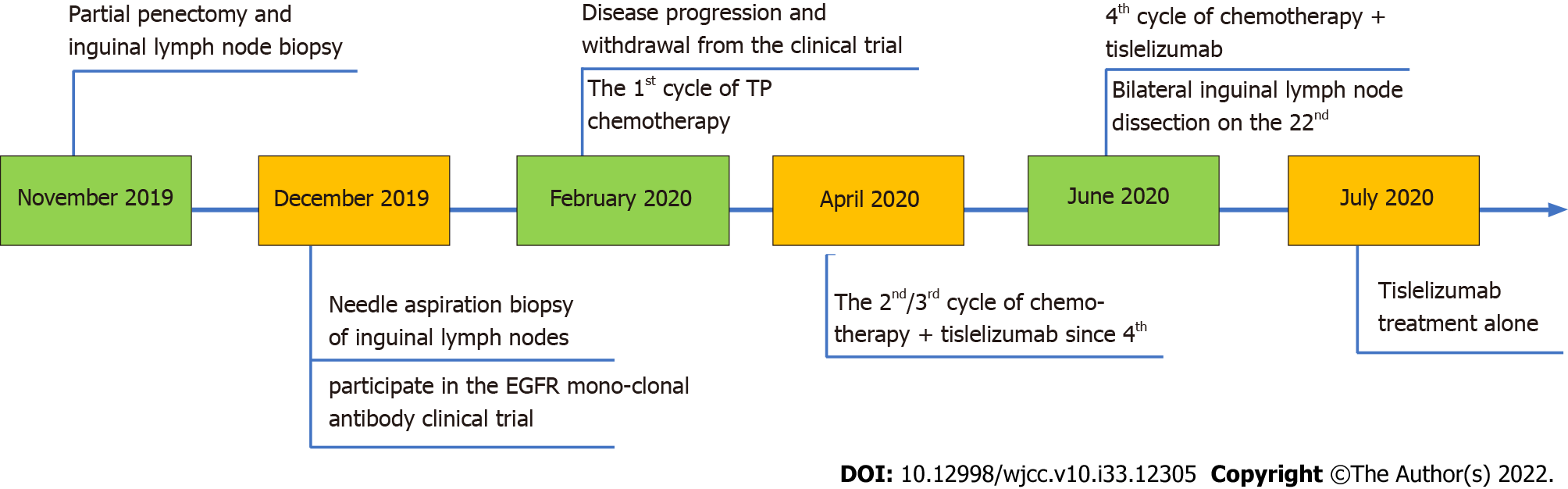
In February 2019, the patient went to Hubei Provincial People’s Hospital and complained of a subpreputial mass. The patient underwent partial penile resection plus partial biopsy of the left inguinal lymph node on November 12, 2019. The patient was admitted to the West China Hospital of Sichuan University 21 d after surgery, where an enhanced computed tomography (CT) of the pelvis showed multiple enlarged lymph nodes in the left inguinal region; the largest lymph node was 4.0 cm × 2.5 cm (Figure 1A). Inguinal lymph node biopsy was performed on December 11, 2019, and cancer cells were detected. The patient had a repeat CT scan on February 25, 2020, which indicated that the lesion had grown.
The patient had a history of hypertension for 10 years, and the highest blood pressure was 180/100 mmHg. The blood pressure was well controlled by oral amlodipine daily. Also, the patient had multiple sexual partners.
Family and personal history were unremarkable. He had no significant medical history or drug allergy.
A poor general condition, Eastern Cooperative Oncology Group score of 2 points. A subpreputial mass located in the left groin was palpable, with a size of 10 cm × 6 cm. The mass was hard, fixed and inactive. No other apparently positive signs were found.
The patient was admitted to our hospital, and the relevant blood tests were normal.
The patient was admitted to the West China Hospital of Sichuan University 21 d after surgery, where an enhanced CT of the pelvis showed multiple enlarged lymph nodes in the left inguinal region; the largest lymph node was 4.0 cm × 2.5 cm (Figure 1A). After consultation with a multidisciplinary team, the decision was reached that it would be difficult to perform a complete inguinal lymph node dissection. The patient was enrolled in a clinical trial of CDP1, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody. However, a larger inguinal lesion developed after 6 cycles of CDP1. The lesion was evaluated as progressive disease by CT scan (Figure 1B). Next, the patient received chemotherapy (paclitaxel 240 mg D1 plus cisplatin 40 mg D1-3) after withdrawal from the clinical trial. After the 1st cycle of chemotherapy, no change in the tumor mass was found (Figure 1C).
Locally advanced PSCC with unresectable inguinal lymph node metastasis (T3N3M0, IV).
Distal penectomy and biopsy of the left inguinal lymph node were subsequently performed. The postoperative pathological biopsy showed high-/medium-differentiated squamous carcinoma of the penis invading the penile corpus cavernosum, with no involvement of the uroepithelium. Pathological biopsy of the left inguinal lymph node showed loss of normal lymph node structure and multifocal cancerous infiltration of fibrous tissue. The final pathological stage was IV (pT3N3M0), and the timeline of treatment and procedures is shown in Figure 2.
Tislelizumab, a humanized IgG4 anti-PD-1 monoclonal antibody[22], was added to the chemotherapy regimen. After three cycles of treatment with tislelizumab plus chemotherapy, the inguinal ulcer in the patient healed. Moreover, enhanced pelvic CT (Figure 1D) indicated that the inguinal mass was significantly smaller, and the effect of conversion therapy was obvious. Based on the effects of the combination therapy, bilateral inguinal lymph node dissection was performed.
Postoperative pathological examination indicated no tumor in 21 inguinal lymph nodes on the left side or in 18 inguinal nodes on the right side. Some lymph nodes presented as necrotic with multinucleated giant cell reactions, indicating a complete response to the treatment strategy. After surgery, the patient continued taking 200 mg tislelizumab alone for three cycles and requested to discontinue immunotherapy due to irritating side effects (diarrhea/abdominal pain). The patient had no evidence (Figure 1E) of tumor recurrence at regular postoperative follow-ups over 12 mo.
Chemotherapy is commonly used as a translational therapy for patients with locally advanced unresectable PSCC. However, despite a meaningful response to systemic chemotherapy, long-term survival rates are disappointing, with 2-year progression-free survival and disease-specific survival rates of 12% and 28%, respectively[10,23,24]. Moreover, patients with disease that is resistant to chemotherapy have a worse prognosis. Therefore, there is an urgent need to find new translational therapeutic strategies with higher efficacy and low toxicity profiles. Based on the experience with advanced non-small cell lung cancer and melanoma, PD-(L)1 blockade plus chemotherapy may be a promising option[12-16].
Theoretically, in the primary tumor, PD-(L)1 blockade relieves the suppressive immune microenvironment, restores the activity of exhausted cytotoxic T cells and mediates tumor regression[25]. Simultaneously, chemotherapy causes tumor cell necrosis and the release of more tumor antigens. In the presence of ICIs, dendritic cells can present antigens to T cells more efficiently, thereby initiating tumor-specific T cell proliferation and activation. Activated T cells enter the bloodstream from tumor-draining lymph nodes and migrate to tumor sites and distant micrometastases, shrinking the primary lesion and reducing postoperative distant recurrence[26]. In addition, the preoperatively induced systemic immune response generates long-term immune memory and prevents tumor recurrence[27]. These results suggest that immunotherapy combined with chemotherapy is a promising translational treatment strategy.
Numerous factors are involved in the effectiveness of immunotherapy. To explore treatment options, we performed immunohistochemistry and next-generation sequencing analysis of samples from our patient. PD-L1 expression in tumor tissues is a validated companion diagnostic test for predicting the efficacy of treatment with ICIs. Several studies have shown that 40%-60% of PSCC patients express PD-L1 (PD-L1 positivity defined by > 5% tumor cell expression), and high PD-L1 expression is positively associated with worse stage and prognosis[28] and with fewer tumor-infiltrating lymphocytes in tumor tissue[29]. The PD-L1 expression score for our patient was 40% (tumor proportion score), which provided the rationale for the therapeutic use of ICIs for patients with PSCC.
Tumor mutation burden (TMB) is the number of nonsynonymous somatic mutations in coding regions of tumor cells per megabase of DNA. PSCC is a heterogeneous disease, harboring approximately 5.45 genomic alterations per tumor[30]. TMB has been suggested as a promising immunotherapeutic marker in many cancer types[31]. However, the TMB cutoff values are not the same in different cancers[32]. In our case, the patient had a TMB of 5.0 mutations per megabase, slightly higher than the median value (4.5 mutations per megabase) in PSCC, and a recent report suggested that mutations in select genes may be a better predictor than TMB[33]. High microsatellite instability is another valid marker of sensitivity to ICIs. In our case, the tumor was microsatellite stable; the literature reports a low incidence of high microsatellite instability in PSCC[34].
More interestingly, laboratory testing for our patient revealed the presence of HPV16 DNA. Patients with HPV+ PSCC have a better prognosis than those with HPV- disease[35]. The possible mechanism is that the virus increases the production of neoantigens while increasing the number of infiltrating CD8+ T cells in the tumor microenvironment[36]. Does this mean that HPV could be a meaningful biomarker for immunotherapy? Considering the impact of HPV on cancer, several clinical trials of combination immunotherapy with HPV-targeted vaccines have been conducted in HPV-associated malignancies. Current results regarding the association between the status of HPV and the expression of PD-L1 are conflicting and need to be confirmed by more studies. However, the differences in TMB between patients with HPV- and HPV+ PSCC are minimal[28].
In addition, our patient had high expression of EGFR by immunohistochemistry and was treated experimentally with CDP1. Several studies have shown that high EGFR expression detected by immunohistochemistry is frequent in PSCC[37]. High expression of EGFR may be associated with poor prognosis, implying the potential for better effects upon targeting EGFR[38]. Retrospective studies[39] have shown that the use of cetuximab or dacomitinib (a pan-HER tyrosine kinase inhibitor) provides benefit to only a small number of patients. Our patient experienced disease progression after attempting CDP1 therapy. Next-generation sequencing of this case showed the p.E453K mutation in PIK3CA. In colon cancer, PIK3CA mutations are significantly associated with clinical resistance to anti-EGFR monoclonal antibodies[40].
In conclusion, this case report described HPV+ locally advanced inoperable PSCC that responded well to ICI plus chemotherapy. The tumor was converted to operable, and the patient underwent inguinal lymph node dissection. The patient achieved a pathological complete response. Postoperative disease-free survival exceeded 12 mo, with the expectation of continued prolongation of survival. For this group, immunotherapy combined with chemotherapy is a promising translational treatment option. However, more clinical trials are needed to validate this hypothesis, and effective biomarkers need to be further explored.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Boopathy Vijayaraghavan KM, India; Kanat O, Turkey; Rotondo JC, Italy S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Flaherty A, Kim T, Giuliano A, Magliocco A, Hakky TS, Pagliaro LC, Spiess PE. Implications for human papillomavirus in penile cancer. Urol Oncol. 2014;32:53.e1-53.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Olesen TB, Sand FL, Rasmussen CL, Albieri V, Toft BG, Norrild B, Munk C, Kjær SK. Prevalence of human papillomavirus DNA and p16INK4a in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 2019;20:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Minhas S, Manseck A, Watya S, Hegarty PK. Penile cancer--prevention and premalignant conditions. Urology. 2010;76:S24-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Dillner J, von Krogh G, Horenblas S, Meijer CJ. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl. 2000;189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Philippou P, Shabbir M, Malone P, Nigam R, Muneer A, Ralph DJ, Minhas S. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Graafland NM, van Boven HH, van Werkhoven E, Moonen LM, Horenblas S. Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J Urol. 2010;184:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Wang JY, Zhu Y, Tang SX, Zhang HL, Qin XJ, Zhang SL, Dai B, Ye DW. Prognostic significance of the degree of extranodal extension in patients with penile carcinoma. Asian J Androl. 2014;16:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Bandini M, Pederzoli F, Necchi A. Neoadjuvant chemotherapy for lymph node-positive penile cancer: current evidence and knowledge. Curr Opin Urol. 2020;30:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Azizi M, Aydin AM, Hajiran A, Lai A, Kumar A, Peyton CC, Minhas S, Sonpavde GP, Chahoud J, Pagliaro LC, Necchi A, Spiess PE. Systematic Review and Meta-Analysis-Is there a Benefit in Using Neoadjuvant Systemic Chemotherapy for Locally Advanced Penile Squamous Cell Carcinoma? J Urol. 2020;203:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Paz Rojas JF, Ballestas Almario CA, García-Perdomo HA. Effectiveness and safety of adjuvant chemotherapy compared to neoadjuvant chemotherapy in patients with penile cancer and positive lymph nodes regarding overall survival and free disease survival: a systematic review and meta-analysis. Urol Oncol. 2022;40:200.e11-200.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC, Gonzalez R, Hodi FS, Ascierto PA, Salama AKS, Margolin KA, Gangadhar TC, Wei Z, Ebbinghaus S, Ibrahim N, Ribas A. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 13. | Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2106] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 14. | Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol. 2022;13:823618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 15. | Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab vs docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 5052] [Article Influence: 561.3] [Reference Citation Analysis (0)] |
| 16. | Mazziotta C, Lanzillotti C, Gafà R, Touzé A, Durand MA, Martini F, Rotondo JC. The Role of Histone Post-Translational Modifications in Merkel Cell Carcinoma. Front Oncol. 2022;12:832047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10350] [Article Influence: 796.2] [Reference Citation Analysis (34)] |
| 18. | Xu K, Yang H, Ma W, Fan L, Sun B, Wang Z, Al-Hurani MF, Schmid RA, Yao F. Neoadjuvant immunotherapy facilitates resection of surgically-challenging lung squamous cell cancer. J Thorac Dis. 2021;13:6816-6826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Tarhini AA, Eads JR, Moore KN, Tatard-Leitman V, Wright J, Forde PM, Ferris RL. Neoadjuvant immunotherapy of locoregionally advanced solid tumors. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 20. | Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N; CheckMate 816 Investigators. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022;386:1973-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1597] [Article Influence: 532.3] [Reference Citation Analysis (0)] |
| 21. | Tarantino P, Gandini S, Trapani D, Criscitiello C, Curigliano G. Immunotherapy addition to neoadjuvant chemotherapy for early triple negative breast cancer: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2021;159:103223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Ye D, Liu J, Zhou A, Zou Q, Li H, Fu C, Hu H, Huang J, Zhu S, Jin J, Ma L, Guo J, Xiao J, Park SH, Zhang D, Qiu X, Bao Y, Zhang L, Shen W, Bi F. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 2021;112:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 23. | Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M, Wen S, Thall PF, Pettaway CA. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28:3851-3857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 24. | Djajadiningrat RS, Bergman AM, van Werkhoven E, Vegt E, Horenblas S. Neoadjuvant taxane-based combination chemotherapy in patients with advanced penile cancer. Clin Genitourin Cancer. 2015;13:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, Li J, Li F, Tan HB. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 940] [Article Influence: 156.7] [Reference Citation Analysis (0)] |
| 26. | Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 704] [Article Influence: 140.8] [Reference Citation Analysis (0)] |
| 27. | Keung EZ, Ukponmwan EU, Cogdill AP, Wargo JA. The Rationale and Emerging Use of Neoadjuvant Immune Checkpoint Blockade for Solid Malignancies. Ann Surg Oncol. 2018;25:1814-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Ottenhof SR, Djajadiningrat RS, Thygesen HH, Jakobs PJ, Jóźwiak K, Heeren AM, de Jong J, Sanders J, Horenblas S, Jordanova ES. The Prognostic Value of Immune Factors in the Tumor Microenvironment of Penile Squamous Cell Carcinoma. Front Immunol. 2018;9:1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18:842-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 573] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 30. | Ali SM, Pal SK, Wang K, Palma NA, Sanford E, Bailey M, He J, Elvin JA, Chmielecki J, Squillace R, Dow E, Morosini D, Buell J, Yelensky R, Lipson D, Frampton GM, Howley P, Ross JS, Stephens PJ, Miller VA. Comprehensive Genomic Profiling of Advanced Penile Carcinoma Suggests a High Frequency of Clinically Relevant Genomic Alterations. Oncologist. 2016;21:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1568] [Cited by in RCA: 1877] [Article Influence: 312.8] [Reference Citation Analysis (0)] |
| 32. | McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB, Lim B, Ueno NT, Litton JK, Ferrarotto R, Chang JT, Moulder SL, Lin SY. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 755] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 33. | Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1661] [Cited by in RCA: 2510] [Article Influence: 313.8] [Reference Citation Analysis (0)] |
| 34. | Stoehr R, Wendler O, Giedl J, Gaisa NT, Richter G, Campean V, Burger M, Wullich B, Bertz S, Hartmann A. No Evidence of Microsatellite Instability and Loss of Mismatch-Repair-Protein Expression in Squamous Cell Carcinoma of the Penis. Pathobiology. 2019;86:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Lont AP, Kroon BK, Horenblas S, Gallee MP, Berkhof J, Meijer CJ, Snijders PJ. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer. 2006;119:1078-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Ahmed ME, Falasiri S, Hajiran A, Chahoud J, Spiess PE. The Immune Microenvironment in Penile Cancer and Rationale for Immunotherapy. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Gu W, Zhu Y, Ye D. Beyond chemotherapy for advanced disease-the role of EGFR and PD-1 inhibitors. Transl Androl Urol. 2017;6:848-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Carthon BC, Ng CS, Pettaway CA, Pagliaro LC. Epidermal growth factor receptor-targeted therapy in locally advanced or metastatic squamous cell carcinoma of the penis. BJU Int. 2014;113:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Necchi A, Lo Vullo S, Perrone F, Raggi D, Giannatempo P, Calareso G, Nicolai N, Piva L, Biasoni D, Catanzaro M, Torelli T, Stagni S, Togliardi E, Colecchia M, Busico A, Gloghini A, Testi A, Mariani L, Salvioni R. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int. 2018;121:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 595] [Article Influence: 37.2] [Reference Citation Analysis (0)] |