Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12230
Peer-review started: August 23, 2022
First decision: October 17, 2022
Revised: October 19, 2022
Accepted: October 24, 2022
Article in press: October 24, 2022
Published online: November 26, 2022
Processing time: 92 Days and 6.7 Hours
The association between pretreatment serum ferritin concentration (SFC) and long-term survival in lung cancer remains unclear now.
To identify the prognostic value of pretreatment SFC in lung cancer patients based on current evidence.
The PubMed, EMBASE and Web of Science databases were searched from inception to May 29, 2022 for relevant studies. The primary endpoint was overall survival (OS) and the hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were combined to assess the predictive role of pretreatment SFC for long-term survival of lung cancer patients. The data were then extracted and assessed on the basis of the Reference Citation Analysis (https://www.referencecitationanalysis.com/).
Twelve retrospective studies involving 1654 patients were analyzed. The results manifested that increased pretreatment SFC was associated with worse OS (HR = 1.09, 95%CI: 1.03-1.15, P = 0.004). Subgroup analysis stratified by the country (China vs non-China) showed similar results. However, subgroup analysis stratified by tumor type revealed inconsistent results (lung cancer: HR = 1.39, P = 0.008; small cell lung cancer: HR = 1.99, P = 0.175; non-small cell lung cancer: HR = 1.03, P = 0.281).
Pretreatment SFC might serve as a promising prognostic indicator in lung cancer patients and elevated pretreatment SFC predicts worse prognosis. However, more high-quality studies with big sample sizes are still needed to further verify its prognostic value in lung cancer.
Core Tip: Our results manifested that increased pretreatment serum ferritin concentration (SFC) was significantly associated with worse overall survival (P = 0.004). Subgroup analysis based on the country (China vs non-China) showed similar results. However, subgroup analysis stratified by tumor type revealed inconsistent results (lung cancer: HR = 1.39, P = 0.008; small cell lung cancer: HR = 1.99, P = 0.175; non-small cell lung cancer: HR = 1.03, P = 0.281). Pretreatment SFC might serve as a promising prognostic indicator in lung cancer patients and elevated pretreatment SFC predicts worse prognosis. However, more high-quality studies with big sample sizes are still needed to further verify its prognostic value in lung cancer.
- Citation: Gao Y, Ge JT. Prognostic role of pretreatment serum ferritin concentration in lung cancer patients: A meta-analysis. World J Clin Cases 2022; 10(33): 12230-12239
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12230.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12230
Lung cancer is the most common malignancy in China and remains the leading cause of cancer-related death[1]. Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) account for about 85% and 15% of all lung cancer cases, respectively[2,3]. Despite the great advances in the screening and therapy strategies of lung cancer, the overall prognosis of lung cancer patients is still not optimistic[1,4,5]. The tumor-node-metastasis (TNM) remains the most authoritative tool for prediction of long-term survival and formulation of treatment strategy. However, in some cases, TNM stage system is not enough for clinical guidance because of the significant heterogeneity between lung cancer patients with the same TNM stage. Thus, it is necessary to identify more valuable prognostic indicators in lung cancer.
Iron is an essential trace element for the human body. It is not only necessary for oxygen supply to all cells, but also participates in redox reactions and cell growth processes[6]. Besides, it also plays an important role in antioxidant defense reactions[6]. However, elevated serum iron levels would accelerate redox reactions and the production of reactive oxygen species like hydroxyl radicals. The hydroxyl radical is a highly active substance which can induce lipid peroxidation and DNA damage, leading to the occurrence and development of some cancers[7-10]. Compared with normal cells, tumor cells are highly dependent on iron, which is called iron addiction[11]. It is known that serum ferritin is a reliable indicator reflection the iron level. A number of studies have manifested that the serum ferritin concentration (SFC) is obviously increased and also associated with long-term survival in cancer patients[12-16]. Up to now, a number of studies explored the predictive role of pretreatment SFC for prognosis in lung cancer with inconsistent results[17-28].
Therefore, we aimed to further verify the prognosis value of pretreatment SFC in lung cancer.
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2009) checklist[29].
In this meta-analysis, the PubMed, EMBASE and Web of Science electronic databases were searched from inception to May 29, 2022 for studies exploring the prognostic value of pretreatment SFC in lung cancer. The following key words were used during the search process: Ferritin, lung, pulmonary, tumor, cancer, neoplasm, carcinoma, prognosis, prognostic and survival. The specific search strategy in the PubMed was as follows: (ferritin) AND (lung OR pulmonary) AND (tumor OR cancer OR neoplasm OR carcinoma) AND (prognosis OR prognostic OR survival). Furthermore, the references in the included studies were also reviewed for availability. Meanwhile, Reference Citation Analysis (https://www.referencecitationanalysis.com/) was used to supplement the search.
The inclusion criteria were as follows: (1) Patients were pathologically diagnosed with primary lung cancer; (2) the SFC was calculated before anti-tumor treatment including the surgery, chemotherapy, radiotherapy and others; (3) the overall survival (OS) or other similar survival indexes of patients were compared between increased and decreased pretreatment SFC groups; and (4) the hazard ratios (HRs) with 95% confidence intervals (CIs) were reported in the articles or enough data were provided to calculate them.
The exclusion criteria were as follows: (1) Reviews, meeting abstracts, editorials, letters or animal trials; (2) insufficient data for assessment of study quality; and (3) the HRs with corresponding 95%CIs were not available.
The following information was extracted from each included studies: the name of first author, publication year, country, sample size, tumor type, TNM stage, treatment (surgery vs non-surgery), cutoff value of SFC, endpoint, HR and 95%CI.
The methodological quality of included studies was evaluated according to the Newcastle Ottawa Scale (NOS)[30]. The NOS consisted of three parameters of quality: Selection (representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure and outcome of interest), comparability (comparability of cohorts), and outcome (assessment of outcome, time of follow-up and adequacy of follow-up). Studies with a NOS score of 6 or higher were defined as high-quality studies[30].
Besides, the literature search, selection, data extraction and quality assessment were all conducted by two authors independently and any disagreement was resolved by team discussion.
All statistical analysis of this study were performed by STATA 15.0 software (College Station, TX, United States). The HRs with 95%CIs were combined to identify the association of pretreatment SFC with prognosis of lung cancer patients. The heterogeneity was evaluated by Cochran’s Q test and Higgins I2 statistic; P < 0.10 and/or I2 > 50% was defined as significant heterogeneity among studies, and the random-effects model was used for the pooled effect estimates, otherwise the fixed-effects model was used[31]. Subgroup analyses stratified by the country (non-China vs China) and tumor type (SCLC vs lung cancer vs NSCLC) were conducted. Sensitivity analysis for OS was performed by removing individual study from the meta-analysis each time. Besides, the Begg’s funnel plot and Egger’s test were also conducted to detect the publication bias[32]. If publication bias was observed by presenting a P < 0.05, the nonparametric trim-and-fill method was applied to re-estimate a corrective effect size after publication bias was adjusted.
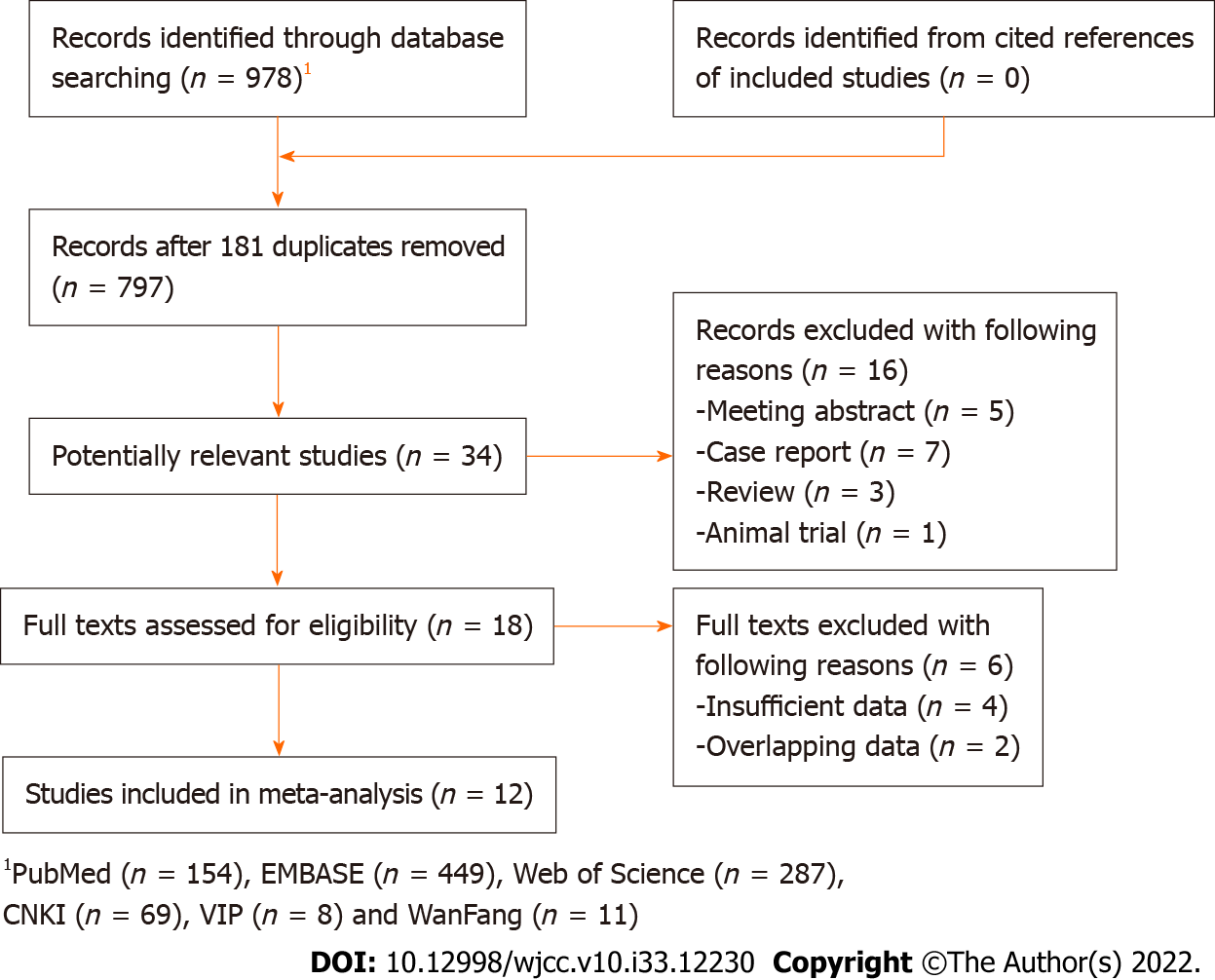
Initially, 978 records were found in databases and 181 duplicated records were removed. Then 763 irrelevant publications were excluded. Subsequently, the full texts of 18 publications were reviewed after removing 16 records due to the design nature. Eventually, only 12 publications were included [17-28]. The detailed selection process was presented in Figure 1.
Among 12 retrospective included studies, 1654 patients were involved with the sample size ranged from 25 to 400. Most studies were from Asian countries. Five[19,23-26] and four[17,19,22,27] studies focused on NSCLC and SCLC, separately. The cutoff values of SFC ranged from 114.1 ng/dL to 400 ng/dL. All included studies were high-quality studies with a NOS score ≥ 6. Detailed information was presented in Table 1.
| Ref. | Country | Sample size | Tumor type | TNM stage | Treatment | Cutoff value of ferritin (ng/dL) | NOS | Endpoint | |||
| Selection | Comparability | Outcome | Overall | ||||||||
| Milman et al[17], 1991 | Denmark | 31 | SCLC | Limited and advanced | Non-surgery | 400 | 3 | 0 | 3 | 6 | OS |
| Ferrigno et al[18], 1992 | Italy | 168 | LC | I-IV | Mixed | 236 | 4 | 0 | 2 | 6 | OS |
| Milman et al[19], 2002 | Denmark | 90 | NSCLC | I-IV | Mixed | 300 | 4 | 0 | 3 | 7 | OS |
| Milman et al[19], 2002 | Denmark | 25 | SCLC | Limited and advanced | Mixed | 300 | 3 | 0 | 3 | 6 | OS |
| Erbaycu et al[20], 2008 | Turkey | 41 | LC | I-IV | Mixed | 220 | 3 | 0 | 3 | 6 | OS |
| Zhao et al[21], 2014 | China | 69 | LC | IV | Non-surgery | NR | 4 | 0 | 3 | 7 | OS |
| Xie et al[22], 2018 | China | 72 | SCLC | Limited and advanced | NR | NR | 4 | 0 | 3 | 7 | OS |
| Lee et al[23], 2019 | Republic of Korea | 138 | NSCLC | IIIB-IV | Non-surgery | 200 | 4 | 0 | 3 | 7 | OS |
| Ma et al[24], 2019 | China | 393 | NSCLC | I-III | NR | 382.65 | 4 | 0 | 3 | 7 | OS |
| Sun[25], 2020 | China | 72 | NSCLC | III-IV | Non-surgery | Male: 200, female: 150 | 3 | 0 | 3 | 6 | OS |
| Ji et al[26], 2021 | China | 69 | NSCLC | IIIB-IV | Non-surgery | 311.1 | 3 | 0 | 3 | 6 | OS |
| Xiao[27], 2021 | China | 86 | SCLC | NR | NR | NR | 3 | 0 | 3 | 6 | OS |
| Zhu[28], 2021 | China | 400 | LC | I-IV | NR | 114.1 | 4 | 0 | 3 | 7 | OS |
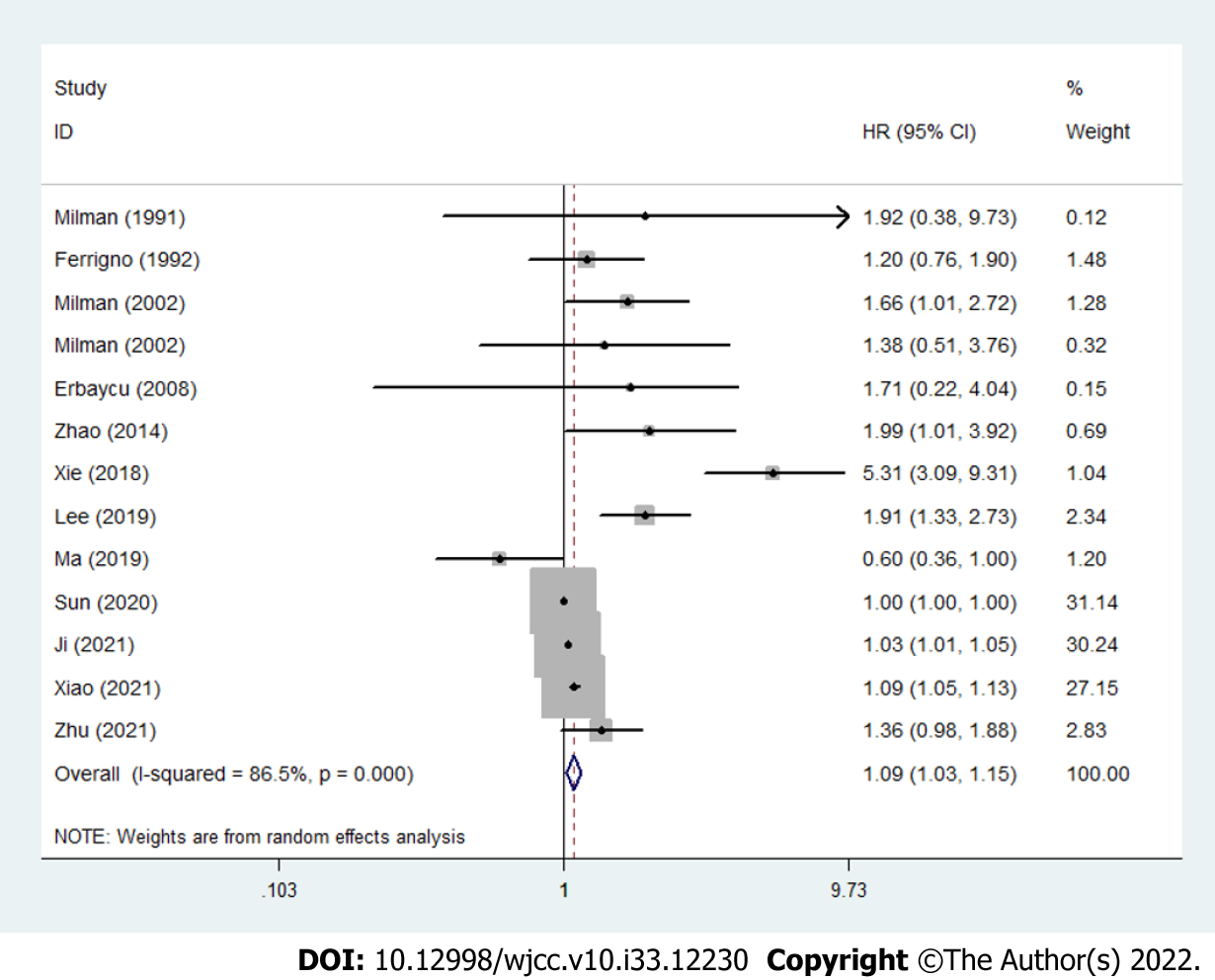
The results demonstrated that increased pretreatment SFC was significantly associated with poorer OS in lung cancer (HR = 1.09, 95%CI: 1.03-1.15, P = 0.004; I2 = 86.5%, Pheterogeneity < 0.001) (Figure 2).
Subgroup analysis based on the country was performed and the results manifested that pretreatment SFC had high prognostic value in both non-Chinese (HR = 1.61, 95%CI: 1.27-2.03, P < 0.001) and Chinese patients (HR = 1.06, 95%CI: 1.00-1.12, P = 0.042) (Table 2). Besides, subgroup analysis stratified by the tumor type showed inconsistent results. Pretreatment SFC was related to the OS in lung cancer (HR = 1.39, 95%CI: 1.09-1.77, P = 0.008), but no significant association between pretreatment SFC and OS in SCLC (HR = 1.99, 95%CI: 0.74-5.35, P = 0.175) and NSCLC (HR = 1.03, 95%CI: 0.98-1.07, P = 0.281) (Table 2).
| No. of studies | HR | 95%CI | P value | I2 (%) | Pheterogeneity | |
| Overall survival | 12 (17-28) | 1.09 | 1.03-1.15 | 0.004 | 86.5 | < 0.001 |
| Country | ||||||
| Non-China | 5 (17-20, 23) | 1.61 | 1.27-2.03 | < 0.001 | 0.0 | 0.760 |
| China | 7 (21, 22, 24-28) | 1.06 | 1.00-1.12 | 0.042 | 91.5 | < 0.001 |
| Tumor type | ||||||
| SCLC | 4 (17, 19, 22, 27) | 1.99 | 0.74-5.35 | 0.175 | 90.7 | < 0.001 |
| LC | 4 (18, 20, 21, 28) | 1.39 | 1.09-1.77 | 0.008 | 0.0 | 0.666 |
| NSCLC | 5 (19, 23-26) | 1.03 | 0.98-1.07 | 0.281 | 86.2 | < 0.001 |
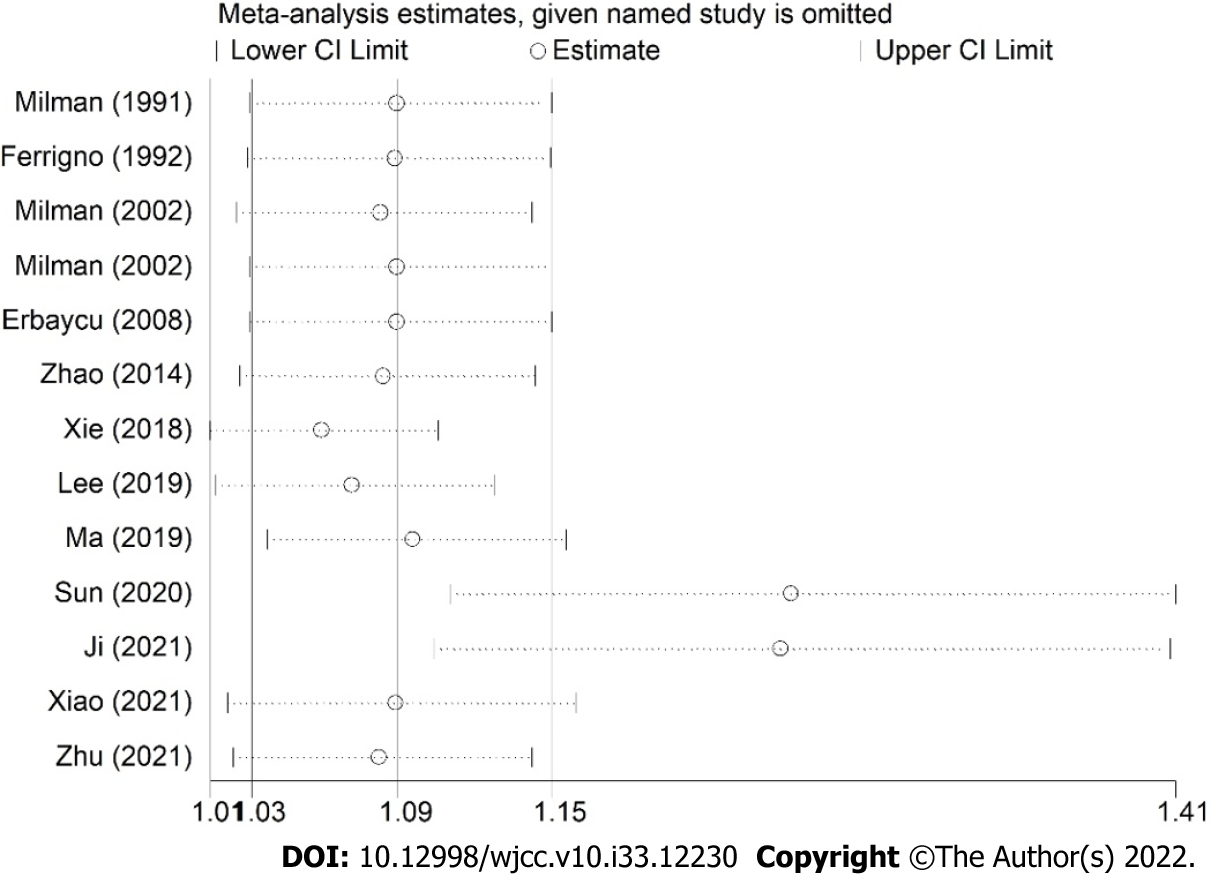
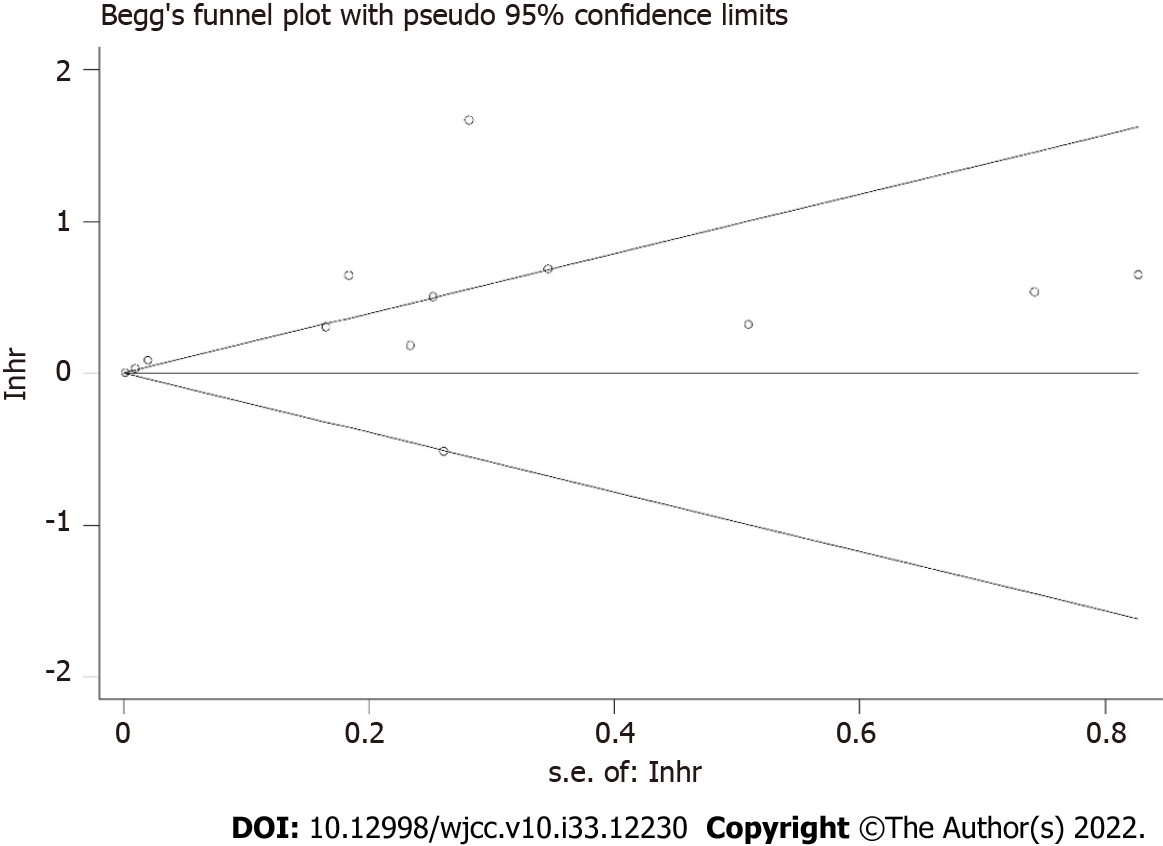
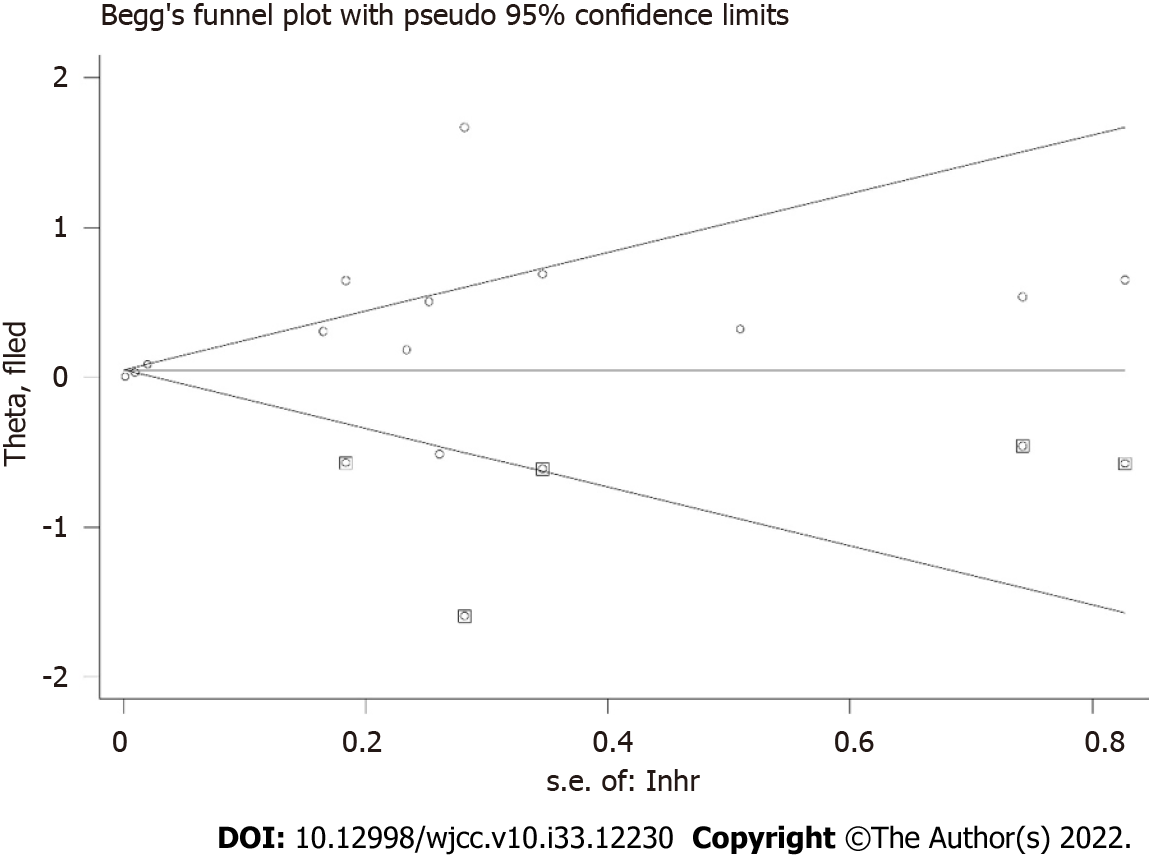
The sensitivity analysis demonstrated that the results were stable and relatively reliable (Figure 3). However, the studies by Sun et al[25] and Ji et al[26] showed relatively obvious impacts on the overall results. Furthermore, due to the asymmetric Begg’s funnel plot (Figure 4) and P = 0.009 of Egger’s test, significant publication bias was observed. Then the trim-and-fill method was used and we detected five potentially unpublished studies (Figure 5). After combining these five studies, the new estimated HRs were 1.003 (95%CI: 1.000-1.006, P = 0.031) for fixed-effects model and 1.051 (95%CI: 0.984-1.123, P = 0.139) for random-effects model, separately. Thus, more prospective high-quality studies are still needed to further explore the prognostic value of pretreatment SFC in lung cancer patients.
In the current meta-analysis, 12 studies involving 1654 Lung cancer patients were included and the results demonstrated that elevated pretreatment SFC was a significant predictor for worse OS in lung cancer. Subgroup analysis based on the country (China vs non-China) showed similar results, but the subgroup analysis stratified by the tumor type (SCLC vs lung cancer vs NSCLC) showed inconsistent findings. Therefore, in overall, pretreatment SFC is believed to serve as a promising prognostic indicator in lung cancer. However, more prospective high-quality studies are still needed to further explore the prognostic role of pretreatment SFC in lung cancer.
Serum ferritin is the main indicator which reflects the amount of iron storage in the human body. The elevation of SFC is mainly caused by clearance barriers or increased sources of ferritin. In clinics, liver disease which results in the decreased liver function is a major cause of impaired clearance for ferritin[33]. On the other hand, tumor is the main cause of excess production of ferritin and it has been widely reported that cancer patients show obvious increased SFC compared with normal population[34,35]. Sukiennicki et al[36] conducted a case control study involving 200 Lung cancer patients and 200 matched healthy people, which indicated that lung cancer patients had significantly higher mean SFC (P = 0.007). The studies by Cobanoglu et al[37] and Zhu et al[28] manifested similar results. However, this phenomenon is different in male and female patients and positive association between increased SFC and incidence of lung cancer is only observed in male patients[36,37]. Meanwhile, in male lung cancer patients, smoking obviously contributes to the elevation of SFC (P = 0.036)[38,39]. However, for female lung cancer patients, menstruation is one of the important factors affecting SFC[40]. Besides, some other parameters may also affect the SFC like the tumor stage[40]. Thus, the baseline SFC varies in different populations and is regulated by many mechanisms.
Up to now, the prognostic value of pretreatment SFC in several types of cancers has been verified. Lin et al[41] included seven relevant studies involving 1244 patients and demonstrated that elevated pretreatment SFC was related to worse OS (HR = 1.60, P < 0.001) and RFS/progression-free survival (PFS)/time to recurrence (HR = 1.70, P = 0.008) in hepatobiliary and pancreas cancers. Besides, Demir et al[12] manifested that high SFC was associated with worse survival. Furthermore, Kim et al[42] manifested that elevated SFC was related to poor prognosis in patients with diffuse large B cell lymphoma.
Actually, there are still many fields worthy of further investigation about the SFC in lung cancer. First, the current meta-analysis only revealed that pretreatment SFC was related to prognosis of lung cancer. Thus, it is necessary to explore the association between the changes of SFC during the anti-tumor treatment and long-term survival. Second, as mentioned above, it is believed that the baseline SFC varies in different people. Therefore, the optimal cutoff value of SFC should be specific in different populations. Third, it is also needed to explore the clinical value of interfering with SFC.
There are several limitations in this meta-analysis. First, all included studies are retrospective and the overall sample size is relatively small, which may cause some bias. Second, we were unable to conduct more subgroup analyses based on other important parameters such as the TNM stage, age, sex and smoking history due to the unobtainable original data. Third, obvious heterogeneity existed in this meta-analysis. However, subgroup analysis did not find sources of heterogeneity. Four, in this type of meta-analysis, the optimal cutoff value of pretreatment SFC could not be determined. Five, the significant publication bias was observed in our meta-analysis, although the potentially unpublished studies did not have a significant impact on the pooled results.
In overall, pretreatment SFC might serve as a promising prognostic indicator in lung cancer patients and elevated pretreatment SFC predicts worse prognosis. However, more prospective high-quality studies with big sample sizes are still needed to further verify its prognostic value in lung cancer.
The association between pretreatment serum ferritin concentration (SFC) and long-term survival in lung cancer remains unclear now.
Whether the pretreatment SFC could play a role in predicting long-term survival in lung cancer remains unclear.
To identify the prognostic value of pretreatment SFC in lung cancer patients based on current evidence.
The PubMed, EMBASE and Web of Science databases were searched from inception to May 29, 2022 for relevant studies. The primary endpoint was overall survival (OS) and the hazard ratios (HRs) with 95% confidence intervals (CIs) were combined to assess the predictive role of pretreatment SFC for long-term survival of lung cancer patients. All statistical analysis was conducted by STATA 15.0.
A total of 12 retrospective studies involving 1654 patients were included. The pooled results manifested that increased pretreatment SFC was significantly associated with worse OS (HR = 1.09, 95%CI: 1.03-1.15, P = 0.004). Subgroup analysis based on the country (China vs non-China) showed similar results. However, subgroup analysis stratified by tumor type revealed inconsistent results (lung cancer: HR = 1.39, P = 0.008; small cell lung cancer: HR = 1.99, P = 0.175; non-small cell lung cancer: HR = 1.03, P = 0.281).
Pretreatment SFC might serve as a promising prognostic indicator in lung cancer patients and elevated pretreatment SFC predicts worse prognosis.
The pretreatment SFC might contribute to the clinical management and treatment of lung cancer patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andriolo LG, Italy; Gupta P, United States; Yang X, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Li H, Guo J, Liang H, Zhang T, Zhang J, Wei L, Shi D, Wang Z. The Burden of Trachea, Bronchus, and Lung Cancer Attributable to Occupational Exposure From 1990 to 2019. Front Public Health. 2022;10:928937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Thill PG, Goswami P, Berchem G, Domon B. Lung cancer statistics in Luxembourg from 1981 to 2008. Bull Soc Sci Med Grand Duche Luxemb. 2011;43-55. [PubMed] |
| 3. | Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1259] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 4. | Oncology Society of Chinese Medical Association; Chinese Medical Association Publishing House. [Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2022 edition)]. Zhonghua Zhong Liu Za Zhi. 2022;44:457-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Wang L, Liu A, Wang Z, Xu N, Zhou D, Qu T, Liu G, Wang J, Yang F, Guo X, Chi W, Xue F. A Prognostic Model of Non-Small Cell Lung Cancer With a Radiomics Nomogram in an Eastern Chinese Population. Front Oncol. 2022;12:816766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliövaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer. 1994;56:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 217] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Hino K, Yanatori I, Hara Y, Nishina S. Iron and liver cancer: an inseparable connection. FEBS J. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Schöttker B, Gào X, Jansen EH, Brenner H. Associations of Human Colorectal Adenoma with Serum Biomarkers of Body Iron Stores, Inflammation and Antioxidant Protein Thiols. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yu YC, Luu HN, Wang R, Thomas CE, Glynn NW, Youk AO, Behari J, Yuan JM. Serum Biomarkers of Iron Status and Risk of Hepatocellular Carcinoma Development in Patients with Nonalcoholic Fatty Liver Disease. Cancer Epidemiol Biomarkers Prev. 2022;31:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, Hegde P, Brewer M, Wang X, Miller LD, Dyment N, Torti FM, Torti SV. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene. 2017;36:4089-4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 12. | Demir H, Beypinar I, Urvay S, Davarcı SE, Baykara M. Prognostic role of pre-operative serum ferritin level in stage 2 colon cancer. Eur Rev Med Pharmacol Sci. 2021;25:6473-6479. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Karakatsanis S, Panitsas F, Arapaki M, Galopoulos D, Asimakopoulos JV, Liaskas A, Chatzidimitriou C, Belia M, Konstantinou E, Vassilopoulos I, Papadatos SS, Sachanas S, Efstathopoulou M, Yiakoumis X, Pardalis V, Iliakis T, Giannakopoulou N, Dimou M, Chatzidavid S, Boutsikas G, Petevi K, Kanellopoulos A, Gainaru G, Variamis E, Siakantaris MP, Kyrtsonis MC, Plata E, Tsaftaridis P, Dimopoulou MN, Viniou NA, Syrigos KN, Pangalis GA, Panayiotidis P, Konstantopoulos K, Angelopoulou MK, Vassilakopoulos TP. Serum ferritin levels in previously untreated classical Hodgkin lymphoma: correlations and prognostic significance. Leuk Lymphoma. 2022;63:799-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Park JM, Mau CZ, Chen YC, Su YH, Chen HA, Huang SY, Chang JS, Chiu CF. A case-control study in Taiwanese cohort and meta-analysis of serum ferritin in pancreatic cancer. Sci Rep. 2021;11:21242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Ramírez-Carmona W, Díaz-Fabregat B, Yuri Yoshigae A, Musa de Aquino A, Scarano WR, de Souza Castilho AC, Avansini Marsicano J, Leal do Prado R, Pessan JP, de Oliveira Mendes L. Are Serum Ferritin Levels a Reliable Cancer Biomarker? Nutr Cancer. 2022;74:1917-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Zhuge X, Zhou H, Chen L, Chen H, Chen X, Guo C. The association between serum ferritin levels and malignant intraductal papillary mucinous neoplasms. BMC Cancer. 2021;21:1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Milman N, Sengeløv H, Dombernowsky P. Iron status markers in patients with small cell carcinoma of the lung. Relation to survival. Br J Cancer. 1991;64:895-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ferrigno D, Buccheri G. A comprehensive evaluation of serum ferritin levels in lung cancer patients. Lung Cancer. 1992;8:85-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Milman N, Pedersen LM. The serum ferritin concentration is a significant prognostic indicator of survival in primary lung cancer. Oncol Rep. 2002;9:193-198. [PubMed] |
| 20. | Erbaycu AE, Ucar H, Uslu O, Tuksavul F, Kazanci MN, Batum O, Kalenci D, Guclu SZ. Prognostic significance of serum iron, iron-binding capacity, ferritin and bronchoalveolar lavage ferritin levels in primary lung cancer. UHOD - Uluslararasi Hematoloji-Onkoloji Dergisi. 2008;18:217-225 Available from: http://www.uhod.org/summary_en.php3?id=337. |
| 21. | Zhao W, Shi H, Wu C, Ji M. Association of serum ferritin expression and prognosis in patients with advanced lung carcinoma. Jiangsu Med J. 2014;40:2978-2980. [DOI] [Full Text] |
| 22. | Xie J, Ji H, Chen G, Wang Y, Shen Y. Relationship between serum ferritin, erythrocyte sedimentation rate, mean corpuscular indexes and prognosis in patients with small cell lung cancer. J Int Oncol. 2018;45:465-469. [DOI] [Full Text] |
| 23. | Lee S, Jeon H, Shim B. Prognostic Value of Ferritin-to-Hemoglobin Ratio in Patients with Advanced Non-Small-Cell Lung Cancer. J Cancer. 2019;10:1717-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Ma C, Zuo W. Preoperative serum bilirubin levels associated with stage and prognosis in patients with stages i-iii of non-small cell lung cancer in jiangxi province, china. International Journal of Clinical and Experimental Medicine. 2019;12:10433-10442 Available from: https://e-century.us/web/journal_toc.php?journal=ijcem&volume=12&number=8. |
| 25. | Sun A. Changes and clinical significance of serum CEA, Fer, A125 and CA199 in patients with NSCLC. Contemporary Medicine. 2020;26:39-42 Available from: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202002276860074699. |
| 26. | Ji D, Duan A, Li C. Predictive Value of Baseline Serum Ferritin Level for the Prognosis of NSCLC Patients Treated with EGFR-TKIs. Zhongliu Yaoxue. 2021;11 Available from: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202102229574263950. |
| 27. | Xiao J. Correlation between serum SF, ESR and average red blood cell index levels and the prognosis of patients with small cell lung cancer. Qingdao Yiyaoweisheng. 2021;53:105-107 Available from: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202102233347731725. |
| 28. | Zhu M. The Correlation Study of Serum Ferritin in Patients with Lung Cancer. Hangkong Hangtian Yixue Zazhi. 2021;32:899-901 Available from: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202202246720602222. |
| 29. | Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8:e83138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 609] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 30. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12664] [Article Influence: 844.3] [Reference Citation Analysis (0)] |
| 31. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46545] [Article Influence: 2115.7] [Reference Citation Analysis (3)] |
| 32. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 33. | Cao P, Wu Y, Wu S, Wu T, Zhang Q, Zhang R, Wang Z, Zhang Y. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers. 2021;26:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Shi HB, Li XD, Jiang JT, Zhao WQ, Ji M, Wu CP. Serum ferritin is elevated in advanced non-small cell lung cancer patients and is associated with efficacy of platinum-based chemotherapy. J Cancer Res Ther. 2014;10:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 35. | Zhang XZ, Su AL, Hu MQ, Zhang XQ, Xu YL. Elevated serum ferritin levels in patients with hematologic malignancies. Asian Pac J Cancer Prev. 2014;15:6099-6101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Sukiennicki GM, Marciniak W, Muszyńska M, Baszuk P, Gupta S, Białkowska K, Jaworska-Bieniek K, Durda K, Lener M, Pietrzak S, Gromowski T, Prajzendanc K, Łukomska A, Waloszczyk P, Wójcik JZ, Scott R, Lubiński J, Jakubowska A. Iron levels, genes involved in iron metabolism and antioxidative processes and lung cancer incidence. PLoS One. 2019;14:e0208610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Cobanoglu U, Demir H, Sayir F, Duran M, Mergan D. Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pac J Cancer Prev. 2010;11:1383-1388. [PubMed] |
| 38. | Li S, Lin L, Mo Z, Qin X, Lv H, Gao Y, Tan A, Yang X, Huang S, Chen Z. Reference values for serum ferritin in Chinese Han ethnic males: results from a Chinese male population survey. Clin Biochem. 2011;44:1325-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Lapenna D, de Gioia S, Mezzetti A, Ciofani G, Consoli A, Marzio L, Cuccurullo F. Cigarette smoke, ferritin, and lipid peroxidation. Am J Respir Crit Care Med. 1995;151:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Kukulj S, Jaganjac M, Boranic M, Krizanac S, Santic Z, Poljak-Blazi M. Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer. Med Oncol. 2010;27:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Lin S, Fang Y, Lin Y, Mo Z, Hong X, Jian Z, Ji C. Meta-analysis of the prognostic value of pretreatment serum ferritin in hepatobiliary and pancreas (HBP) cancers. BMJ Open. 2021;11:e040801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Kim DJ, Kim T, Jeong JY, Jo JC, Lee WS, Shin HJ, Lee JH, Lee HS. Poor prognostic impact of high serum ferritin levels in patients with a lower risk of diffuse large B cell lymphoma. Int J Hematol. 2020;111:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |