Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12156
Peer-review started: August 13, 2022
First decision: September 23, 2022
Revised: September 29, 2022
Accepted: October 24, 2022
Article in press: October 24, 2022
Published online: November 26, 2022
Processing time: 102 Days and 9.1 Hours
The combination therapy of transarterial chemoembolization and radiofrequency ablation (TACE-RFA) shows promising efficacy in large hepatocellular carcinoma (HCC). Data on the clinical efficacy and safety of TACE-RFA for large HCC with barcelona clinic liver cancer (BCLC) stage C are lacking in China.
To determine the safety and efficacy of TACE-RFA for large, advanced HCC.
Patients of HCC with BCLC stage C who were treated with TACE-RFA or TACE alone at our institute from August 2008 to January 2017 were retrospectively reviewed. The complications were observed. The associations between overall survival (OS) and treatment method were analysed.
Data were collected from 102 HCC patients. Among them, 64 underwent TACE-RFA and 38 underwent TACE. The combination of TACE and RFA was safe. All complications were controllable. The median OS in the TACE-RFA group was significantly longer than that in the TACE group (8.0 mo vs 4.0 mo, P = 0.000). The 6-, 12- and 24-mo survival rates of the combination group were 68.8%, 34.4%, and 10.9%, respectively, while those of the TACE group were 36.8%, 7.9%, and 0% (P < 0.05).
TACE-RFA has an advantage over TACE alone in improving OS in large HCC patients with BCLC stage C.
Core Tip: Few reports have focused on the effect of transarterial chemoembolization and radiofrequency ablation (TACE-RFA) in patients with large hepatocellular carcinoma (HCC) of barcelona clinic liver cancer (BCLC) stage C. Thus, the aim of this retrospective study was to evaluate the safety and efficacy of a combined therapy strategy with TACE-RFA in large HCC of BCLC C group. We found that TACE-RFA had an advantage over TACE alone in improving overall survival in large HCC patients with BCLC C.
- Citation: Sun SS, Li WD, Chen JL. Transarterial chemoembolization combined with radiofrequency ablation in the treatment of large hepatocellular carcinoma with stage C. World J Clin Cases 2022; 10(33): 12156-12163
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12156.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12156
Hepatocellular carcinoma (HCC) was the sixth common malignancy and the third leading cause of cancer death worldwide[1]. Due to the hidden onset of HCC, many patients are initially diagnosed as advanced stage and lose the opportunity for curative surgery[2]. According to guidelines, systemic therapy and transcatheter arterial chemoembolization (TACE) are the recommended treatments for patients with barcelona clinic liver cancer (BCLC) stage C HCC[3,4]. In reality, some BCLC stage C HCC patients cannot undergo systemic therapy, such as sorafenib or lenvatinib, in China because of economic or other reasons. It is now understood that TACE plays an important role in the nonoperative treatment of advanced HCC[5,6]. However, TACE alone has difficulty causing complete tumor necrosis[7]. Radiofrequency ablation (RFA) is a valid treatment strategy for early HCC because of its effectiveness and safety[8], but it has a limitation that complete ablation is difficult to achieve in large HCC[9]. Some researchers have found that the combination therapy of TACE and RFA (hereafter, TACE-RFA) have better overall survival (OS) than either RFA or TACE treatment alone in large HCC patients[10,11]. Nevertheless, few reports have focused on the effect of TACE-RFA in HCC patients with stage C. Therefore, this retrospective study was to explore the efficacy and safety of TACE-RFA in stage C HCC.
Between August 2008 and January 2017, this retrospective study analysed 102 consecutive HCC patients who were diagnosed with BCLC stage C at Capital Medical University Affiliated Beijing Ditan Hospital. The patients underwent TACE-RFA or TACE alone. Written informed consent was obtained from every patient before treatment.
The eligibility criteria were as follows: (1) Cytological/histological diagnosis by biopsy or two imaging techniques showing typical features of HCC; (2) Age 18 to 75 years; (3) Child-Pugh class A or B; (4) Multiple (three or fewer) HCC lesions and one of the tumors having a maximum diameter ≥ 5 cm; (5) BCLC stage C, no extrahepatic metastases, portal vein tumor thrombi of Cheng type I or II[12]; and (6) Eastern Cooperative Oncology Group performance status 0-1. Exclusion criteria: (1) Child-Pugh class C; (2) Severe underlying cardiac or renal diseases, oesophageal or gastric variceal bleeding, hepatic encephalopathy, or active infection; (3) Applied systemic therapy; (4) Malignancies other than HCC; and (5) Diffuse liver cancer.
TACE was administered to the TACE-RFA group and the TACE group using the Seldinger technique. Angiography was performed to identify the tumor-feeding artery, and then the catheter was advanced to the target artery as superselectively as possible. First, 10 mL iodized oil was infused, and then a mixture of epirubicin (15 mg/m2) and hydroxycamptothecin (10 mg/m2) was administered into the target vessels. Finally, gelatine sponge particles were introduced.
RFA was administered to the combination group 5-10 d after TACE treatment. Analgesia (10 mg of morphine) and local anaesthesia (10 mL of lidocaine) were administered before RFA. During the procedure, vital signs were monitored by electrocardiogram, and the specification of the radiofrequency therapy needle was selected according to the tumor condition. RFA was performed with a multitoned, 15-cm-long expandable electrode with a maximum dimension of 4.0 cm (welfare multipole conformal needle, Beijing Welfare Electronic, China) or a 17-gauge single-pin bipolar radiofrequency needle with a 3-cm-long exposed tip (Olympus CELON Power, United States). The electrode needle was inserted into the tumor nodule under the guidance of 16-row spiral computed tomography (Siemens Emotion, Siemens Medical, Germany). According to the location and size of the tumor, the appropriate ablation parameters were selected, and each ablation time was approximately 10-15 min. For large tumors, the principle of multiple overlapping ablations and multipoint ablation was followed to ensure that the tumor was effectively damaged[13,14]. Computed tomography was done after ablation to observe whether there were complications such as subcapsular haemorrhage, pneumothorax, and gastrointestinal and gallbladder perforation.
All patients were regularly followed up every 4-6 wk in the first half-year according to institutional practice, including a complete physical examination, haematologic and biochemistry profiles, serum alpha-fetoprotein (AFP), chest X-ray, and abdominal computed tomography or magnetic resonance imaging. In the next half-year, these were done every three months. If the tumor progressed or relapsed, minimally invasive treatment could be continued if it was assessed to be tolerable. OS was assessed from the date of diagnosis until death from any cause. The last follow-up date for this study was April 30, 2017. Safety assessment adopted the Common Terminology Criteria for Adverse Events 4.0. The common adverse events and operation-related serious adverse events were counted.
The data were analysed using SPSS for Windows (Version 16.0, Chicago, United States). The independent χ2 test was used to compare categorical variables. Continuous variables were compared using the unpaired t test. The OS curves were analysed using the Kaplan-Meier method, and the differences between groups were compared by the log-rank test. All statistical tests were two-sided, and differences were considered significant at P < 0.05.
In all, 102 HCC patients were enrolled in the study. The TACE-RFA group was 64, and that TACE group was 38. The baseline clinical characteristics are summarized in Table 1.
| Variables | TACE-RFA (n = 64) | TACE (n = 38) | P value |
| Sex (male/female) | 51/13 | 33/5 | 0.359 |
| Age (yr) | 53.0 ± 9.5 (25-72) | 52.7 ± 8.5 (39-75) | 0.876 |
| HBsAg (+/-) | 54/10 | 35/3 | 0.409 |
| HCV (+/-) | 3/61 | 4/34 | 0.470 |
| ALB (g/L) | 38.1 ± 4.6 (25.0-53.0) | 36.4 ± 5.8 (25.0-52.0) | 0.103 |
| AFP/(ng/mL) | 0.628 | ||
| < 400 | 11 | 8 | |
| ≥ 400 | 53 | 30 | |
| Child-Pugh class | 0.036 | ||
| A | 51 | 23 | |
| B | 13 | 15 | |
| Tumour number | 0.987 | ||
| < 3 | 22 | 13 | |
| 3 | 42 | 25 | |
| TBIL (μmol/L) | 18.2 ± 7.9 (4.9-49.5) | 21.2 ± 9.2 (5.3-45.3) | 0.081 |
| Max-diameter (cm) | 9.5 ± 3.1 (5.0-18.0) | 10.2 ± 3.5 (5.1-17.0) | 0.267 |
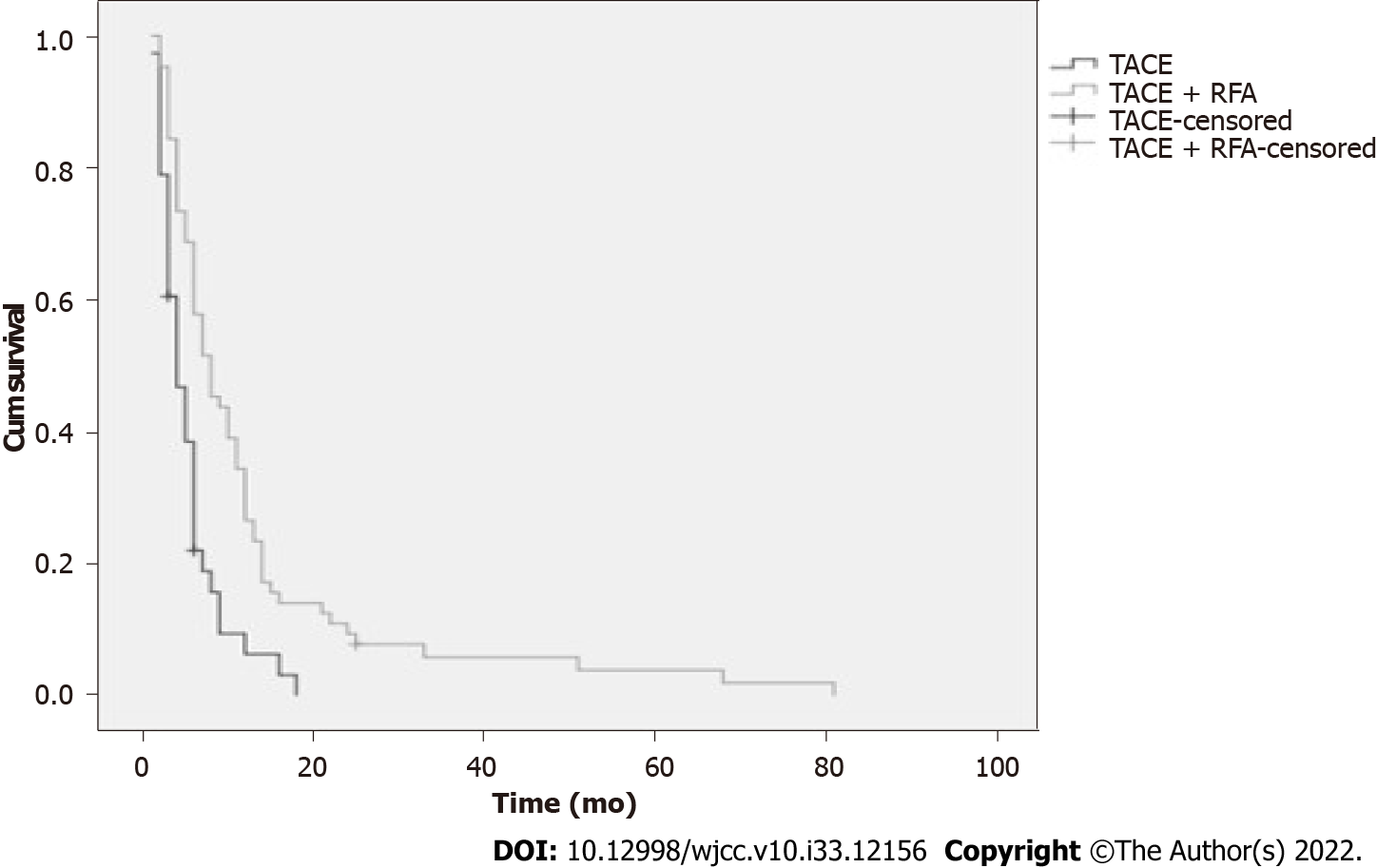
The median OS was 8.0 mo in the TACE-RFA group, while the TACE group was 4.0 mo (Table 2). The OS rates were significantly different between the two groups (Figure 1). The 6-, 12-, and 24-mo survival rates for the TACE-RFA patients were 68.8%, 34.4%, and 10.9%, respectively, and those for the TACE group were 36.8%, 7.9%, and 0%, respectively (P < 0.05, Table 3). From Figure 1, we see that the 1-, 3-, and 5-year OS rates for TACE-RFA group patients were significantly higher than those of the TACE group (P = 0.000).
| Group | Mean (mo) | Estimate SD | 95%CI | Median (mo) | Estimate SD | 95%CI |
| TACE | 5.385 | 0.657 | 4.096-6.673 | 4.000 | 0.743 | 2.544-5.456 |
| TACE-RFA | 12.504 | 1.939 | 8.704-16.304 | 8.000 | 1.327 | 5.398-10.602 |
| Mo | TACE (%) | TACE-RFA (%) | χ2 | P value |
| 6 | 36.8 | 68.8 | 9.986 | 0.002 |
| 12 | 7.9 | 34.4 | 9.036 | 0.003 |
| 24 | 0 | 10.9 | 4.462 | 0.035 |
There were no treatment-related deaths. Common complications, including fever, pain, fatigue, vomiting, and transient liver function injury, were observed in most patients (Table 4). The complications were mild or moderate and improved after symptomatic treatments. Asymptomatic ascites was present in two cases after combination treatment. However, none of these patients required interventional drainage procedures. There was one case of gastrointestinal bleeding in each group. Two patients had a small amount of bleeding, which improved after medical treatment. Serious adverse events, including biliary tract haemorrhage, gastrointestinal tract perforation, diaphragm perforation, liver abscess, and acute liver failure, were not present.
| Parameter | TACE-RFA (n = 64) | TACE (n = 38) |
| Grade 1-2/3-4 | Grade 1-2/3-4 | |
| Fever | 48/2 | 31/1 |
| Omitting | 28/3 | 16/1 |
| Pain | 45/3 | 26/2 |
| Liver function injury | 52/2 | 30/1 |
| Ascites | 2/0 | 0/0 |
| Gastric haemorrhage | 1/0 | 1/0 |
| Fatigue | 53/0 | 29/0 |
In China, some late-stage HCC patients who are not suitable for hepatectomy could not afford systemic therapy before 2017. It is critical to maximize the reduction in tumor load to improve their quality of life and prolong their survival. Therefore, minimally invasive treatment (such as TACE and RFA) is a promising alternative. TACE has been well accepted as a palliative therapy for unresectable HCC. TACE can improve the blockade of the tumor blood supply and locate small lesions that imaging examination miss. However, complete necrosis is difficult to achieve in HCC following TACE alone, so tumor relapse is common. RFA has been proposed as a nonsurgical treatment option for small HCCs, but its therapeutic effect is limited by the tumor size and location[15]. The complete necrosis rates in larger HCC patients treated by RFA alone range from 29% to 70%[16]. Thus, combination therapy is a promising therapy for large unresectable HCC. The combination of TACE-RFA can lead to a large coagulation area that improves the complete tumor ablation rate[17,18]. TACE plus RFA of late-stage large liver cancer can improve the ablation effect[19].
Therefore, this study retrospectively analysed clinical data from 102 HCC patients with large BCLC stage C who underwent TACE-RFA or TACE for primary exploration. The results showed that combination therapy for TACE followed by RFA appeared to be suitable for patients with advanced liver cancer. The median OS of TACE-RFA group was significantly higher than that of TACE group (8 mo vs 4 mo, P = 0.000). The 6-, 12-, and 24-mo survival rates for the combination group patients were higher than those of the TACE group. This OS time seemed to be unsatisfactory, but since all enrolled patients were in BCLC stage C and the tumor size was larger than 5 cm, many larger than 10 cm, we believe these results are acceptable. Ren et al[17] demonstrated that TACE-RFA had good survival benefits for HCC patients with BCLC stages A and B. Our results further show that TACE-RFA could also be applied in the BCLC stage C group.
The application of RFA in the tumor size of HCC patients is still controversial. RFA has been accepted as a curative treatment for early-stage small HCC[20]. Owing to its limited ablation range, it is less favorable for complete tumor necrosis of tumors greater than 5 cm in diameter[21]. However, our results showed that HCC patients who received TACE-RFA on tumor diameters larger than 5 cm and even greater than 10 cm exhibited better OS than those who had only TACE. These are similar to Ke et al’s clinical findings[22]. Hence, we should use RFA flexibly in patients with liver cancer after fully evaluating their condition, regardless of tumor size.
Like other studies[14,23-26], our study indicated that TACE-RFA was relatively safe. First, we found that the patients could tolerate TACE-RFA treatment, with no deaths. Second, all complications were controllable in each group. Our study has some limitations. Its retrospective design and selection bias are the main limitations. The study was conducted only in our institution, so the results may be influenced by the experience of the researchers. Therefore, the observations need to be further validated by prospective, multicenter, randomized studies.
In conclusion, comparing with TACE alone, TACE-RFA could improve the survival of large HCC patients with BCLC stage C. Moreover, combination therapy of TACE followed by RFA appeared to be relatively safe. Since this was only a retrospective study, a large clinical trial is necessary to assess curative effects.
Systemic therapy and transcatheter arterial chemoembolization (TACE) are the recommended treatments for hepatocellular carcinoma (HCC) patients with barcelona clinic liver cancer (BCLC) stage C in China. However, some late stage HCC patients because of economic reason can’t apply systemic therapy. It is known that TACE plays an important role in the non-operative treatment of advanced HCC. But TACE alone is difficult to cause complete tumor necrosis. Radiofrequency ablation (RFA) is a valid treatment strategy for early HCC. Nevertheless, it also has a limited range, and for large HCCs, complete ablation is difficult to achieve. Some studies have shown that the combination of TACE and RFA(TACE-RFA) can improve overall survival (OS) in patients with large HCC with a better efficacy than either RFA or TACE alone.
There have been no studies to explore the efficacy of TACE-RFA in large HCC patients with stage C.
This study aimed to determine the safety and efficacy of TACE-RFA in HCC with large (≥ 5.0 cm in diameter) and BCLC stage C.
The complications were observed. The associations between OS and treatment method were analysed.
The combination of TACE and RFA was safe. The median OS of the TACE-RFA group was significantly higher than that of the TACE group.
TACE-RFA was better than TACE alone in improving survival for large HCC patients with BCLC stage C. Moreover, the combination therapy appeared to be relatively safe.
A large clinical trial is necessary to assess curative effects.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E, E
P-Reviewer: Boninsegna E, Italy; Lordelo P, Brazil; Mizuguchi T, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63888] [Article Influence: 15972.0] [Reference Citation Analysis (174)] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3280] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 5990] [Article Influence: 855.7] [Reference Citation Analysis (3)] |
| 4. | Cheng S, Yang J, Shen F, Zhou W, Wang Y, Cong W, Yang GS, Cheng H, Hu H, Gao C, Guo J, Li A, Meng Y, Jiang X, Yang Y, Qian G, Luo M, Hu B, Man X, Zhang B, Su C, Zhou F, Li N, Shi J, Wang M, Zheng Y, Guo W, Sun J, Wang H, Lau WY, Wu MC. Multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus - Eastern Hepatobiliary Surgical Hospital consensus statement. Oncotarget. 2016;7:40816-40829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Yuan-Dong S, Hao Z, Hui-Rong X, Jing-Zhou L, Hui-Yong W, Jian-Jun H, Yu JM. Combination therapy: Meta-analysis of the effects of TACE and cryoablation on hepatocellular carcinoma. Medicine (Baltimore). 2019;98:e18030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 7. | Yan L, Ren Y, Qian K, Kan X, Zhang H, Chen L, Liang B, Zheng C. Sequential transarterial chemoembolization and early radiofrequency ablation improves clinical outcomes for early-intermediate hepatocellular carcinoma in a 10-year single-center comparative study. BMC Gastroenterol. 2021;21:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Lurje I, Czigany Z, Bednarsch J, Roderburg C, Isfort P, Neumann UP, Lurje G. Treatment Strategies for Hepatocellular Carcinoma ⁻ a Multidisciplinary Approach. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 9. | Chen J, Peng K, Hu D, Shen J, Zhou Z, Xu L, Chen J, Pan Y, Wang J, Zhang Y, Chen M. Tumor Location Influences Oncologic Outcomes of Hepatocellular Carcinoma Patients Undergoing Radiofrequency Ablation. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Jiang C, Cheng G, Liao M, Huang J. Individual or combined transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma: a time-to-event meta-analysis. World J Surg Oncol. 2021;19:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Chai B, Wang W, Wang F, Zhou G, Zheng C. Transcatheter chemoembolization plus percutaneous radiofrequency ablation versus laparoscopic radiofrequency ablation: improved outcome for inoperable hepatocellular carcinoma. Int J Hyperthermia. 2021;38:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, Lau WY, Wu M. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer. 2020;9:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Yuan P, Zhang Z, Kuai J. Analysis on efficacy and safety of TACE in combination with RFA and MWA in the treatment of middle and large primary hepatic carcinoma. J BUON. 2019;24:163-170. [PubMed] |
| 15. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4066] [Article Influence: 580.9] [Reference Citation Analysis (6)] |
| 16. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 740] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 17. | Ren Y, Cao Y, Ma H, Kan X, Zhou C, Liu J, Shi Q, Feng G, Xiong B, Zheng C. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study. BMC Cancer. 2019;19:983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Haochen W, Jian W, Li S, Tianshi L, Xiaoqiang T, Yinghua Z. Transarterial chemoembolization plus multi-imaging-guided radiofrequency ablation for elimination of hepatocellular carcinoma nodules measuring 3.1 to 5.0 cm: a single-center study. J Int Med Res. 2018;46:2650-2657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 20. | Sulaiman AS, Gani RA, Hasan I, Lesmana CRA, Kurniawan J, Jasirwan COM, Kalista KF, Nababan SHH, Aprilicia G, Lesmana LA. Overall Survival of Hepatocellular Carcinoma Patients Underwent Radiofrequency Ablation (RFA) Treatment: a Retrospective Cohort Study from Two Referral Hospitals in Indonesia. J Gastrointest Cancer. 2022;53:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Zhu K, Huang J, Lai L, Huang W, Cai M, Zhou J, Guo Y, Chen J. Medium or Large Hepatocellular Carcinoma: Sorafenib Combined with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;288:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Ke S, Gao J, Kong J, Ding XM, Niu HG, Xin ZH, Ning CM, Guo SG, Li XL, Zhang L, Dong YH, Sun WB. Repeated Radiofrequency Ablation Combined With Ablated Lesion Elimination and Transarterial Chemoembolization Improves the Outcome of Solitary Huge Hepatocellular Carcinomas 10 m or Larger. Medicine (Baltimore). 2016;95:e3393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Tang C, Shen J, Feng W, Bao Y, Dong X, Dai Y, Zheng Y, Zhang J. Combination Therapy of Radiofrequency Ablation and Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma: A Retrospective Study. Medicine (Baltimore). 2016;95:e3754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Saviano A, Iezzi R, Giuliante F, Salvatore L, Mele C, Posa A, Ardito F, De Gaetano AM, Pompili M; HepatoCATT Study Group. Liver Resection versus Radiofrequency Ablation plus Transcatheter Arterial Chemoembolization in Cirrhotic Patients with Solitary Large Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Liu W, Xu H, Ying X, Zhang D, Lai L, Wang L, Tu J, Ji J. Radiofrequency Ablation (RFA) Combined with Transcatheter Arterial Chemoembolization (TACE) for Patients with Medium-to-Large Hepatocellular Carcinoma: A Retrospective Analysis of Long-Term Outcome. Med Sci Monit. 2020;26:e923263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Yuan P, Wang F, Zhu G, Chen B. The clinical efficiency of TACE combined with simultaneous computed tomography-guided radiofrequency ablation for advanced hepatocellular carcinoma. Invest New Drugs. 2021;39:1383-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |