Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11486
Peer-review started: April 15, 2022
First decision: July 14, 2022
Revised: September 14, 2022
Accepted: September 29, 2022
Article in press: September 29, 2022
Published online: November 6, 2022
Processing time: 194 Days and 19.2 Hours
Combined pituitary hormone deficiency 3 (CPHD3; OMIM: 221750) is caused by mutations within the LHX3 gene (OMIM: 600577), which located on the subtelomeric region of chromosome 9 at band 9q34.3, has seven coding exons and six introns. LIM homeobox (LHX) 3 protein is the key regulator of pituitary development in fetal life.
We have diagnosed and treate an 11-year-old boy with combined pituitary hormone deficiency (CPHD). The main clinical manifestations were pituitary hormone deficiency, hydrocele of the tunica vaginalis, pituitary dwarfism, gonadal dysplasia, micropenis, clonic convulsion, and mild facial dysmorphic features. We collected peripheral blood from the patient, the patient's older brother, as well as their parents, and sequenced them by using high-throughput whole-exosome sequencing, which was verified by Sanger sequencing. The results showed that there were two compound heterozygous variants of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene. c.613G>C (p.V205L) was inherited from his mother and c.220T>C (p.C74R) from his father. His brother also has both variants and symptoms.
This study reported ununreported genetic mutations of LHX3, and recorded the treatment process of the patients, providing data for the diagnosis and treatment of CPHD.
Core Tip: We report an 11-yar-old boy with combined pituitary hormone deficiency (CPHD). DNA sequencing showed that there were two compound heterozygous variants in the LHX3 gene. This study extends the mutation spectrum of the LHX3 gene, and provides a molecular basis for the etiological diagnosis of CPHD and genetic consultation for the family.
- Citation: Lin SZ, Ma QJ, Pang QM, Chen QD, Wang WQ, Li JY, Zhang SL. Novel compound heterozygous variants in the LHX3 gene caused combined pituitary hormone deficiency: A case report. World J Clin Cases 2022; 10(31): 11486-11492
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11486.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11486
Combined pituitary hormone deficiency (CPHD) is an autosomal recessive inheritance genetic disease caused by mutation of the LHX3 gene. LHX3 mutations are associated with growth hormone (GH), gonadotropin, and thyroid-stimulating hormone (TSH) deficiency; abnormal pituitary morphology; and may be accompanied with limited neck rotation and sensorineural hearing loss[1-3].
The LHX3 gene, located on the subtelomeric region of chromosome 9 at band 9q34.3, consists of seven coding exons and six introns. It is a transcription factor and key regulator of pituitary development in early fetal life[1]. In this case, two novel heterozygous mutations of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene were found by whole-exosome sequencing (WES) in an 11-year-old patient with pituitary hormone deficiency, hydrocele of the tunica vaginalis, pituitary dwarfism, gonadal dysplasia, micropenis, clonic convulsion, and mild facial dysmorphic features.
A 11-year-old boy presented to our hospital because of two epileptic attacks in the last 7 mo, and he was found pituitary hormone deficiency since birth.
The patient came to our hospital in December 2021, and he had two epileptic attacks in the last 7 mo. In May 2021, the child had convulsions without obvious inducement, no fever, manifested as stiff limbs, oral cyanosis, loss of consciousness, no salivation, maintained for 30 min, and the seizures stopped after sedation. In August, he had similar convulsions, maintained for 10 min, and had remission without treatment. In the last 8 d, he began to develop dizziness. His cognitive function was normal, but he could not control falling on two occasions.
The patient was diagnosed with hypopituitarism aged 2 mo because of jaundice and pituitary hormone deficiency for > 1 mo. He was treated with oral levothyroxine (Euthyrox) and hydrocortisone and GH, and followed up regularly, and now has stopped GH treatment for > 7 mo. He had intermittent oral treatment with testosterone undecanoate.
The patient was G2P2, had normal delivery at 41W+3, with body weight of 3350 g. There was no history of asphyxiation and resuscitation. His parents were clinically normal, but had another, 15-year-old son with hypopituitarism. There was no intermarriage or family history. The history of vaccination was normal, and the history of trauma, infectious disease, blood transfusion, and drug allergy were denied.
On physical examination at the patient’s visit in August 2021, his weight was 33.0 kg and length was 133.1 cm, with head circumference of 57 cm. The occipital bulges and forehead were prominent, with blepharoptosis. Neurological physical examination was normal. Reproductive system examination showed the scrotum was hypogenetic, while the testicular volume was 1 mL, and the penis length was 2 cm, in no pubic hair period.
In August, 2021, the patient was subjected to a detailed laboratory examination. Insulin-like growth factor-1 was 66.10 ng/mL. Sex hormone levels were: estradiol < 5.0 pg/mL, follicle-stimulating hormone < 0.10 IU/L, luteinizing hormone < 0.10 IU/L, testosterone < 0.025 ng/mL, prolactin 0.1 ng/mL, progesterone < 0.050 ng/mL, and cortisol 572.0 nmol/L. Thyroid function was evaluated as follows: triiodothyronine 4.07 pmol/L, free thyroxine 16.20 pmol/L, and TSH 0.02 mIU/L. In addition, the biochemical examination of this patient was generally normal.
In December 2021, we examined the patient. Electrocardiography showed incidental atrial premature beats. Pituitary magnetic resonance imaging (MRI) showed that intracranial space occupying lesion, located in the sellar region of the brain, though the nature of the pathology was unknown. It was accompanied by agnogenic cystic lesions and signs of an irregular pituitary gland. Electroencephalography was abnormal, showing medium amplitude spike, slow wave sporadic or paroxysmal in the left occipital area. Computed tomography of the head showed: Signs of skull base depression, combined with a cerebellar subtonsillar hernia the foramen magnum was narrow the brain anterior and posterior diameter was widened small dense nodules in the anterior margin of the pituitary gland and no calcification signs of typical craniopharyngioma. The patient underwent a skeletal examination, which showed that the bone age was 11 years.
DNA samples were extracted from peripheral blood taken from the child and his parents to detect whole-exome sequences and whole-genome copy number variations. The results revealed two novel heterozygous variants of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene. Application of the human gene mutation database (and the OMIM database confirmed the reported pathogenic gene locus. The American College of Medical Genetics and Genomics (ACMG) sequence variation interpretation standards and guidelines were used for a comprehensive evaluation of the pathogenicity of mutation sites[4]. Informed consent was obtained from the guardian for all information collection and publication involved in this article.
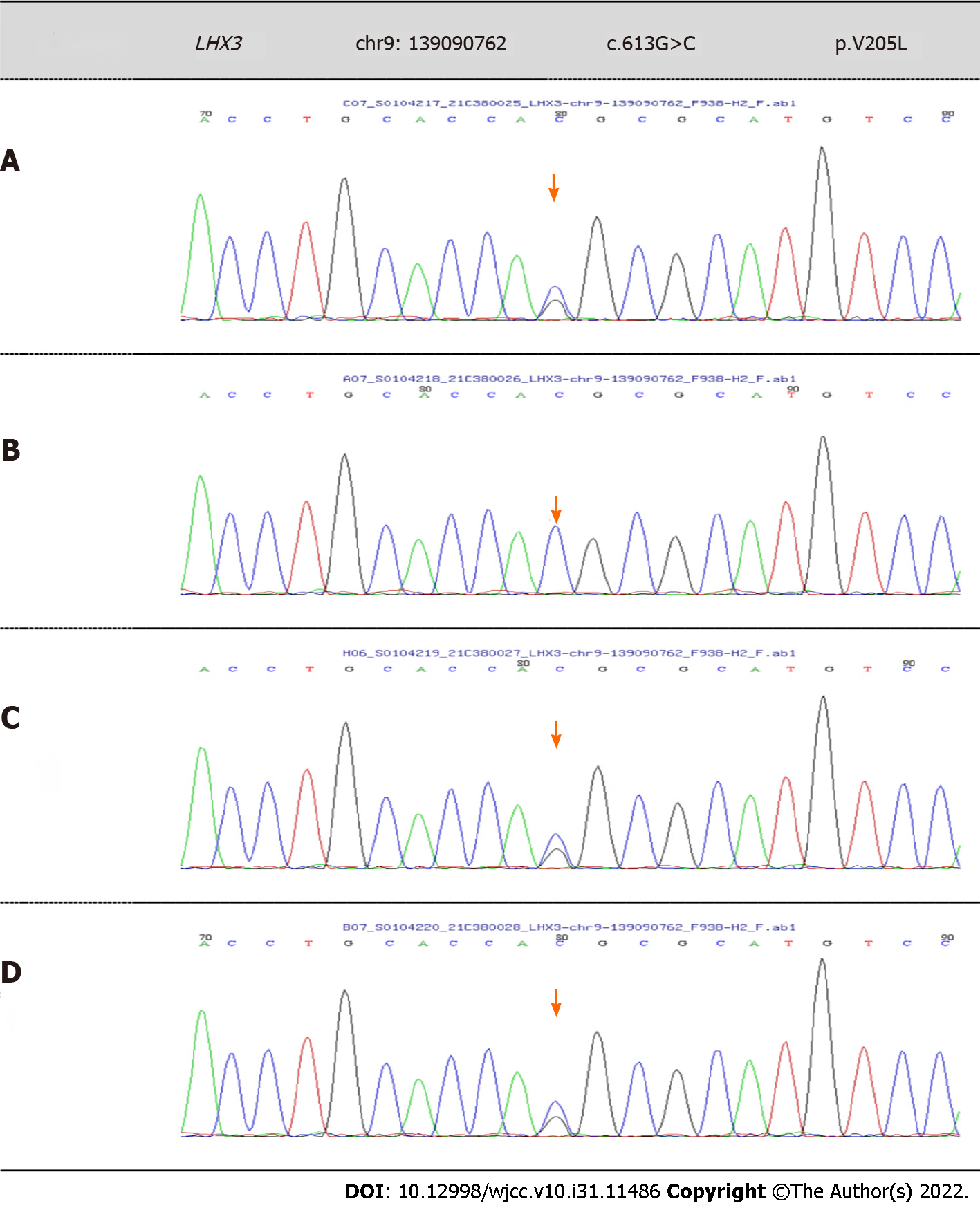
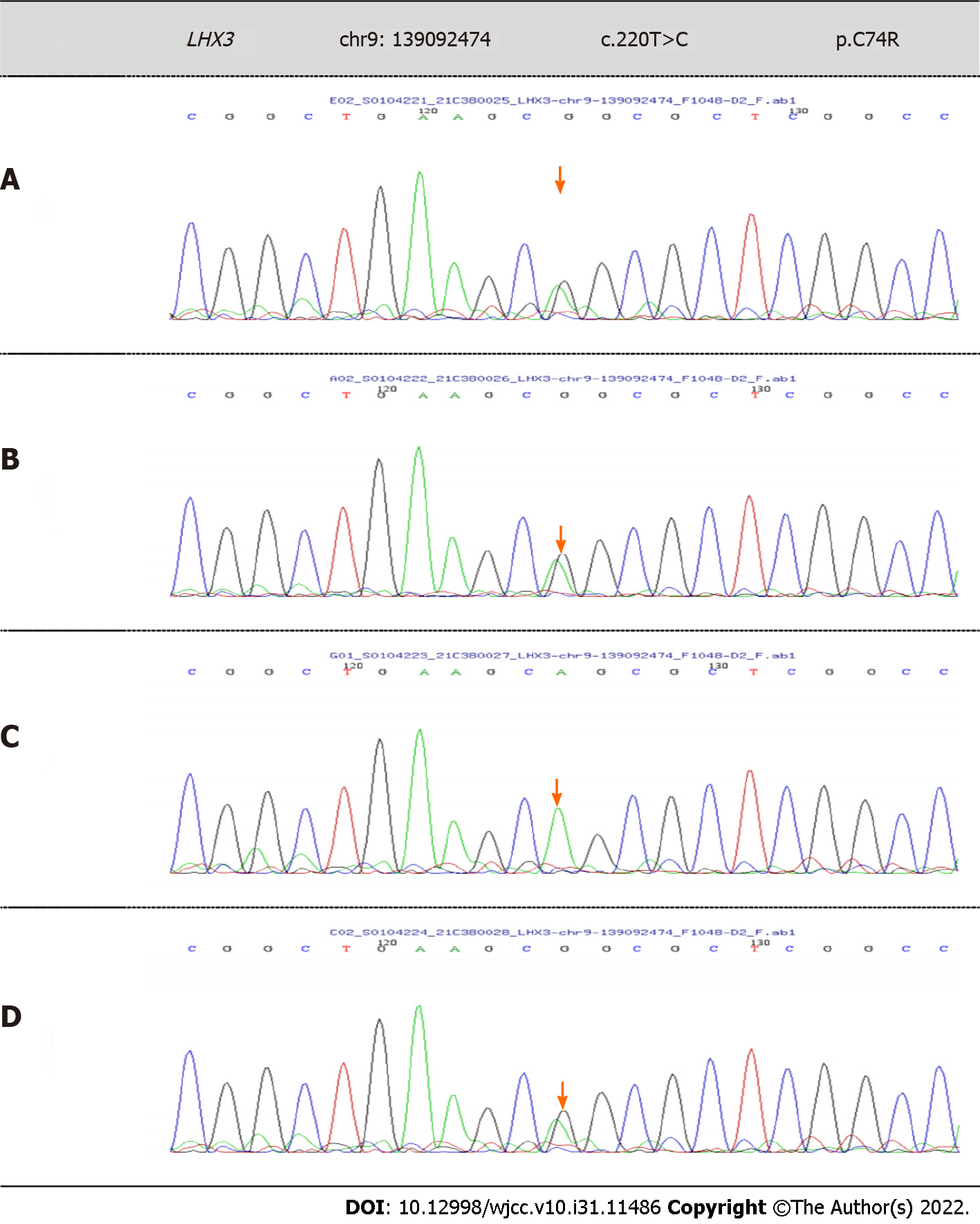
WES showed that there were two novel variants of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene in this patient, which were both unreported variants, and may lead to change in gene function. According to the ACMG guidelines, the mutations were both uncertain. The patient’s older brother also had the same variants. c.613G>C (p.V205L) is a low-frequency variation, with a frequency in the normal population of 0.0003676. According to Sanger sequencing, the patient’s father did not have this mutation, but his mother had the heterozygous mutation (Figure 1). c.220T>C (p.C74R) is also a low-frequency variation. According to Sanger sequencing, the patient’s mother did not have this mutation, but his father had the heterozygous mutation (Figure 2).
Sanger sequencing showed that there were two novel heterozygous variations of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene. Based on clinical presentation, laboratory tests and gene sequencing results, the clinical phenotype was CPHD3.
Considering the patient had sellar space-occupying lesions, a pituitary tumor resection was performed in our hospital, and he recovered well after surgery. Topiramate (3.97 mg/kg/d) was started in January 2022.
The patient is now 12 years old, and his weight is 37.2 kg. He had received topiramate treatment for nearly 3 mo, but his intellectual development is still slightly behind, with poor learning ability. Considering the short duration of medication and the insignificant improvement of the patient's symptoms, we will continuously follow this patient and record the relevant data to get a clear assessment of the treatment effect.
The LHX3 gene maps to chromosome 9 and is located on 9q34.3. It consists of seven coding exons and six introns, and encodes a protein that contains two tandemly repeated amino-terminal LIM motifs (involved in protein–protein interactions modulating the transcriptional activity) and a carboxy-terminal homeodomain with DNA-binding activity[1,2,5]. Expression of the LHX3 gene is one of the earliest markers implicated in the development of the anterior and intermediate lobes, and its expression plays an important role for the formation of gonadotrophs, thyrotrophs, somatotrophs, and lactotrophs[6,7]. It is involved in the development of the pituitary gland, motor neurons, inner ear, and placenta in mice[8]. Previous studies have shown that homozygous variants of LHX3 Lead to CPHD[9].
CPHD is an autosomal recessive genetic disease that is caused by both genetic and nongenetic factors. CPHD includes a heterogeneous group of disorders in which there is a deficiency of GH and also of one or more of other anterior pituitary hormones. The clinical manifestations of this disease include sensorineural deafness, short neck, GH deficiency, pituitary dwarfism, mental deficiency, and short stature. The prevalence of CPHD is estimated to be 1 in 8000 individuals worldwide, and is usually sporadic, but nearly 5%-30% of cases are familial[10]. The genetic defects causing CPHD typically result in misdevelopment of the anterior pituitary gland and insufficient hormone secretion, which manifests in early childhood[11,12].
According to previous studies, deficiency of growth hormone and deficiency of one or more other anterior pituitary hormones constitute the criteria for the diagnosis of CPHD. A retrospective study in Turkey found that short stature was present in 84.2% of patients. GH deficiency was present in > 75% of patients, followed by TSH and/or adrenocorticotropic hormone (ACTH) deficiency, and few patients developed symptoms of pituitary enlargement[10]. This varies slightly from the data obtained from a Korean study. In CPHD patients, endocrine dysfunction other than GH deficiency, including central hypothyroidism, was seen in approximately 82.6%, ACTH deficiency in 78.3%, hypogonadotropic hypogonadism in 73.9%, and central diabetes insipidus in 13.0%. Sellar MRI findings demonstrated structural abnormalities in 21 patients[13].
According to the literature, in 2008, Rajab published the first description of four patients with LHX3 mutations, restricted neck rotation, and sensorineural hearing loss[3]. Although most patients develop severe hormone deficiency after birth, a milder form is observed, and restricted neck rotation is not a universal feature of patients with LHX3 mutations[7].
For the LHX3 gene, we performed the search of the relevant case reports. We found that, except for the base-pair substitutions described here, the insertion and deletion of some gene fragments could lead to CPHD[10,12,13]. Although LHX3 mutations are a rare cause of CPHD, there is sufficient clinical evidence and theoretical support, and in the most cases they involve GH, TSH and LH/FSH defects.
In this case report, this child conformed to the typical clinical presentation of CPHD, and had his own special manifestations. He had short stature and genital dysplasia caused by GH and sex hormone deficiency, with dysmorphic facial features (prominent occipital bulge and forehead). In addition, TSH, cortisol and corticotropin were lower than normal, and renin activity was higher than normal, indi
We described a Chinese patient with CPHD, with two novel compound heterozygous variants of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene identified by WES, along with subsequent treatment. We noted the specific familial genetic situation of the patient, and will further schedule the examination and gene function determination.
Previous studies have demonstrated hypopituitarism in the LHX3-related phenotype of CPHD. Our patient was found to have two novel compound heterozygous variants of c.613G>C (p.V205L) and c.220T>C (p.C74R) in the LHX3 gene, which were unreported before. Typical features of CPHD include sensorineural deafness, short neck, GH deficiency, pituitary dwarfism, mental deficiency, and short stature, and other mild dysmorphic features. We encourage clinicians to consider CPHD in patients with similar clinical features. Genetic testing can not only assist in diagnosis, but also guide patients' prognosis and family genetic disease counseling.
We would like to thank the family members for agreeing to participate in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhattacharya S, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sloop KW, Walvoord EC, Showalter AD, Pescovitz OH, Rhodes SJ. Molecular analysis of LHX3 and PROP-1 in pituitary hormone deficiency patients with posterior pituitary ectopia. J Clin Endocrinol Metab. 2000;85:2701-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Bonfig W, Krude H, Schmidt H. A novel mutation of LHX3 is associated with combined pituitary hormone deficiency including ACTH deficiency, sensorineural hearing loss, and short neck-a case report and review of the literature. Eur J Pediatr. 2011;170:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Rajab A, Kelberman D, de Castro SC, Biebermann H, Shaikh H, Pearce K, Hall CM, Shaikh G, Gerrelli D, Grueters A, Krude H, Dattani MT. Novel mutations in LHX3 are associated with hypopituitarism and sensorineural hearing loss. Hum Mol Genet. 2008;17:2150-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22513] [Article Influence: 2251.3] [Reference Citation Analysis (0)] |
| 5. | Sobrier ML, Attié-Bitach T, Netchine I, Encha-Razavi F, Vekemans M, Amselem S. Pathophysiology of syndromic combined pituitary hormone deficiency due to a LHX3 defect in light of LHX3 and LHX4 expression during early human development. Gene Expr Patterns. 2004;5:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Bosch I Ara L, Katugampola H, Dattani MT. Congenital Hypopituitarism During the Neonatal Period: Epidemiology, Pathogenesis, Therapeutic Options, and Outcome. Front Pediatr. 2020;8:600962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Pfaeffle RW, Savage JJ, Hunter CS, Palme C, Ahlmann M, Kumar P, Bellone J, Schoenau E, Korsch E, Brämswig JH, Stobbe HM, Blum WF, Rhodes SJ. Four novel mutations of the LHX3 gene cause combined pituitary hormone deficiencies with or without limited neck rotation. J Clin Endocrinol Metab. 2007;92:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Sobrier ML, Brachet C, Vié-Luton MP, Perez C, Copin B, Legendre M, Heinrichs C, Amselem S. Symptomatic heterozygotes and prenatal diagnoses in a nonconsanguineous family with syndromic combined pituitary hormone deficiency resulting from two novel LHX3 mutations. J Clin Endocrinol Metab. 2012;97:E503-E509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Ramzan K, Bin-Abbas B, Al-Jomaa L, Allam R, Al-Owain M, Imtiaz F. Two novel LHX3 mutations in patients with combined pituitary hormone deficiency including cervical rigidity and sensorineural hearing loss. BMC Endocr Disord. 2017;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Baş F, Uyguner ZO, Darendeliler F, Aycan Z, Çetinkaya E, Berberoğlu M, Şiklar Z, Öcal G, Darcan Ş, Gökşen D, Topaloğlu AK, Yüksel B, Özbek MN, Ercan O, Evliyaoğlu O, Çetinkaya S, Şen Y, Atabek E, Toksoy G, Aydin BK, Bundak R. Molecular analysis of PROP1, POU1F1, LHX3, and HESX1 in Turkish patients with combined pituitary hormone deficiency: a multicenter study. Endocrine. 2015;49:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Budny B, Zemojtel T, Kaluzna M, Gut P, Niedziela M, Obara-Moszynska M, Rabska-Pietrzak B, Karmelita-Katulska K, Stajgis M, Ambroziak U, Bednarczuk T, Wrotkowska E, Bukowska-Olech E, Jamsheer A, Ruchala M, Ziemnicka K. SEMA3A and IGSF10 Are Novel Contributors to Combined Pituitary Hormone Deficiency (CPHD). Front Endocrinol (Lausanne). 2020;11:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Fang Q, George AS, Brinkmeier ML, Mortensen AH, Gergics P, Cheung LY, Daly AZ, Ajmal A, Pérez Millán MI, Ozel AB, Kitzman JO, Mills RE, Li JZ, Camper SA. Genetics of Combined Pituitary Hormone Deficiency: Roadmap into the Genome Era. Endocr Rev. 2016;37:636-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 13. | Choi JH, Jung CW, Kang E, Kim YM, Heo SH, Lee BH, Kim GH, Yoo HW. Rare Frequency of Mutations in Pituitary Transcription Factor Genes in Combined Pituitary Hormone or Isolated Growth Hormone Deficiencies in Korea. Yonsei Med J. 2017;58:527-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |