Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12268
Peer-review started: June 9, 2022
First decision: July 29, 2022
Revised: September 6, 2022
Accepted: October 20, 2022
Article in press: October 20, 2022
Published online: November 26, 2022
Processing time: 167 Days and 4.7 Hours
Following the global outbreak of coronavirus disease 2019 (COVID-19), unlike other vaccines, COVID-19 vaccines were developed and commercialized in a relatively short period of time. The large-scale administration of this vaccine in a short time-period led to various unexpected side effects, including severe cytopenia and thrombosis with thrombocytopenia syndrome. Despite many reports on adverse reactions, vaccination was necessary to prevent the spread of COVID-19; thus, it is essential to understand and discuss various cases of adverse reactions after vaccination.
A 77-year-old woman was administered the second dose of Pfizer mRNA COVID-19 vaccine. After vaccination she experienced fever, myalgia, and weakness. Antibiotics were subsequently administered for several days, but there was no improvement in the symptoms. The patient showed severe thrombocytopenia and leukocytosis. Thoracic and abdominopelvic computed tomography showed no infection related findings, but splenomegaly and cirrhotic liver features were observed. A large number of immature cells were observed in the peripheral blood smear; thus, bone marrow examination was performed for acute leukemia. However, there were no abnormalities. The patient recovered after administration of hepatotoxins and transfusion treatment for cytopenia and was diagnosed with an adverse reaction to COVID-19 vaccination.
Adverse reactions of vaccination could be mistaken for hematologic malignancies including leukemia. We report a patient with leukocytosis following COVID-19 vaccination.
Core Tip: Cases of cytopenia or thrombosis with thrombocytopenia syndrome after coronavirus disease vaccination have been reported. We report a case of suspected hematologic malignancy, i.e., leukemia after vaccination in a female patient. Adverse reactions of vaccination could be mistaken for hematologic malignancies.
- Citation: Lee SB, Park CY, Park SG, Lee HJ. Case mistaken for leukemia after mRNA COVID-19 vaccine administration: A case report. World J Clin Cases 2022; 10(33): 12268-12277
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12268.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12268
Since the coronavirus disease 2019 (COVID-19) outbreak at the end of 2019, there have been more than 200 million infections and over 4.5 million deaths worldwide. Several people suffer from COVID-19 complications following recovery. Autoimmune hematologic disorders such as immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA), leukocytosis, thrombocytopenia, and eosinopenia have been reported as hematologic complications of COVID-19[1-5]. COVID-19 vaccination campaigns are conducted worldwide. Most adverse reactions after vaccination were mild and the vaccines are effective in the prevention of COVID-19. Severe adverse events include anaphylaxis, pericarditis, neurologic diseases such as Guillain-Barre syndrome, and hematologic diseases [hemolytic anemia, thrombosis with thrombocytopenic syndrome (TTS) such as cerebral sinus venous thrombosis, splanchnic vein thrombosis, and ITP][6-12]. Considering hematologic disorders, most cases are those of ITP or hemolysis in patients with underlying hematologic diseases[13-16]. Cases of blood-related adverse reactions have been reported even among individuals without underlying hematologic disease, and most of these cases were related to cytopenia[17-21].
Leukemoid reaction is a rare clinical condition defined as leukocytosis. This term was initially used by Krumbhaar[22] in 1926. Since then, it has been used to refer to reactive leukocytosis above 50 × 109/L with neutrophilia and a marked left shift (presence of immature neutrophilic forms) with non-hematologic malignancies[23].
We report a case with an adverse reaction that was mistaken for a hematologic malignancy due to an increased proportion of immature cells along with severe leukocytosis after COVID-19 vaccination.
A healthy 77-year-old woman with no known comorbidities and no medication use was transferred to the emergency room due to severe thrombocytopenia.
After the second dose of the BNT162b2 (Pfizer-BioNTech) vaccine, the patient visited a local clinic complaining of fever, myalgia, and weakness. The patient had no history of overseas travel, outdoor activity, or contact with wild animals. She was treated with antibiotics for a week due to elevated infection marker levels and fever. Despite continuous antibiotic administration, the patient's symptoms did not improve; this was followed by the occurrence of dyspnea along with thrombocytopenia. The patient was referred to our clinic for further evaluation of newly diagnosed thrombocytopenia and dyspnea.
Prior to vaccination, the patient had no history of disease, including malignancy, and there was no medication administration. There was no history of any infectious disease, including COVID-19.
The patient is a housewife and has never been exposed to certain occupational risks. She denied tobacco smoking, alcohol drinking, and drug abuse. There was also no confirmed family history.
Except for fever, the patient's vital signs were stable. Despite dyspnea, there was no oxygen demand. Physical examination revealed splenomegaly of three-finger width.
The complete blood count results were as follows (normal ranges are shown in parentheses): White blood cells, 11590 × 103/μL (4.0-10.0 × 103/μL); hemoglobin, 8.6 g/dL (12-16 g/dL); platelets, 38 × 103/μL (150-400 × 103/μL). The blood biochemistry results were as follows: Total bilirubin, 6.5 mg/dL (0.2-1.1 mg/dL); aspartate aminotransferase (AST), 242 U/L (5-40 U/L); alanine aminotransferase (ALT), 74 U/L (5-40 U/L); albumin, 2.06 g/dL (3.5-5.2 g/dL); blood urea nitrogen, 23.0 mg/dL (8-20 mg/dL); creatinine, 1.27 mg/dL (0.5-1.3 mg/dL); C-reactive protein (CRP), > 16 mg/dL (0-0.3 mg/dL). The coagulation profile results were as follows: Prothrombin time, 20.5 s (9.4-12.5 s); activated partial thromboplastin time, 41.3 s (28.0-44.0 s), fibrinogen 350 mg/dL (200-400 mg/dL), D-dimer 5830 (0-255 ng/mL) (Table 1). The real-time reverse transcription-polymerase chain reaction results were negative for COVID-19. The results were also negative for Hantavirus, Letospira, Rickettsia, and Scrub typhus. Further virological laboratory tests for human immunodeficiency virus and hepatitis B, C, and A were negative. Urine and blood cultures showed no bacterial growth (Table 2).
| Laboratory parameter | Result | Normal range |
| WBC (/μL) | 11590 | 4000-10000 |
| Neutrophil (%) | 58.7 | 40-80 |
| Lymphocyte (%) | 31.2 | 25-50 |
| Monocyte (%) | 9.8 | 0-9 |
| Eosinophil (%) | 0.1 | 0-7 |
| Basophil (%) | 0.2 | 0-1.8 |
| Platelet (/μL) | 38000 | 150000-400000 |
| AST (U/L) | 242 | 5-40 |
| ALT (U/L) | 73.5 | 5-40 |
| Total bilirubin (mg/dL) | 6.5 | 0.2-1.2 |
| CRP (mg/dL) | > 16 | 0.0-0.3 |
| Diseases | Result |
| COVID-19 | Negative |
| Ebstein-Barr virus | Negative |
| Cytomegalovirus | Negative |
| Hepatitis A | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Hantavirus | Negative |
| HIV | Negative |
| Rickettsia tsutsugamushi | Negative |
| Leptospira | Negative |
| Blood bacterial culture | Negative |
| Urine bacterial culture | Negative |
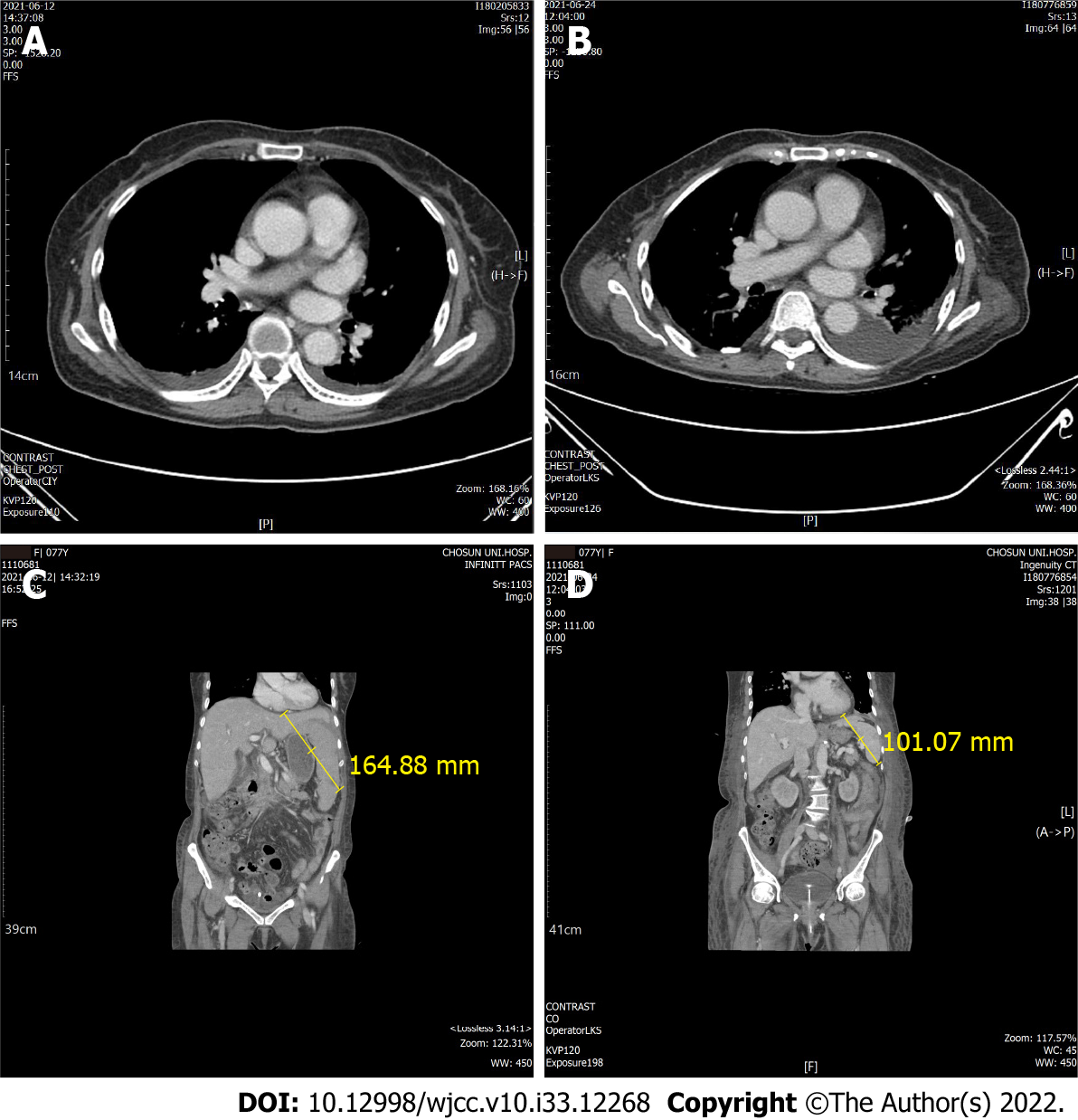
Thoracic and abdominopelvic computed tomography (CT) was performed to check for infection focus and the cause of dyspnea. Thoracic CT revealed mild pleural effusion, but no findings indicated infection, such as pneumonia or bronchitis (Figure 1). On abdominopelvic CT, liver cirrhosis was suspected with splenomegaly (16.5 cm) and moderate ascites (Figure 1).
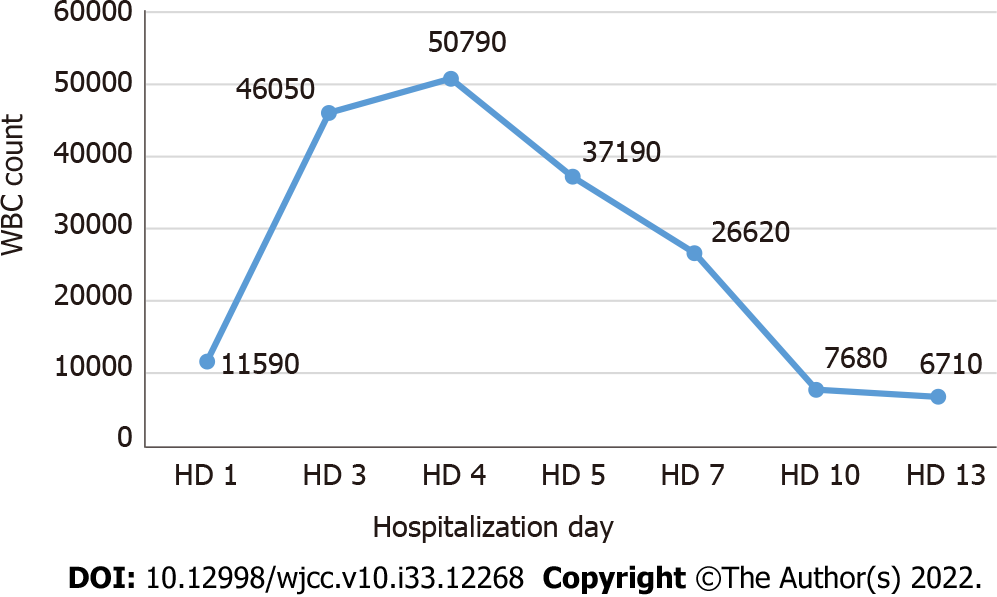
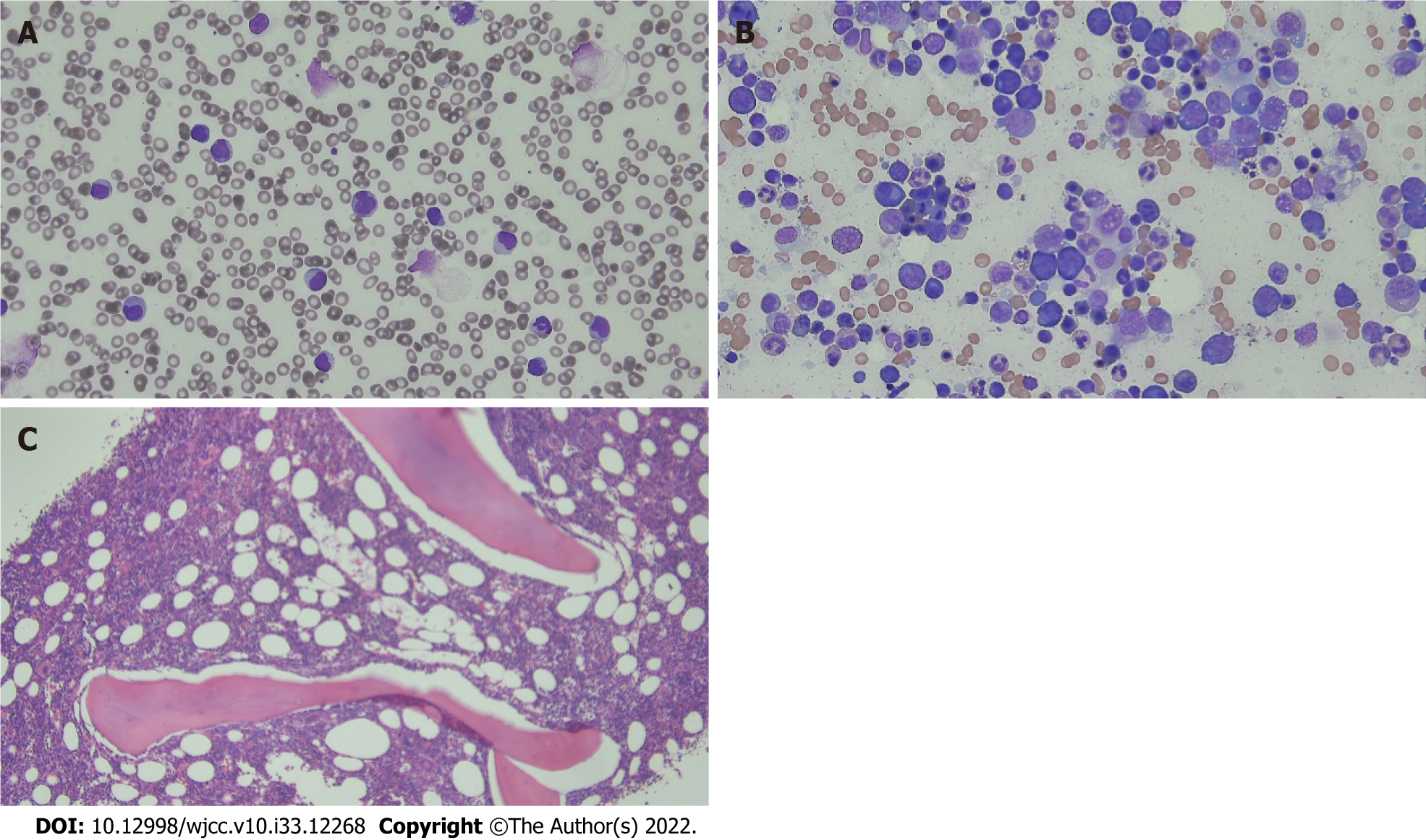
Most infectious diseases were not considered to be the cause of the patient’s symptoms; thus, the causes of cirrhosis and splenomegaly were evaluated. All tests for autoimmune hepatitis were negative (Table 3). Although no evidence of infectious disease was found, ceftriaxone administration was continued due to leukocytosis, CRP elevation, and persistent febrile symptoms. On day 2 of hospitalization, continuous renal replacement treatment (CCRT) was started due to decreased urine output accompanied by metabolic acidosis, and CCRT was stopped due to recovery of kidney function on day 5 of hospitalization. On day 4, the white blood cell count was elevated to 50790 × 103/μL (Figure 2) and immature cells were observed in the peripheral blood smear. To rule out acute leukemia, we performed bone marrow biopsy, but there were no abnormalities (Figure 3). On day 5 of hospitalization, the total bilirubin increased to 10.0 mg/dL and the LDH level also increased to 1053 mg/dL, with a low haptoglobin level. In the peripheral blood smear, schistocytes were observed in trace amounts, but both direct and indirect Coombs’ test results were negative.
| Laboratory parameter | Results | Normal range |
| Anti LKM-1 Ab | Negative | Negative |
| Anti-mitochondria Ab | Negative | Negative |
| ANA (titer) | Centromere 1:1280 | |
| Anti dsDNA antibody (IU/mL) | Negative < 10 | 10-15 |
| p-ANCA (IU/mL) | Negative < 0.1 | 0-3.5 |
The patient was diagnosed with an adverse reaction to COVID-19 vaccination and not with a hematologic malignancy such as acute leukemia.
Hepatotoxins, platelets and fresh-frozen plasma transfusion, and intravascular fluid were only administered due to liver cirrhosis, splenomegaly, changes in blood count, and CRP elevation observed at the time of hospitalization.
AST, ALT, and bilirubin levels decreased from day 7 of hospitalization, and the coagulation panel also started to improve. From day 5 of hospitalization, the leukocyte count started decreasing and recovered to the normal level on day 10; the platelet count also recovered to > 100000 showing a normal blood cell count profile from day 11. On day 13 of hospitalization, we performed abdomino-pelvic CT again and it was confirmed that the ascites had decreased and splenomegaly had improved. The patient was discharged in good condition on day 16 of hospitalization and is currently undergoing regular follow-up as an outpatient.
Various adverse events of COVID-19 vaccines like those of many other vaccines have been reported. There are mild adverse events such as fever, fatigue, headache, myalgia, and arthralgia, and more severe events such as anaphylactic shock, myocarditis, and TTS. Although one case of TTS related to mRNA-based vaccine has been reported, TTS is mainly reported in relation to adenoviral vector vaccines[17-20]. ITP and hemolytic anemia mainly occur in relation to mRNA-based vaccines[24-28].
Cases of ITP and one case of AIHA related to the mRNA-1273 (Moderna) vaccine have been reported[24]. One case of ITP was reported in a patient with Evans syndrome, and AIHA was observed in a healthy elderly man[13]. Adverse events related to the Pfizer-BionTech vaccine included several cases of ITP, one case of AIHA, and four cases of severe hemolysis in paroxysmal nocturnal hemoglobinuria (Table 4)[25-27]. Although the specific vaccine type is unknown, one case of hemolytic crisis in a patient with primary cold agglutinin disease and AIHA in a patient with clinically insignificant cryoglobulinemia have been reported[15]. However, to the best of our knowledge, there are no reports of severe leukocytosis. Cases of leukemoid reaction with COVID-19 have been reported, but there are no reports of similar cases related to vaccination[4,5]. The major causes of leukemoid reaction are severe infection, malignancies, intoxication, or hemorrhage. There were no findings that indicated malignancy or infection on CT performed at the time of admission when the patient was evaluated for all possible infectious diseases at the Department of Infectious Diseases; however, this was not confirmed. The patient showed negative real-time polymerase chain reaction test results for severe acute respiratory syndrome coronavirus 2, eliminating the possibility of COVID-19. With findings including thrombocytopenia, fever, dyspnea, and pleural effusion, a disease such as dengue fever can also be suspected. However, South Korea is not an endemic area of dengue fever and its residents have no history of travel to a country where the disease occurs; thus, this disease was excluded.
| Types of hematologic adverse events | Patient No. and Ref. | Age, yr | Sex | Symptom onset (No. of days after vaccination) | Symptoms | Underlying diseases | Type of vaccine | Outcome |
| ITP | 1, Tarawneh and Tarawneh[31] | 22 | M | 3 | Petechia, gum bleeding | None | Pfizer | Recovery |
| 2-9, Lee et al[26] | NA | NA | NA | NA | NA | Pfizer | NA | |
| 10-20, Lee et al[26] | NA | NA | NA | NA | NA | Moderna | NA | |
| 21, Shah et al[27] | 53 | M | 8 | Petechia rash, myalgia | Crohn’s disease | Pfizer | Recovery | |
| 22, Shah et al[27] | 67 | M | 2 | Melena | Chronic ITP | Pfizer | Recovery | |
| 23, Shah et al[27] | 59 | F | 2 | Bloody diarrhea | SLE, chronic ITP | J&J | Recovery | |
| 24, Ganzel and Ben-Chetrit[25] | 53 | M | 14 | Epistaxis | DM, HTN, otitis | Pfizer | Recovery | |
| 25, Toom et al[32] | 36 | F | 14 | Petechia, bruising, gum bleeding, headache | ITP | Moderna | Recovery | |
| 26, Paulsen et al[28] | 72 | M | 11 | Petechia, epistaxis, headache | Autoimmune thyroiditis | AZD1222 | NA | |
| 27 Paulsen et al[28] | 71 | F | 11 | Petechia, hyposphagma | Latent hyperthyroidism, breast cancer, stroke | AZD1222 | NA | |
| 28 Paulsen et al[28] | 66 | M | 2 | Petechia | HTN, mild thrombocytopenia | AZD1222 | NA | |
| 29 Paulsen et al[28] | 64 | F | 15 | None | HTN, COPD, steatosis hepatitis | AZD1222 | NA | |
| 30, Ghosh et al[33] | 63 | F | 2 | Bruise | COPD, Type 2 DM | Pfizer | Recovery | |
| AIHA | 30, Gaignard et al[13] | 56 | M | 3 | Painless petechia | Evans syndrome | Moderna | Recovery |
| 31, Gaignard et al[13] | 77 | M | 5 | Weakness, fatigue, shortness of breath | none | Moderna | Recovery | |
| 32, Murdych[16] | 84 | M | 19 | Urinary frequency, dizziness | Prostate & colon cancer, CAD, HTN, trace cryoglobulinemia, emphysema, mild chronic anemia, major depression and/or anxiety | Pfizer | Recovery | |
| 33, Brito et al[24] | 88 | F | 2 | Asthenia, jaundice | Insomnia | mRNA vaccine | Recovery | |
| Hemolytic crisis | 35, Pérez-Lamas et al[15] | 57 | F | 2 | Chills, weakness, exertional dyspnea, jaundice, mild hemoglobinurina | Cold agglutinin disease | mRNA vaccine | Recovery |
| Hemolysis | 36, Gerber et al[14] | 25 | M | 5 | Abdominal pain | PNH | Pfizer | NA |
| 37, Gerber et al[14] | 45 | M | 0 | Fever, headache, myalgia, fatigue, hemoglobinuria | PNH | Pfizer | NA | |
| 37, Gerber et al[14] | 32 | F | 0 | Fever, rigor | PNH | Moderna | NA | |
| 38, Gerber et al[14] | 63 | M | 0 | Fatigue, darkening urine | PNH | Moderna | NA |
Our findings suggested the occurrence of cirrhosis from the early stage of hospitalization; all possible causes were evaluated, but the exact cause was not identified. There were no risk factors such as alcohol drinking history, drug abuse, or stick injury. The patient was transferred from the Department of Infectious Diseases to the Department of Hematology due to leukocytosis with immature cells that persisted without evidence of infection. Bone marrow examination was performed to differentiate malignant diseases such as acute leukemia; no abnormal cells including blasts were identified, and the Department of Laboratory Medicine reported that it was a reactive bone marrow according to the patient’s disease state. The patient’s condition improved with only supportive treatment, such as fluid therapy and blood transfusion, without any special treatment except for antibiotic administration. The detailed pathogenesis of leukocytosis and splenomegaly is unknown. The diagnosis of liver cirrhosis was presumed from initial CT findings such as splenomegaly with ascites; however, liver biopsy was not performed to rule out liver cirrhosis. Autoimmune hepatitis developing after COVID-19 vaccination has been reported. This report postulated that autoinflammatory dysregulation was the cause of tissue damage[29]. In our case, organ damage such as liver cirrhosis was observed by a similar mechanism. Further studies on the pathogenesis and confirmation in more cases are needed.
No case of severe leukocytosis after COVID-19 vaccination has been reported so far. There have been reports of leukocytosis after pneumococcal polysaccharide vaccine administration wherein it was hypothesized that the leukocytosis was the result of an inflammatory response due to increased cytokines in the body after vaccination. However, further studies on the pathogenesis have not yet been conducted[30]. An excessive inflammatory response can also be assumed in the present case, which could have been caused by increased cytokines after vaccination; however, additional research is needed regarding this.
The patient was suspected to have infection due to fever, leukocytosis and CRP elevation. All infectious agents were excluded and immature cells were observed in the peripheral blood smear with leukocytosis; thus, other causes of leukemoid reaction were also investigated, but all results were negative. The patient had a history of COVID-19 vaccination prior to symptom onset, no specific underlying disease or medication history, and no special findings in the overall evaluation including bone marrow examination. The patient’s symptoms were considered to be adverse events due to vaccination, and this is the first report of a leukemoid-like reaction that occurred after COVID-19 vaccination.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Salim J, Indonesia; Zhang JX, China S-Editor: Wang DM L-Editor: Webster JR P-Editor: Wang DM
| 1. | Bhattacharjee S, Banerjee M. Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr Clin Med. 2020;2:2048-2058. [PubMed] [DOI] [Full Text] |
| 2. | Mahévas M, Moulis G, Andres E, Riviere E, Garzaro M, Crickx E, Guillotin V, Malphettes M, Galicier L, Noel N, Darnige L, Terriou L, Guerveno C, Sanchis-Borja M, Moulinet T, Meunier B, Ebbo M, Michel M, Godeau B. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190:e224-e229. [PubMed] [DOI] [Full Text] |
| 3. | Algassim AA, Elghazaly AA, Alnahdi AS, Mohammed-Rahim OM, Alanazi AG, Aldhuwayhi NA, Alanazi MM, Almutairi MF, Aldeailej IM, Kamli NA, Aljurf MD. Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2 infection. Ann Hematol. 2021;100:37-43. [PubMed] [DOI] [Full Text] |
| 4. | Tarekegn K, Colon Ramos A, Sequeira Gross HG, Yu M, Fulger I. Leukemoid Reaction in a Patient With Severe COVID-19 Infection. Cureus. 2021;13:e13598. [PubMed] [DOI] [Full Text] |
| 5. | Tabassum S, Bibi T, Tariq F, Tariq S, Raza S, Hafeez M, Rana M. Unusual leukemoid reaction in a COVID-19 patient: a case report. Biol Clin Sci Res J. 2020;. [DOI] [Full Text] |
| 6. | Rosenberg HF, Foster PS. Eosinophils and COVID-19: diagnosis, prognosis, and vaccination strategies. Semin Immunopathol. 2021;43:383-392. [PubMed] [DOI] [Full Text] |
| 7. | Lee DS, Kim JW, Lee KL, Jung YJ, Kang HW. Adverse events following COVID-19 vaccination in South Korea between February 28 and August 21, 2021: A nationwide observational study. Int J Infect Dis. 2022;118:173-182. [PubMed] [DOI] [Full Text] |
| 8. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [PubMed] [DOI] [Full Text] |
| 9. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [PubMed] [DOI] [Full Text] |
| 10. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. [PubMed] [DOI] [Full Text] |
| 11. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [PubMed] [DOI] [Full Text] |
| 12. | Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325:1101-1102. [PubMed] [DOI] [Full Text] |
| 13. | Gaignard ME, Lieberherr S, Schoenenberger A, Benz R. Autoimmune Hematologic Disorders in Two Patients After mRNA COVID-19 Vaccine. Hemasphere. 2021;5:e618. [PubMed] [DOI] [Full Text] |
| 14. | Gerber GF, Yuan X, Yu J, Cher BAY, Braunstein EM, Chaturvedi S, Brodsky RA. COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood. 2021;137:3670-3673. [PubMed] [DOI] [Full Text] |
| 15. | Pérez-Lamas L, Moreno-Jiménez G, Tenorio-Núñez MC, Velázquez-Kennedy K, Jiménez-Chillón C, Astibia-Mahillo B, Núñez-Torrón C, García-Gutiérrez V, Jiménez-Martín A, Vallés-Carboneras A, López-Jiménez JF. Hemolytic crisis due to Covid-19 vaccination in a woman with cold agglutinin disease. Am J Hematol. 2021;96:E288-E291. [PubMed] [DOI] [Full Text] |
| 16. | Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine. Int J Lab Hematol. 2022;44:e10-e12. [PubMed] [DOI] [Full Text] |
| 17. | Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384:2092-2101. [PubMed] [DOI] [Full Text] |
| 18. | Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021;384:1964-1965. [PubMed] [DOI] [Full Text] |
| 19. | Bang SM, Na SH, Kim JH, Kim SR, Jang S. Platelet count as an important prognostic factor for vaccine-induced immune thrombotic thrombocytopenia. Blood Res. 2021;56:129-133. [PubMed] [DOI] [Full Text] |
| 20. | Sangli S, Virani A, Cheronis N, Vannatter B, Minich C, Noronha S, Bhagavatula R, Speredelozzi D, Sareen M, Kaplan RB. Thrombosis With Thrombocytopenia After the Messenger RNA-1273 Vaccine. Ann Intern Med. 2021;174:1480-1482. [PubMed] [DOI] [Full Text] |
| 21. | Shimabukuro T, Nair N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA. 2021;325:780-781. [PubMed] [DOI] [Full Text] |
| 22. | Krumbhaar EB. Leukemoid blood pictures in various clinical conditions. Am J Med Sci. 1926;172:519-532. [DOI] [Full Text] |
| 23. | Portich JP, Faulhaber GAM. Leukemoid reaction: A 21st-century cohort study. Int J Lab Hematol. 2020;42:134-139. [PubMed] [DOI] [Full Text] |
| 24. | Brito S, Ferreira N, Mateus S, Bernardo M, Pinto B, Lourenço A, Grenho F. A Case of Autoimmune Hemolytic Anemia Following COVID-19 Messenger Ribonucleic Acid Vaccination. Cureus. 2021;13:e15035. [PubMed] [DOI] [Full Text] |
| 25. | Ganzel C, Ben-Chetrit E. Immune Thrombocytopenia Following the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Isr Med Assoc J. 2021;23:341. [PubMed] |
| 26. | Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert MP, Bussel JB. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96:534-537. [PubMed] [DOI] [Full Text] |
| 27. | Shah SRA, Dolkar S, Mathew J, Vishnu P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp Hematol Oncol. 2021;10:42. [PubMed] [DOI] [Full Text] |
| 28. | Paulsen FO, Schaefers C, Langer F, Frenzel C, Wenzel U, Hengel FE, Bokemeyer C, Seidel C. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19). Blood. 2021;138:996-999. [PubMed] [DOI] [Full Text] |
| 29. | Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75:222-224. [PubMed] [DOI] [Full Text] |
| 30. | von Elten KA, Duran LL, Banks TA, Collins LC. Systemic inflammatory reaction after pneumococcal vaccine: a case series. Hum Vaccin Immunother. 2014;10:1767-1770. [PubMed] [DOI] [Full Text] |
| 31. | Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021;96:E133-E134. [PubMed] [DOI] [Full Text] |
| 32. | Toom S, Wolf B, Avula A, Peeke S, Becker K. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 Covid-19 vaccine. Am J Hematol. 2021;96:E134-E135. [PubMed] [DOI] [Full Text] |
| 33. | Ghosh AK, Bhushan S, Lopez LDR, Sampat D, Salah Z, Hatoum CA. BNT162b2 COVID-19 Vaccine Induced Immune Thrombocytopenic Purpura. Case Rep Med. 2022;2022:5603919. [PubMed] [DOI] [Full Text] |