Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11617
Peer-review started: July 14, 2022
First decision: August 1, 2022
Revised: August 19, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: November 6, 2022
Processing time: 104 Days and 20.2 Hours
There is no established treatment for primary pulmonary lymphoepithelioma-like carcinoma (LELC) until now.
In this study, the patient responded well to sintilimab combined with paclitaxel and carboplatin, showing no obvious side effects. Meantime, the values of carbohydrate antigen 15-3 (CA15-3) and carbohydrate antigen 72-4 (CA72-4) gradually returned to normal.
Immunotherapy combined with chemotherapy in advanced-stage LELC may be more effective than immunotherapy or chemotherapy alone. CA15-3 and CA72-4 are biomarkers for evaluating therapeutic effects for LELC.
Core Tip: Herein, we present the case of a lymphoepithelioma-like carcinoma (LELC) patient who was successfully treated with sintilimab combined with paclitaxel and carboplatin. Recent literature on LELC is also reviewed and discussed for future avenues for LELC research.
- Citation: Zeng SY, Yuan J, Lv M. Favorable response of primary pulmonary lymphoepithelioma-like carcinoma to sintilimab combined with chemotherapy: A case report. World J Clin Cases 2022; 10(31): 11617-11624
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11617.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11617
Lymphoepithelioma-like carcinoma (LELC) is a rare subtype of NSCLC and first identified as a unique Epstein-Barr virus (EBV)-associated tumor by Bégin et al[1]. EBV infection is generally acknowledged as the most important etiology for LELC. Multi-dimensional comparison has revealed that pulmonary LELC resembles nasopharyngeal carcinoma (NPC) but is clearly different from other lung cancers[2]. Most LELC cases have been documented in Southeast Asia, including Hong Kong, Taiwan, Guangdong, and Singapore, with sporadic cases having been seen in the West[3]. Currently, specific treatment guidelines for LELC have not been established[3]. In recent years, dramatic progress has been made in immunotherapy for cancer.
Herein, we present the case of a LELC patient who was successfully treated with sintilimab combined with paclitaxel and carboplatin. Recent literature on LELC is also reviewed and discussed.
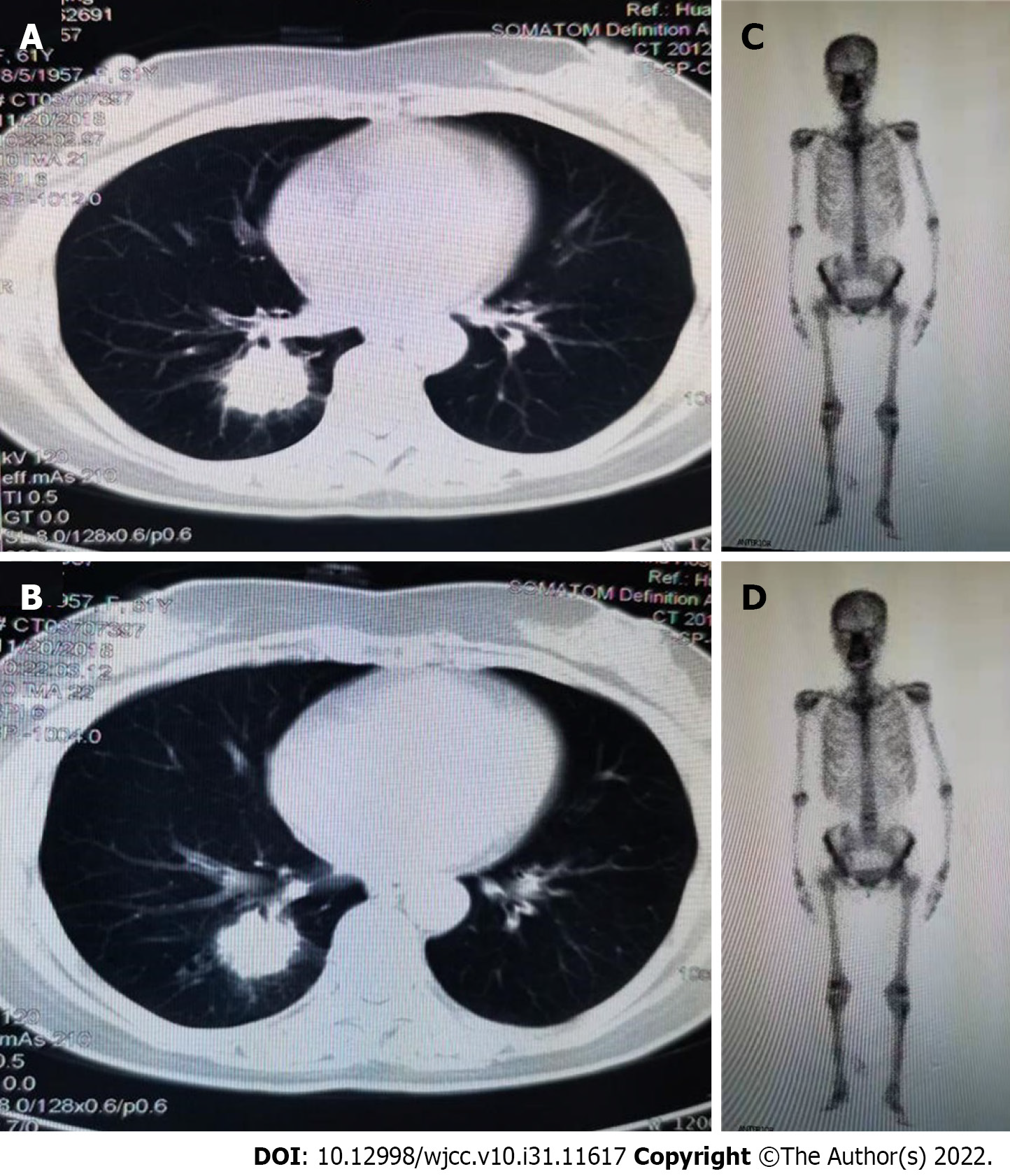
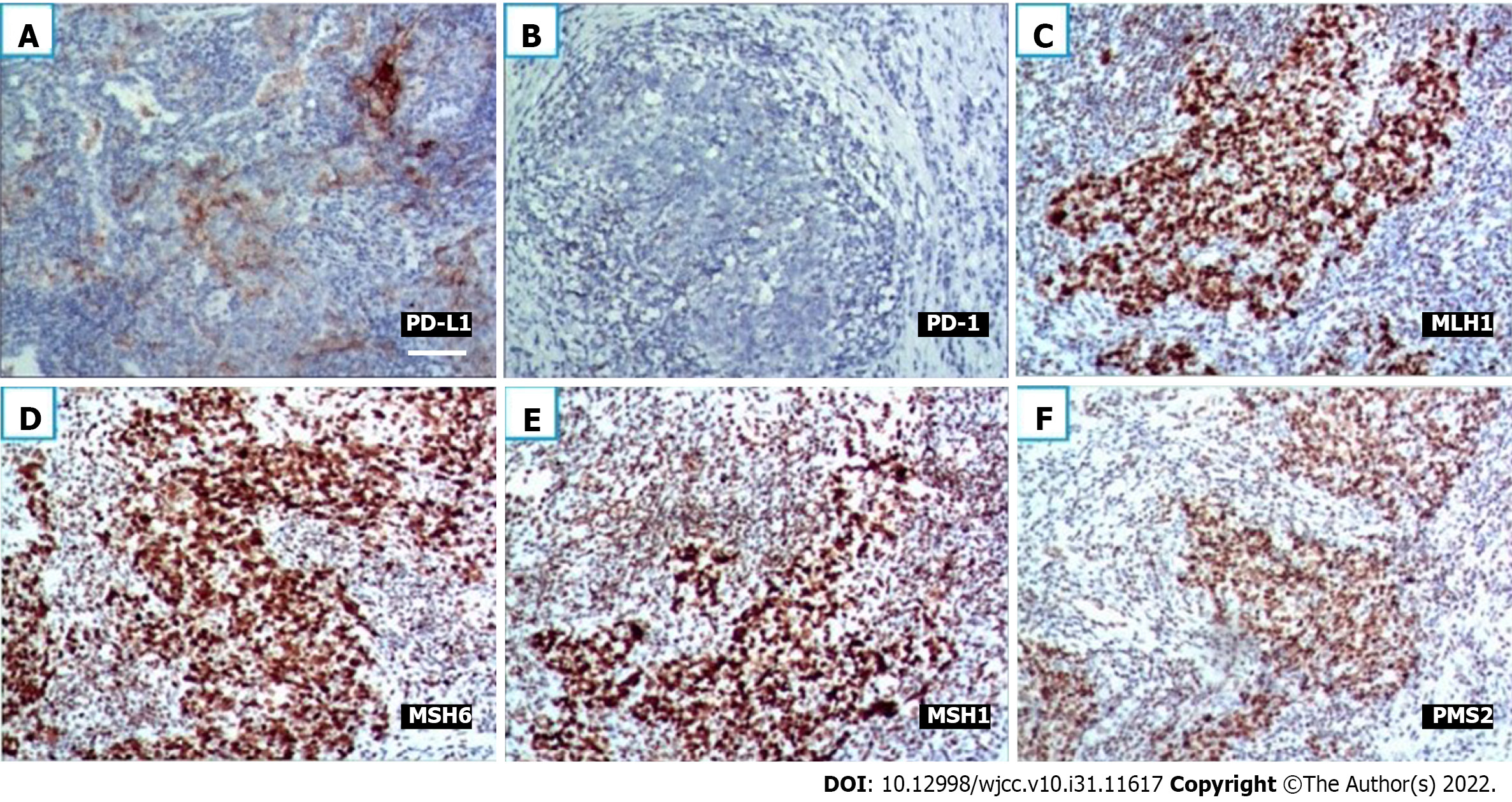
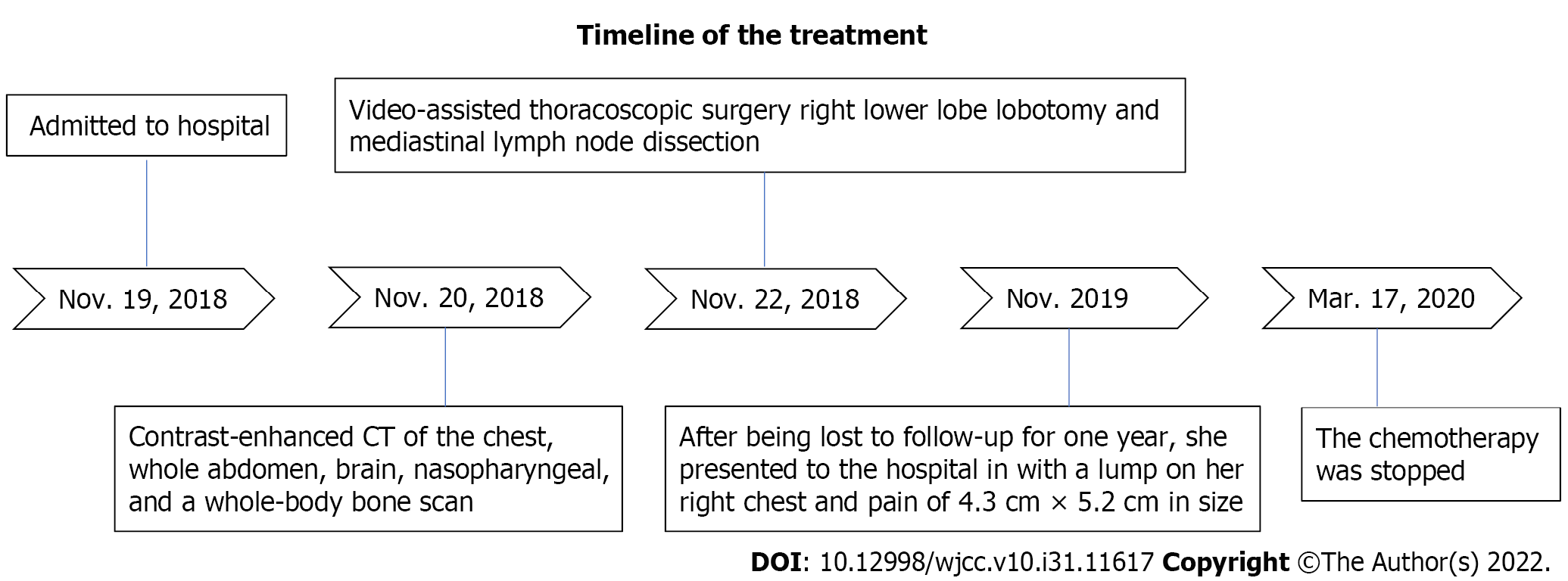
A 61-year-old nonsmoker Chinese woman was admitted to our hospital on November 19, 2018. She had no symptoms, but her chest computed tomography (CT) during her annual healthy check-up showed a mass in the right lung lower lobe. Contrast-enhanced CT of the chest, brain, whole abdomen, and nasopharynx and a whole body bone scan were performed on November 20, 2018. The contrast-enhanced CT of the chest revealed a 2.8 cm × 2.9 cm mass in the right lower lobe, showing superficial lobules with unclear boundaries; the adjacent oblique fissure was pulled (Figure 1A and B). The contrast-enhanced CT of the brain, whole abdomen, and nasopharynx and whole body bone scan revealed no abnormality (Figure 1C and D). At the same time, the serum tumor markers carbohydrate antigen 125 (CA-125) and neural specificity enolase (NSE) were higher than normal (53.46 U/mL vs < 35 U/mL, and 23.96 ng/ mL vs < 15 ng/mL, respectively); other tumor markers were negative. Physical examination showed an eastern cooperative oncology group performance status of 0. Surgical contraindications were excluded; video-assisted thoracoscopic surgery, right lower lobe lobotomy, and mediastinal lymph node dissection were performed under general anesthesia on November 22, 2018. Pathologic examination indicated a stage IIA (PT1aN1M0) disease based on the tumor size and interlobular lymph node involvement. Pathology of surgical specimen of the right lower lobe suggested LELC based on hematoxylin-eosin (HE) and immunohistochemical (IHC) staining; IHC staining demonstrated CK5/6 (+), P40 (+), PCK (+), TTF-1 (-), CK7 (-), NapsinA (-), CgA (-), Syn (-), Ki67 (+ 40%), EBER1/2-ISH (+), programmed death-1 (PD-L1) (+, 70%, SP142, ZSGB-BIO) (Figure 2A and B), and mismatch repair (MMR) proteins (integrity) (Figure 2C-F). No genetic mutations of KRAS, BRAF, EGFR, and ERBB2 or rearrangements in ALK, ROS1, and RET were seen after next-generation sequencing of the right lower lobe specimen was performed.
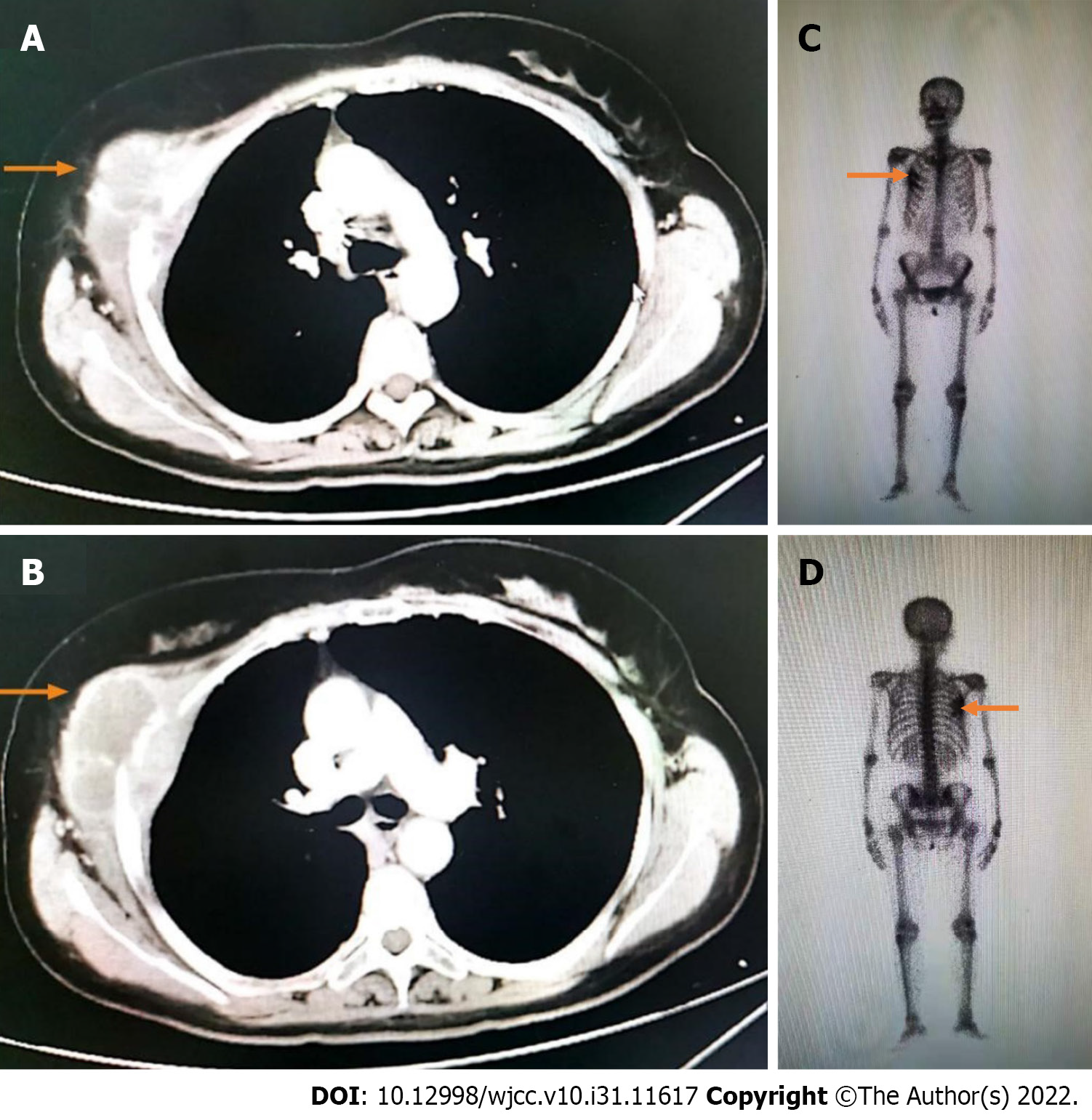
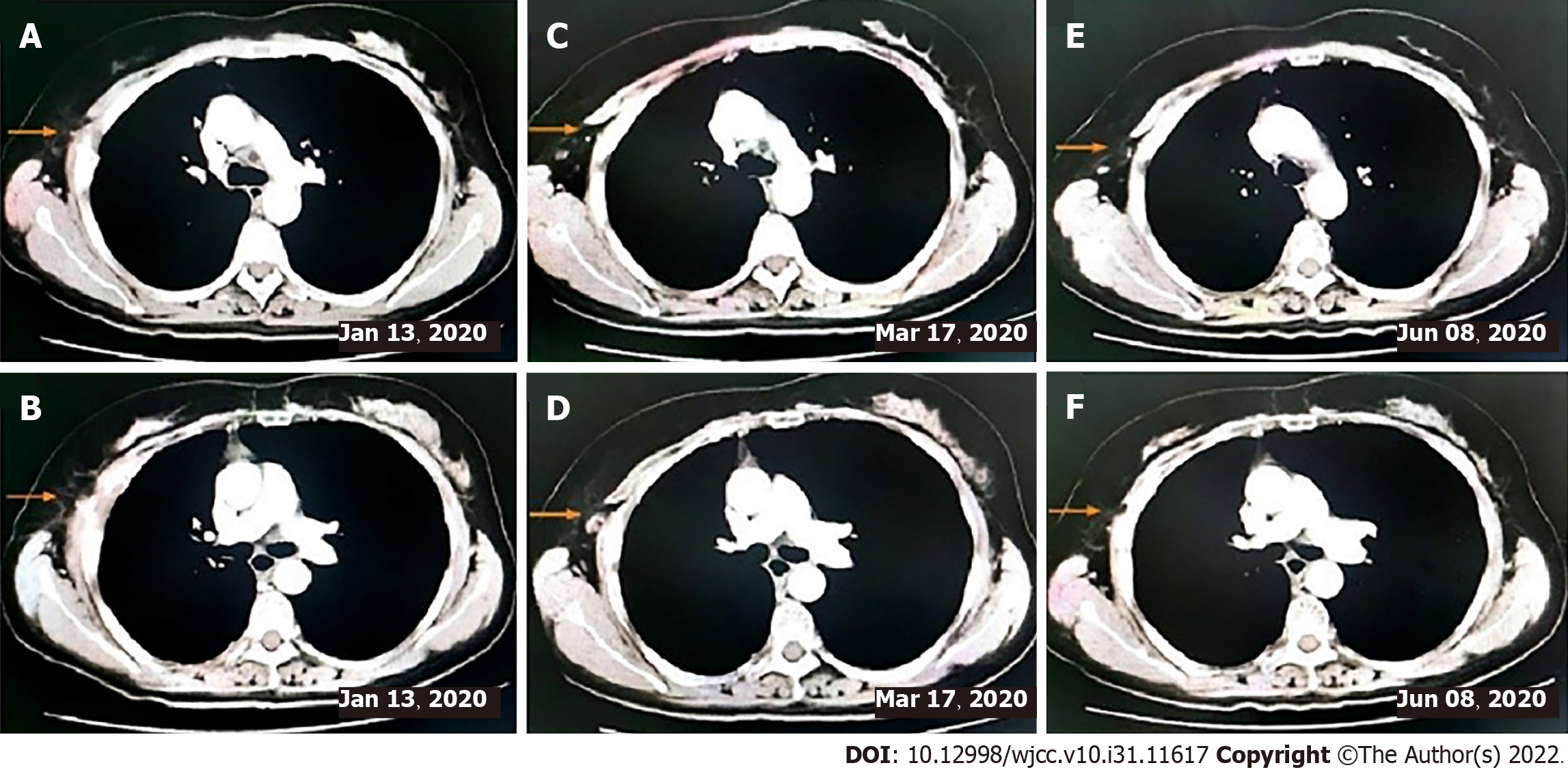
This patient did not undergo postoperative adjuvant chemotherapy. After being lost to follow-up for 1 year, she presented to the hospital in November 2019 with a lump of 4.3 cm × 5.2 cm in size on her right chest and pain. Contrast-enhanced CT indicated bone destruction of the right 4th rib with a soft tissue mass and cortical thickening of the adjacent 5th rib (Figure 3A and B). A whole-body bone scan suggested increased bone metabolism in the right 3rd-5th coastal axillary segments and possible bone metastasis (Figure 3C and D). At the same time, the serum tumor markers carbohydrate antigen 15-3 (CA15-3) and carbohydrate antigen 72-4 (CA72-4) were higher than normal (36 U/mL vs < 28 U/mL, and 14 ng/mL vs < 3.3 ng/mL, respectively). After multidisciplinary consultation and obtaining the patient’s informed consent, she was treated with sintilimab (Tyvyt®, 200 mg ivgtt d0), paclitaxel (210 mg ivgtt d1), and carboplatin (550 mg ivgtt d1) every 3 wk. After two cycles, her lump on the right chest and pain resolved, and she did not experience any toxicity. Interestingly, the mass size was significantly reduced, and the patient achieved partial remission (PR) (according to RECIST 1.1). We checked contrast-enhanced CT again after six cycles of sintilimab combined with chemotherapy, which still revealed PR. Subsequently, the chemotherapy was stopped on March 17, 2020 and sintilimab maintenance treatment of 200 mg ivgtt (given every 3 wk) was initiated.
So far, the patient has finished six cycles of sintilimab combined with chemotherapy and ten cycles of sintilimab maintenance treatment (Figure 4). The values of CA15-3 and CA72-4 gradually returned to normal (10.4 U/mL vs < 28 U/mL, and 2.1 ng/ mL vs < 3.3 ng/mL, respectively). The patient was in a continuous PR state. No obvious side effects were observed during the treatment process.
A 61-year-old nonsmoker Chinese woman was admitted to our hospital on November 19, 2018, and contrast-enhanced CT of the chest revealed a 2.8 cm × 2.9 cm mass in the right lower lobe, showing superficial lobules with unclear boundaries. She underwent video-assisted thoracoscopic surgery, right lower lobe lobotomy, and mediastinal lymph node dissection.
The patient had a history of hypertension and diabetes.
This patient was born in Chengdu and lived here. She denied having a family history of hereditary disease.
The patient’s temperature was 36.7 °C, and her blood pressure was 120/80 mmHg, heart rate was 70 bpm, and oxygen saturation in room air was 99%. Physical examination showed that the breath sound of both lungs was normal. No other positive signs were found in the physical examination.
Laboratory examinations revealed that the patient’s CA15-3 was 36 U/mL, CA72-4 was 4 ng/ mL, CA-125 was 53.46 U/mL, and NSE was 23.96 ng/mL.
Contrast-enhanced CT indicated bone destruction of the right 4th rib with a soft tissue mass and cortical thickening of the adjacent 5th rib. A whole-body bone scan suggested increased bone metabolism in the right 3rd-5th coastal axillary segments and possible bone metastasis.
The patient was diagnosed as having primary pulmonary LELC.
The patient was treated with sintilimab (Tyvyt®, 200mg ivgtt d0), paclitaxel (210 mg ivgtt d1), and carboplatin (550 mg ivgtt d1) every 3 wk. Subsequently, the chemotherapy was stopped and sintilimab maintenance treatment of 200 mg ivgtt (given every 3 wk) was initiated.
The patient was in a continuous PR state. No obvious side effects were observed during the treatment process. The values of CA15-3 and CA72-4 gradually returned to normal. She has been followed for 1 year.
As far as we know, this is the first report of a patient with LELC who positively responded to sintilimab combined with chemotherapy. The patient achieved a partial response to our therapeutic regimen after two cycles. She completed six cycles of sintilimab combined with chemotherapy and ten cycles of sintilimab maintenance treatment. Her tumor burden significantly decreased, without obvious side effects. The timeline summarizing the main events of this case report is showed in Figure 5.
In order to investigate the molecular mechanism underlying the development of LELC, several studies have examined the mutation status of LELC oncogenic driver and tumor suppressor genes, such as EGFR, KRAS, BRAF, ALK, ROS1, and TP53. Yin et al[4] found EGFR mutation in five patients (5/175, 2.9%) and ALK alteration in three patients (3/140, 2.1%). The subtype might not be sensitive to conventional targeted therapy for NSCLC. Several studies revealed that PD-L1 expression is prevalent in pulmonary LELC. Chang et al[5] observed detectable PD-L1 expression in 75.8% (50/66) of pulmonary LELCs. There are high PD-L1 expression and infrequent driver mutations in LELCs compared with conventional NSCLCs. Wu et al[6] reported that among PD-L1-positive cases, 91.5% of primary pulmonary LELC patients (54/59) had a tumor proportion score (TPS) ≥ 5%, while more than half of PLELC patients (36/59, 61.0 %) with a TPS ≥ 50% showed high levels of PD-L1 expression. Patients with positive PD-L1 expression had longer PFS and OS than those with negative PD-L1 expression. Xie et al[7] found that 69% (20/29) of patients had positive PD-L1 expression, whereas three patients benefited from a PD-1 inhibitor. In contrary, Paz-Ares et al[8] showed that the positive rate of PD-L1 in LELC patients was 74.3%, and overexpression of PD-L1 was associated with impaired DFS.
The treatment for PLELC mainly depends on tumor stage. Surgery is the mainstay of treatment in early-stage tumors, while a combination of chemotherapy and radiotherapy is used for advanced patients[3]. The most commonly used chemotherapy include cisplatin or carboplatin combined with 5-fluorouracil, paclitaxel, docetaxel, or gemcitabine[9]. High PD-L1 level and lack of canonical druggable driver mutations raise the potential of checkpoint immunotherapy for PLELC[10]. Our patient revealed the characteristics of the EBER1/2-ISH (+) and PD-L1 (+, 70%), and the lack of any canonical druggable driver mutations, which supported the feasibility of checkpoint immunotherapy.
The immune checkpoint PD-1 blockade is a recent addition to the treatment approach against different types of advanced cancers[8]. Two anti-PD-1 antibodies, nivolumab and pembrolizumab, have shown survival benefits over standard second-line chemotherapy in treating NSCLC[11]. Sintilimab, as a monotherapy, owns a safety profile in adults with relapsed or refractory classical Hodgkin’s lymphoma participating in ORIENT-1[9], which is similar to the safety profiles of nivolumab and pembrolizumab. Gao et al[10] discovered that neoadjuvant sintilimab was well tolerated by patients with NSCLC, and a 40.5% major pathologic response rate is encouraging. Thus far, immunotherapy has rarely been adopted for patients with LELC. At present, there are few reports on immunotherapy for LELC. Kim et al[12] first reported treating a patient with LELC using nivolumab who underwent chemotherapy; side effects, including tachypnea and cough, occurred 10 d after the first treatment. Moreover, Tang et al[11] reported a patient who was given 3 mg/kg nivolumab every 2 wk after first-line chemotherapy; after two cycles, the patient achieved PR. CT revealed that the disease had progressed 5 mo later in our patient, after which nedaplatin and paclitaxel were added to the nivolumab, resulting in a reduction of the tumor.
Our patient achieved PR after two cycles of sintilimab, paclitaxel, and carboplatin every 3 wk. By September 2020, the patient successfully completed six cycles of sintilimab combined with chemotherapy and ten cycles of sintilimab maintenance treatment in a continuous PR state without obvious side effects during the treatment process. To date, five patients with primary advanced LELCs have been reported, including the one reported in this study, who have received immunotherapy combined with chemotherapy or targeted therapy (Table 1). It is possible that immunotherapy combined with chemotherapy in advanced primary pulmonary LELC may be more effective than immunotherapy or chemotherapy alone. Thus, immunotherapy combined with chemotherapy should be considered as the first line of treatment for patients with advanced primary pulmonary LELC.
| Case | Reference | EBV | PD-L1 expression | Previous treatment history | Treatment | Onset time | Best response | PFS (M) |
| 1 | Zhou et al[13] | NA | 60% | 1st-line chemotherapy | 2nd-line nivolumab, 3rd-line nivolumab + anluotinib + gemcibabine | NA | SD | 2, 4 |
| 2 | Zhou et al[13] | NA | 30% | NA | Nivolumab + gemcibabine | NA | SD | NA |
| 3 | Xie et al[7] | NA | 15% | 2nd-line chemotherapy | Camrelizumab + apatinib | NA | SD | 7 + |
| 4 | Tang et al[11] | NA | 10% | 1st-line chemotherapy | 2nd-line nivolumab, 3rd-line | Two cycles | PR | 5 |
| Nivolumab + nedaplatin + paclitaxel | Four cycles | SD | NA | |||||
| 5 | Zenget al (our case) | + | 70% | Surgery | Sintilimab + paclitaxel + carboplation | Two cycles | PR | NA |
Tang et al[11] found that NSE is an effective biomarker for immunotherapy in LELC. In our patients, we found that after effective treatment, CA15-3 and CA72-4 returned to normal. NSE remained normal throughout the relapse. Thus, CA15-3 and CA72-4 are efficient biomarkers for detecting LELC.
Although immune checkpoint inhibitor-based therapy has shown great potential in solid tumors, the shortage of validated biomarkers is still an important issue since only a part of patients could benefit from immunotherapy. In addition, some potential biomarkers should be further explored, such as PD-L1 level, tumor mutational burden, microsatellite instability status, and gut microbiota. Furthermore, the efficacy of immunotherapy in clinical trials may differ in terms of drugs, patients, designs, study phases, and so on.
Our patient achieved PR after immunotherapy combined with chemotherapy, which suggested that the combined treatment regimen may be more effective than immunotherapy or chemotherapy alone. Meanwhile, both CA15-3 and CA72-4 are biomarkers for evaluating therapeutic effects for LELC.
The authors wish to thank the patient for allowing the publication of her case details.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mazilu L, Romania; Rizzo A, Italy S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Bégin LR, Eskandari J, Joncas J, Panasci L. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol. 1987;36:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Hong S, Liu D, Luo S, Fang W, Zhan J, Fu S, Zhang Y, Wu X, Zhou H, Chen X, Chen G, Zhang Z, Zheng Q, Li X, Chen J, Liu X, Lei M, Ye C, Wang J, Yang H, Xu X, Zhu S, Yang Y, Zhao Y, Zhou N, Zhao H, Huang Y, Zhang L, Wu K. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat Commun. 2019;10:3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Qin Y, Gao G, Xie X, Zhu Z, Guan W, Lin X, Xie Z, Ming O, Chen R, Zhong N, Li S, Zhou C. Clinical Features and Prognosis of Pulmonary Lymphoepithelioma-like Carcinoma: Summary of Eighty-five Cases. Clin Lung Cancer. 2019;20:e329-e337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Yin K, Feng HB, Li LL, Chen Y, Xie Z, Lv ZY, Guo WB, Lu DX, Yang XN, Yan WQ, Wu YL, Zhang XC. Low frequency of mutation of epidermal growth factor receptor (EGFR) and arrangement of anaplastic lymphoma kinase (ALK) in primary pulmonary lymphoepithelioma-like carcinoma. Thorac Cancer. 2020;11:346-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Chang YL, Yang CY, Lin MW, Wu CT, Yang PC. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer. 2015;88:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Wu Q, Wang W, Zhou P, Fu Y, Zhang Y, Shao YW, Jiang L. Primary pulmonary lymphoepithelioma-like carcinoma is characterized by high PD-L1 expression, but low tumor mutation burden. Pathol Res Pract. 2020;216:153043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Xie Z, Liu L, Lin X, Xie X, Gu Y, Liu M, Zhang J, Ouyang M, Lizaso A, Zhang H, Feng W, Li B, Han-Zhang H, Chen S, Li S, Zhong N, Liu H, Zhou C, Qin Y. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Mod Pathol. 2020;33:626-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM; KEYNOTE-407 Investigators. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2666] [Article Influence: 380.9] [Reference Citation Analysis (0)] |
| 9. | Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, Zhang W, Gao Y, Jin Z, Zhou J, Jin C, Zou L, Qiu L, Li W, Yang J, Hou M, Zeng S, Zhang Q, Hu J, Zhou H, Xiong Y, Liu P. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019;6:e12-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 10. | Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, Tao X, Zhao J, Mao Y, Wang B, Shao K, Lei W, Wang D, Lv F, Zhao L, Zhang F, Zhao Z, Su K, Tan F, Gao Y, Sun N, Wu D, Yu Y, Ling Y, Wang Z, Duan C, Tang W, Zhang L, He S, Wu N, Wang J, He J. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 11. | Tang Z, Fang R, Tong G, Liu P, Ou Z, Tang Y. Overcoming resistance to anti-PD-1 immunotherapy in lymphoepithelioma-like carcinoma: A case report and review of the literature. Lung Cancer. 2020;146:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Kim C, Rajan A, DeBrito PA, Giaccone G. Metastatic lymphoepithelioma-like carcinoma of the lung treated with nivolumab: a case report and focused review of literature. Transl Lung Cancer Res. 2016;5:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Zhou N, Lin Y, Peng X, Wang Y. Thorough survey and analysis of pulmonary lymphoepithelioma-like carcinoma in Macau and multimodality treatment for advanced disease. Lung Cancer. 2019;138:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |