Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12289
Peer-review started: June 30, 2022
First decision: August 1, 2022
Revised: August 8, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 26, 2022
Processing time: 146 Days and 3 Hours
Several vaccines against the severe acute respiratory syndrome coronavirus 2 have been approved and widely distributed, raising public concerns regarding the side effects of immunization, as the incidence of ease. Although many adverse events following the coronavirus disease 2019 (COVID-19) vaccine have been reported, neurological complications are relatively uncommon. Herein, we report a rare case of multiple cranial palsies following COVID-19 vaccination in an adolescent patient.
A previously healthy, 14-year-old Asian girl with facial palsy presented to the emergency department with inability to close the right eye or wrinkle right side of the forehead, and pain in the right cheek. She had received second dose of the COVID-19 mRNA vaccine (Pfizer-BioNTech) 18 days before onset of symptoms. She was diagnosed with Bell’s palsy and prescribed a steroid (1 mg/kg/day methylprednisolone) based on symptoms and magnetic resonance imaging findings. However, the next day, all sense of taste was lost with inability to swallow solid food; the gag reflex was absent. Horizontal diplopia was also present. Due to worsening of her condition, she was given high-dose steroids (1 g/day methylprednisolone) for 3 days and then discharged with oral steroids. Improvement in the symptoms was noted 4 days post steroid treatment completion. At the most recent follow-up, her general condition was good with no symptoms except diplopia; ocular motility disturbances were noted. Hence, prism glasses were prescribed for diplopia relief.
Small-angle exotropia was observed in the facial, trigeminal, and glossopharyngeal nerve palsies, in our patient. The etiology of this adverse effect following vaccination was thought to be immunological.
Core Tip: Novel mRNA coronavirus disease 2019 (COVID-19) vaccines have been developed against severe acute respiratory syndrome coronavirus 2. These vaccines are highly effective in preventing COVID-19; however, many side effects have been reported following vaccination. Neurological complications are relatively uncommon but have been reported variously. However, the occurrence of multiple cranial nerve (CN) palsy is rare. Based on our knowledge, this is the first pediatric case of multiple CN palsies following COVID-19 vaccination. Our case demonstrated facial, trigeminal, glossopharyngeal nerve palsies with small-angle exotropia were observed. The etiology of CN palsy following vaccination may be immunological.
- Citation: Lee H, Byun JC, Kim WJ, Chang MC, Kim S. Multiple cranial nerve palsies with small angle exotropia following COVID-19 mRNA vaccination in an adolescent: A case report. World J Clin Cases 2022; 10(33): 12289-12294
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12289.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12289
Coronavirus disease 2019 (COVID-19) is an infectious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The global pandemic has continued since the first case was reported in December 2019, and to address this issue, several vaccines against this virus have been approved and widely distributed, raising public concerns regarding the side effects of immunization. Many adverse events following messenger RNA COVID-19 vaccines have been reported, although the underlying mechanism is not fully understood[1,2]. Neurological complications are relatively uncommon but have been reported variously[3-6]. Cranial nerve (CN) palsies after vaccination are rare, and only one case of multiple CN palsies has been reported. There is no standard treatment for CN palsies as a side effect of vaccination; however, the use of steroids for treatment of CN palsy is mentioned in the existing scientific literature[5-7]. To the best of our knowledge, this is the first pediatric case of multiple CN palsies following COVID-19 vaccination. In this case, small-angle exotropia was observed not only in the facial, but also in the trigeminal and glossopharyngeal nerve palsies. The timeline is shown in Table 1.
| Time | Event | Evaluation | Management | |
| -18 day | COVID-19 vaccination; Second dose | |||
| ER visit, admission | 0 | Right facial nerve palsy | Brain MRI + CISS: Focal enhancement at Right IAC | Methylprednisolone 1 mg/kg/day |
| 1 | Right trigeminal nerve palsy; Right glossopharyngeal nerve palsy; Small angle exotropia | Extraocular muscle examination: Small angle exotropia | Methylprednisolone 1000 mg/day for 3 days | |
| Discharge | 3 | Steroid tapering | ||
| 7 | Symptoms started to improve | |||
| Frist, follow-up | 13 | |||
| Last, follow-up | +2 mo | All symptoms had disappeared except diplopia | Extraocular muscle examination: Small angle exotropia (improved) | Prescription of prism glasses |
A previously healthy, 14-year-old Asian girl with facial palsy presented to the emergency department.
She had received a second dose of the mRNA COVID-19 vaccine (Pfizer-BioNTech) 18 days before the onset of symptoms.
Prior to this event, the patient had no history of any illness. She had received routine childhood immunization in Korea and had suffered no side effects.
The patient had no relevant personal or family history.
There were no abnormal findings on the general physical examination. Neurological examination revealed that her right eyelid did not completely close, and the corner of her right mouth was drooping. An asymmetric wrinkle was observed on her right forehead, and tenderness was observed on her right face.
Reverse transcriptase polymerase chain reaction for the detection of SARS-CoV-2 from nasopharyngeal swabs was negative, and routine blood tests, including inflammatory markers, revealed no abnormalities. There were no symptoms or signs of infection, and the inflammation markers (CRP, ESR) were normal, so no other virus tests were performed.
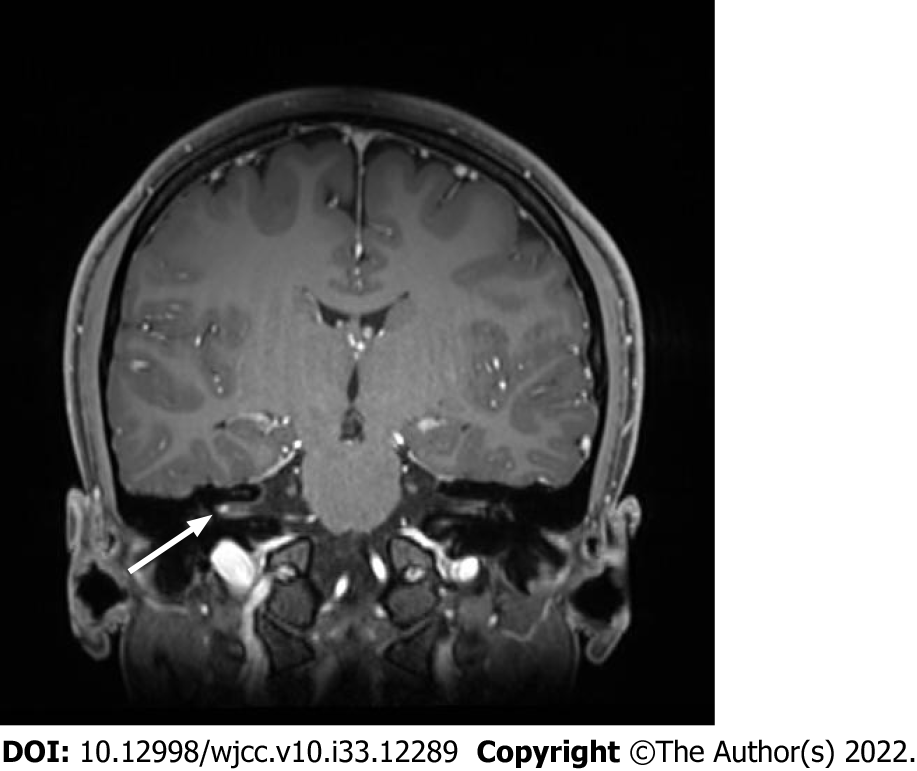
Brain magnetic resonance imaging (MRI) with constructive interference in the steady state was performed to assess the cranial nerves, and a focally enhancing lesion in the right internal acoustic canal was confirmed (Figure 1).
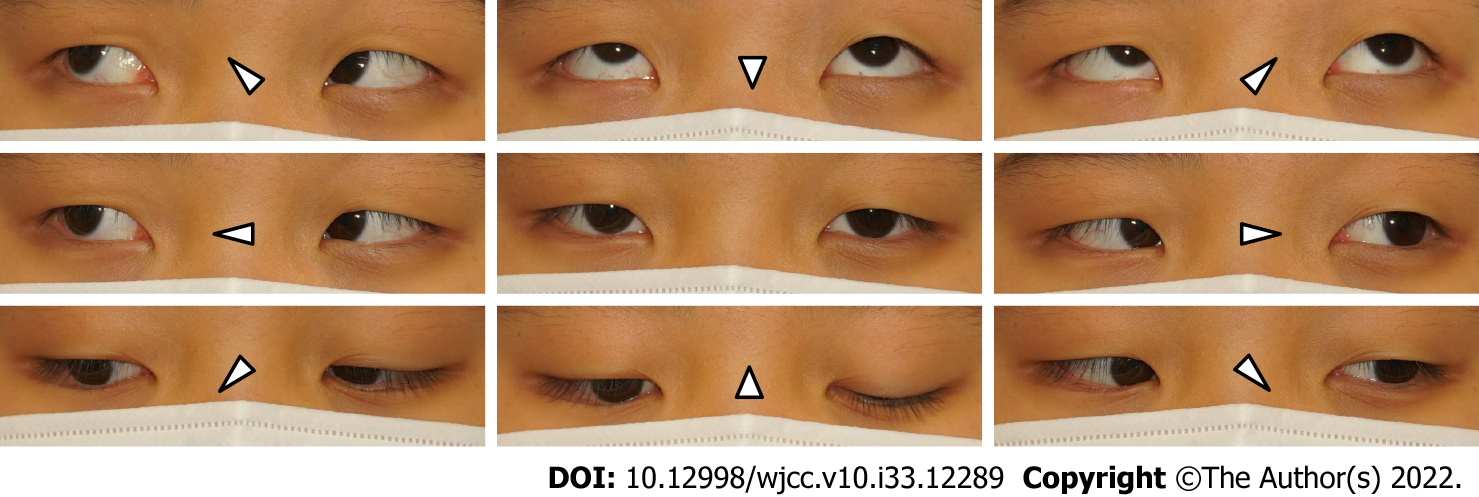
On the first day of admission, she was diagnosed with Bell’s palsy based on the symptoms and MRI findings. However, the following day, trigeminal and glossopharyngeal nerve palsy were additionally confirmed with small-angle exotropia (Figure 2). Considering that there was no evidence of a specific infection and no suspicious history other than vaccination, the most probable etiology was immune-mediated neuropathy.
The patient was diagnosed with Bell’s palsy and received steroid administration (1 mg/kg/day methylprednisolone). However, this standard dose of steroid was ineffective and her condition worsened; therefore, she was given high-dose steroid administration (1 g/day methylprednisolone) for 3 days and was then discharged with oral steroids (with tapering from the high dose over 11 days).
The patient’s symptoms improved 4 days post completion of the steroid course. At the most recent follow-up, her general condition was good, and all the symptoms (except diplopia) disappeared. Follow-up CN examination revealed no abnormal findings with an exception of an ocular motility disturbance; thus, prism glasses were prescribed for diplopia relief.
A novel mRNA COVID-19 vaccine has been developed against SARS-CoV-2, such as Pfizer-BioNTech and Moderna. mRNA induces an immune response against the spike protein present on the viral surface by producing copies of the fake spike protein, resulting in antibody production against this protein[1,7,8]. These vaccines are highly effective in preventing COVID-19; however, many side effects have been reported following vaccination. Local adverse reactions (such as pain and swelling) and systemic reactions (such as fatigue and headaches) are commonly reported[1,2]. Neurological complications are relatively uncommon but have been reported variously. Shafiq et al[3] reviewed 78 patients who experienced such side effects, and facial nerve palsy was the most common event. Other CN palsies have also been reported[4,5]. However, the occurrence of multiple cranial palsies is rare. To date, there has been only one case in which a 29-year-old male patient developed left oculomotor, abducens, trigeminal, and facial palsies 6 days after receiving the first dose of the Pfizer-BioNTech COVID-19 vaccine[6]. The neurological complications following other type of vaccines have also been reported. Cosby et al[9] reviewed immune-related vaccine adverse events by searching PubMed for articles published between January 1945 and August 2018 and reported neuromuscular phenomenon such as Guillain–Barré syndrome (GBS). Other Immunologically mediated neurological complications were also reported[10,11]. About cranial nerve palsies, there are several studies reporting CN palsies following other vaccines. According to US Vaccine Adverse Event reporting system, the most commonly reported vaccine was seasonal influenza vaccine, followed by Hemophilus influenzae type b and Human papillomavirus vaccine[12,13].
To the best of our knowledge, this is the first pediatric case of multiple CN palsies following COVID-19 vaccination. Our case demonstrated facial, trigeminal, and glossopharyngeal nerve palsies. The etiology of CN palsy following vaccination is suspected to be immunological. Previous reports have suggested that the vaccine may damage the myelin sheaths and surrounding axons, causing demyelinating neuropathies such as Guillain–Barré syndrome (GBS), Bell’s palsy, and acute disseminated encephalomyelitis[14]. In the present case, multiple CN palsies and small-angle exotropia were observed. The extraocular muscles are susceptible to neurotoxins because they have a higher ratio of nerve fibers to the extraocular muscle fibers (1:3 to 1:5) compared to that of other skeletal muscles (1:50 to 1:125)[9]. The ocular motility findings of our patient were similar to those of previous reports of exotropia and diplopia caused by snakebites or insect sting[15]. Immune-mediated toxin after vaccination may affect the neuromuscular junction of the extraocular muscles, which leads to exotropia and diplopia. In adults, cardiac complications such as myocarditis and cardiac dysfunction have been reported[16,17]. It is known that the disease course of COVID-19 in children is milder than in adults, but cardiac complications such as cardiogenic shock and myocardial dysfunction also have been[18-20]. Therefore, it is important to consider vaccination according to the patient's underlying disease and risk of complications.
This case highlights a rare adverse effect of the mRNA COVID-19 vaccine in children. Our patient developed multiple CN palsies with small angle exotropia and substantially recovered after a steroid treatment. The most suspected etiology is the vaccine-induced immune response. There are very few cases of side effects caused by COVID-19 vaccine in children. As the incidence of the COVID-19 pandemic continues to increase, more studies on the side effects of vaccination, especially in children, are crucial.
We would like to thank the patient for allowing us to share case information.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Freund O S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021;15:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 2. | Xing K, Tu XY, Liu M, Liang ZW, Chen JN, Li JJ, Jiang LG, Xing FQ, Jiang Y. Efficacy and safety of COVID-19 vaccines: a systematic review. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:221-228. [PubMed] |
| 3. | Shafiq A, Salameh MA, Laswi I, Mohammed I, Mhaimeed O, Mhaimeed N, Paul P, Mushannen M, Elshafeey A, Fares A, Holroyd S, Zakaria D. Neurological Immune-Related Adverse Events After COVID-19 Vaccination: A Systematic Review. J Clin Pharmacol. 2022;62:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Pawar N, Ravindran M, Padmavathy S, Chakrabarty S. Acute abducens nerve palsy after COVID-19 vaccination in a young adult. Indian J Ophthalmol. 2021;69:3764-3766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Kerbage A, Haddad SF, Haddad F. Presumed oculomotor nerve palsy following COVID-19 vaccination. SAGE Open Med Case Rep. 2022;10:2050313X221074454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Manea MM, Dragoș D, Enache I, Sirbu AG, Tuta S. Multiple cranial nerve palsies following COVID-19 vaccination-Case report. Acta Neurol Scand. 2022;145:257-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Finsterer J, Scorza FA, Scorza C, Fiorini A. COVID-19 associated cranial nerve neuropathy: A systematic review. Bosn J Basic Med Sci. 2022;22:39-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20:817-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 804] [Article Influence: 201.0] [Reference Citation Analysis (0)] |
| 9. | Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 10. | Williams SE, Klein NP, Halsey N, Dekker CL, Baxter RP, Marchant CD, LaRussa PS, Sparks RC, Tokars JI, Pahud BA, Aukes L, Jakob K, Coronel S, Choi H, Slade BA, Edwards KM. Overview of the Clinical Consult Case Review of adverse events following immunization: Clinical Immunization Safety Assessment (CISA) network 2004-2009. Vaccine. 2011;29:6920-6927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Wajih Ullah M, Qaseem A, Amray A. Post Vaccination Guillain Barre Syndrome: A Case Report. Cureus. 2018;10:e2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 658] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 13. | Woo EJ, Winiecki SK, Ou AC. Motor palsies of cranial nerves (excluding VII) after vaccination: reports to the US Vaccine Adverse Event Reporting System. Hum Vaccin Immunother. 2014;10:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bettini E, Locci M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 15. | Abdelhady A, Patel BC, Aslam S, Al Aboud DM. Anatomy, Head and Neck, Eye Superior Oblique Muscle. 2021 Aug 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 16. | Freund O, Eviatar T, Bornstein G. Concurrent myopathy and inflammatory cardiac disease in COVID-19 patients: a case series and literature review. Rheumatol Int. 2022;42:905-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology. 2020;94:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 18. | Rodriguez-Gonzalez M, Castellano-Martinez A, Cascales-Poyatos HM, Perez-Reviriego AA. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World J Clin Cases. 2020;8:5250-5283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Zimmermann P, Curtis N. Why is COVID-19 less severe in children? Arch Dis Child. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 20. | Han MS, Choi EH, Chang SH, Jin BL, Lee EJ, Kim BN, Kim MK, Doo K, Seo JH, Kim YJ, Park JY, Suh SB, Lee H, Cho EY, Kim DH, Kim JM, Kim HY, Park SE, Lee JK, Jo DS, Cho SM, Choi JH, Jo KJ, Choe YJ, Kim KH, Kim JH. Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea. JAMA Pediatr. 2021;175:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |