Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11391
Peer-review started: May 31, 2022
First decision: July 14, 2022
Revised: August 1, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: November 6, 2022
Processing time: 148 Days and 13.8 Hours
Patients with lupus nephritis (LN) typically undergo long-term treatment with glucocorticoids (GCs) and immunosuppressants. There is a growing demand for optimal therapy with better remission results and fewer side effects. Sustained traditional Chinese medicine (TCM) might be quite valuable for multitarget therapy, reducing the total dosage of GCs and minimizing the side effects of immunosuppressants.
To evaluate whether Dan Bai Xiao Formula (DBXF) can reduce the exposure to GCs and cyclophosphamide (CYC) and to assess the efficacy and safety of DBXF for the resolution of proteinuria and hematuria in children with LN.
A 24-wk pilot study was conducted at Beijing Children’s Hospital. Children with active LN were divided into either a TCM group or a control group. Children in the TCM group received DBXF combined with GCs and CYC, and the ones in the control group received GCs and CYC every 4 wk for 24 wk. The primary endpoints of this trial were urinary protein excretion of < 150 mg/d and normal serum albumin concentration and renal function.
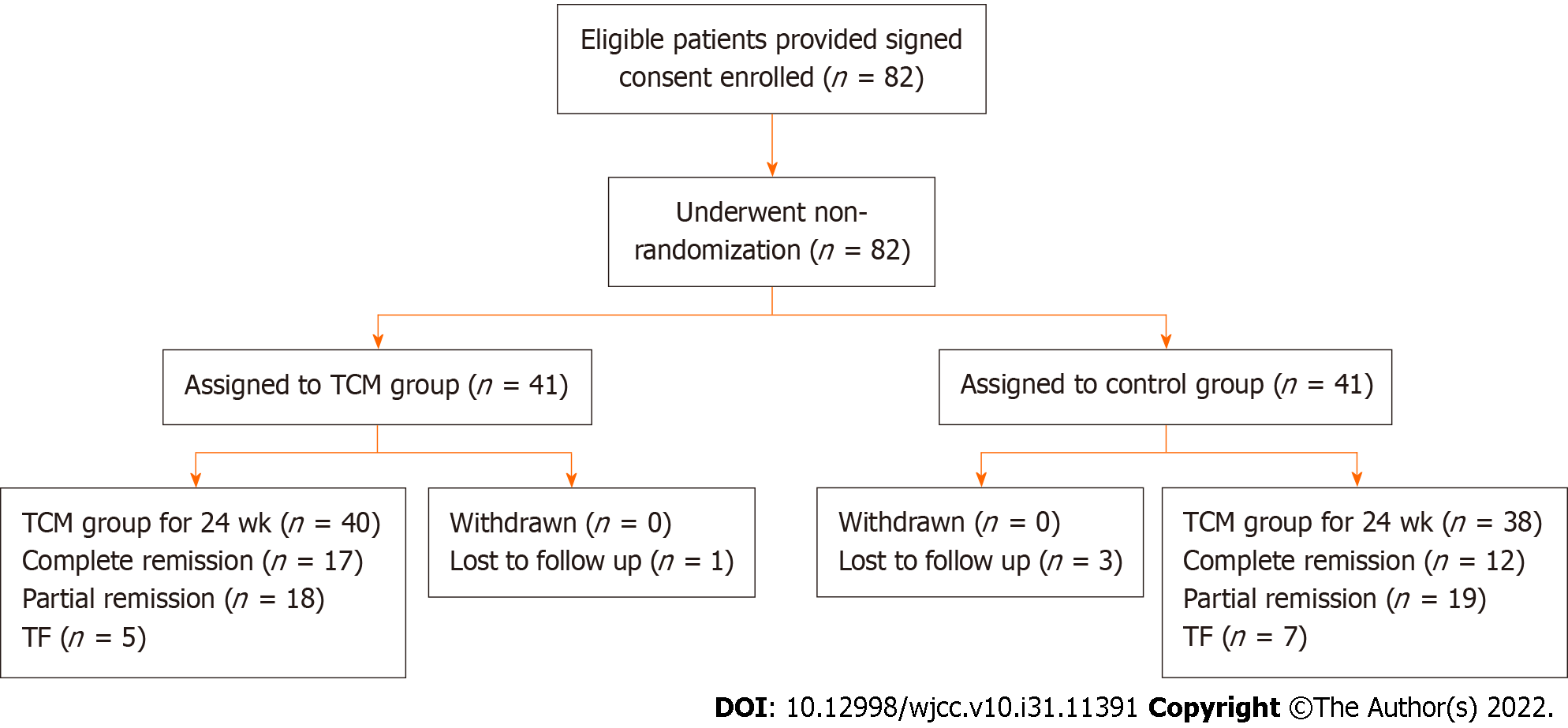
The trial included 78 children, of whom 38 received GCs and CYC treatment (control group) and the remaining 40 received DBXF combined with GCs and CYC treatment (TCM group). At week 24, the TCM group showed a better rate of complete remission (42.5%); however, there was no significant difference compared with the control group (31.5%, P > 0.05). The urine red blood cell count and urine protein level were significantly lower in the TCM group than in the control group at weeks 4, 12, and 24 (P < 0.05). Furthermore, patients in the TCM group had a lower proportion of methylprednisolone pulses than those in the control group (1.30 ± 1.41 vs 3.05 ± 2.02, P < 0.0001). The ending GC dose was significantly lower in the TCM group than in the control group (P < 0.001). Moreover, more hepatic function damage, gastrointestinal adverse effects, and hypertension were observed in the control group than in the TCM group (P < 0.05).
The findings suggest that DBXF treatment is effective and safe as a supplementary therapy for LN and is superior to routine GC and CYC therapy. DBXF containing combination treatment possibly results in a faster resolution of proteinuria and hematuria, smoother GC reduction, fewer methylprednisolone pulses, and fewer adverse events.
Core Tip: Lupus nephritis (LN) is the most common and serious complication of systemic lupus erythematosus. Glucocorticoids (GCs) and immunosuppressants were considered routine treatments for patients with LN. However, there is a widespread consensus regarding the toxicity of immunosuppressive agents and the necessity of preventing children from taking these medications over an extended period. Accordingly, there is an increasing need for holistic and optimal therapy that results in a higher rate of remission and fewer side effects. Our study suggests that Dan Bai Xiao Formula (DBXF) treatment is effective and safe as a supplementary therapy for LN; moreover, this treatment is superior to routine GC and cyclophosphamide therapies. DBXF containing combination treatment may lead to faster proteinuria and hematuria resolution, smoother GC reduction, fewer methylprednisolone pulses, and fewer adverse events.
- Citation: Cao TT, Chen L, Zhen XF, Zhao GJ, Zhang HF, Hu Y. Dan Bai Xiao Formula combined with glucocorticoids and cyclophosphamide for pediatric lupus nephritis: A pilot prospective study. World J Clin Cases 2022; 10(31): 11391-11402
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11391.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11391
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that involves disturbances in the immune system with the production of immune complexes that induce inflammatory injury to multiple organs and tissues, particularly the kidneys[1]. Lupus nephritis (LN) is the most common serious complication of SLE. Compared with adults, SLE diagnosed in childhood or adolescence is more aggressive, and up to 80% of SLE children (also referred to as patients below) have LN[2]. To induce renal remission, patients are routinely treated with glucocorticoids (GCs) and immunosuppressants for 6 mo of aggressive induction immunosuppression. This is followed by years of maintenance immuno
There is a lack of data on the optimal therapy for LN in children and adolescents. Although available therapies, including corticosteroids, hydroxychloroquine, and other immunosuppressive drugs, are effective for LN, there is a widespread agreement on the toxicity of immunosuppressive agents and the need to avoid long-term use of these drugs in children. As a result, there is a growing demand for a holistic and optimal therapy that results in greater remission and fewer side effects. The integrated treatment of traditional Chinese medicine (TCM) has been indicated to be beneficial for LN. Immunosuppression can be combined with TCM to improve the efficacy and relieve the adverse effects during the active period of LN. Meanwhile, TCM could aid in stabilizing the case, preventing recurrence, and decreasing the use of GCs or immunosuppressant drugs.
TCM is used to prevent, treat, and diagnose diseases under the guidance of the TCM theory. It has condensed thousands of years of practical experience. Based on the concept of “systematic holism,” TCM pays equal attention to strengthening the resistance of the body and regulating immunity. According to Yang et al[4], the effects of TCM have also been reported to be beneficial in patients with LN when used in combination with GCs and immunosuppressant agents. Some studies have stated that sustained TCM may be quite useful for multitarget therapy, reducing the total dosage of GCs and minimizing the side effects of immunosuppressive agents[5,6]. Dan Bai Xiao Formula (DBXF) comes from Pei Xueyi’s clinical experience summary; notably, Pei Xueyi is a renowned doctor practicing in China for decades. This formula is beneficial for many children with nephritis. According to TCM, it is believed that the pathogenesis of LN is based on the qi disorder, blood, and water. Therefore, promoting qi and blood circulation should be the focus of the treatment. The effect of DBXF is to diurese and dampen and to correct the pathological phenomenon in qi and blood. The formula can be used in various TCM syndrome types.
The basic composition of DBXF is listed in Table 1. Studies have shown that some herbal components in this recipe exhibit anti-inflammatory and immunoregulatory effects. Oxymatrine, an active constituent of Radix Sophorae Flavescentis, protects the organs and tissues by regulating inflammation, oxidative stress, apoptosis, and fibrosis[7-9]. Shibeshi et al[10] observed that Achyranthis asperae has a regulating effect on serum lipids and hormones (Table 1).
| DBXF meterial | Equivalent pharmaceutical name | Amount (g) |
| Feng Wei Cao | Herba Pteridis Multifidae | 15 |
| Ku Shen | Radix Sophorae Flavescentis | 10 |
| Shi Wei | Folium Pyrrosiae | 12 |
| Dao Kou Cao | Herba Achyranthis Asperae | 15 |
| Lian Xu | Stamen Nelumbinis | 10 |
| Dan Dou Chi | Semen Sojae Preperatum | 12 |
| Chi Xiao Dou | Semen Vignae Angularis | 30 |
| Yi Yi Ren | Semen Coicis | 30 |
Owing to the lack of data on the efficacy and safety of the long-term use of DBXF in LN, we conducted a 6-mo clinical trial assessing the efficacy and safety of DBXF containing combination treatment in patients with LN. The results observed over a period of 24 wk in our patients related to clinical renal remission, serum indexes, proteinuria, dosage of GCs, and adverse events are described herein. We hope that the findings may provide clues for the selection of LN therapy regimens and for other future studies.
This was a prospective study of 82 consecutive patients with LN at the ward of the Children’s National Medical Center. All participants were recruited from the Outpatient Specialist Clinics of Rheumatology at the Beijing Children’s Hospital, Beijing, China from January 2018 to January 2020. Patients who met the entry criteria from our TCM ward were assigned to the TCM group. Patients from the Rheumatology Medical Ward were assigned to the control group[11], which received oral GC therapy at an initial dose of 1-2 mg/kg/d. The GC dosage was reduced on a monthly basis by 5 mg after 4-6 wk; subsequently, the dose was further reduced to a dose of < 10 mg/d according to the patient’s condition. Intravenous cyclophosphamide (CYC) pulse was given at a dose of 1.0 g/m2 of body surface area at 4-wk intervals over 6 mo. Intermittent pulse methylprednisolone was given intravenously at a dose of 20-30 mg/kg for 3 d if the condition was not satisfactory. The maximum bolus dose of methylprednisolone was 1 g. Patients included in the TCM group also received GCs and intravenous CYC pulse, and the dosage of GC was reduced in the same way. The DBXF composition is summarized in Table 1. Each medicine’s dosage in DBXF is based on the Chinese pharmacopoeia and needs to be decocted in water. Patients took the solution orally twice daily for 24 wk. Patients who had treatment failure were retreated by the attending physicians.
The American College of Systemic Lupus International Collaborating Clinics/American College of Rheumatology 2012 classification criteria were used to diagnose LN in eligible patients between 4-15 years of age[12]. Patients with the following medical conditions were excluded from the study: Severe infections within the past 3 mo, creatinine clearance of < 30 mL/min or serum creatinine on repeat tests of > 3.0 mg/dL, human immunodeficiency virus infection, hepatitis B or C virus infection, neuropsychiatric lupus erythematosus, and other severe coexisting conditions during the trial. The ethics committee of the hospital approved the study protocol. Informed consent forms were signed by the patients’ legal representatives.
Patients were seen every 4 wk for a total of 24 wk. Complete remission at 24 wk was the primary endpoint. Clinical and laboratory assessments were performed during the follow-up visits. Patients were evaluated for clinical manifestations of LN and adverse effects related to the treatment at each visit. Blood pressure and body weight were recorded. Blood samples were collected, and urinalysis was performed. Complete blood cell counts, coagulation function, renal and liver function, serum C3 and C4 concentrations, and anti-double-stranded DNA (dsDNA) antibodies were determined. A 24-h urine sample was collected for urinary protein excretion measurement.
The SLE Disease Activity Index 2000 (SLEDAI-2K) score was reported at baseline and at each follow-up visit according to Gladman et al[13]. The care providers comprehensively assessed whether the patient had experienced a disease flare since the last visit and the specific reason for the flare. No specific flare criteria were provided.
Normal urinary protein excretion (< 150 mg/d) and normal urinary sediment were used to define complete remission. Partial remission was defined as a greater than 50% reduction in urinary protein excretion. Treatment failure was defined as follows: Failure to reach complete or partial remission at 24 wk[14].
The Common Terminology Criteria for Adverse Events (CTCAE v4.0) was used to assess adverse events (AEs). AEs were recorded at each study visit.
Prism software version 6.0 was used to perform the statistical analyses. All data are expressed as the mean ± SD or median as appropriate. The Mann-Whitney U Test was used to compare the quantitative variables. Chi-square test and Fisher’s exact test were used to compare the categorical variables as appropriate. A P value of < 0.05 was considered significant to determine the factors that were significantly associated with the response rate.
The trial included 82 patients, with 41 assigned to the control group and an equal number to the TCM group. The baseline characteristics of the two groups were similar, as shown in Table 1. As shown in Figure 1, the numbers of patients who completed the trial were 38 (92.6%) in the control group and 40 (97.5%) in the TCM group. Four patients, including three from the control group and one from the TCM group, dropped out of the trial because of the lack of follow-up. In either group, no patients experienced severe AEs. The trial, including its follow-up, was completed by 78 patients (Figure 1). All patients were followed for at least 24 wk. The average length of follow-up was 12 wk (range, 0-24 wk).
As shown in Table 2, at baseline, 61 patients had high dsDNA antibody concentration (33 in the TCM group and 28 in the control group), 75 had low serum C3 concentration (37 in the TCM group and 38 in the control group), and 66 had low serum C4 concentration (34 in the TCM group and 26 in the control group). No significant difference was found between the control and TCM groups in the baseline clinical characteristics (Table 2).
| Characteristic | Control group (n = 38) | TCM group (n = 40) | P value |
| Age (yr) | 11.07 ± 2.28 | 11.51 ± 2.77 | 0.338 |
| Female sex, n (%) | 25 (65.8) | 28 (70) | 0.690 |
| Weight (kg) | 39.18 ± 11.55 | 41.61 ± 15.55 | 0.582 |
| Duration of SLE (years) | 0.65 ± 1.37 | 0.78 ± 1.21 | 0.607 |
| Duration of LN (years) | 0.15 ± 0.41 | 0.17 ± 0.28 | 0.559 |
| Serum creatinine (μmol/L) | 61.73 ± 46.68 | 51.46 ± 22.52 | 0.127 |
| White blood cell count | 5.03 ± 3.22 | 5.83 ± 3.20 | 0.201 |
| NE (%) | 53.85 ± 19.54 | 57.56 ± 15.33 | 0.489 |
| HGB (g/L) | 100.18 ± 16.93 | 105.85 ± 23.73 | 0.308 |
| PT (s) | 10.45 ± 0.93 | 11.23 ± 2.09 | 0.115 |
| APTT (s) | 35.48 ± 17.25 | 37.6 ± 16.17 | 0.577 |
| D-D (mg/L) | 1.08 ± 1.64 | 1.02 ± 1.32 | 0.161 |
| Serum albumin (g/L) | 29.23 ± 5.99 | 30.92 ± 4.61 | 0.167 |
| Urine protein (g/24 h) | 1.29 ± 1.81 | 1.32 ± 1.11 | 0.314 |
| Urine RBC count/HPF | 26.17 ± 37.92 | 25.65 ± 37.59 | 0.24 |
| Serum C3 (g/L) | 0.28 ± 0.18 | 0.39 ± 0.28 | 0.101 |
| Serum C4 (g/L) | 0.05 ± 0.04 | 0.06 ± 0.05 | 0.782 |
| BUN (IU/mL) | 6.94 ± 3.89 | 5.72 ± 2.31 | 0.057 |
| ALT (mmol/L) | 33.20 ± 28.04 | 32.88 ± 36.34 | 0.730 |
| AST (mmol/L) | 40.00 ± 41.21 | 39.61 ± 43.37 | 0.957 |
| SLEDAI | 15.57 ± 5.01 | 14.07 ± 3.49 | 0.315 |
| ds-DNA postive, n (%) | 28 (73.6) | 33 (82.5) | 0.345 |
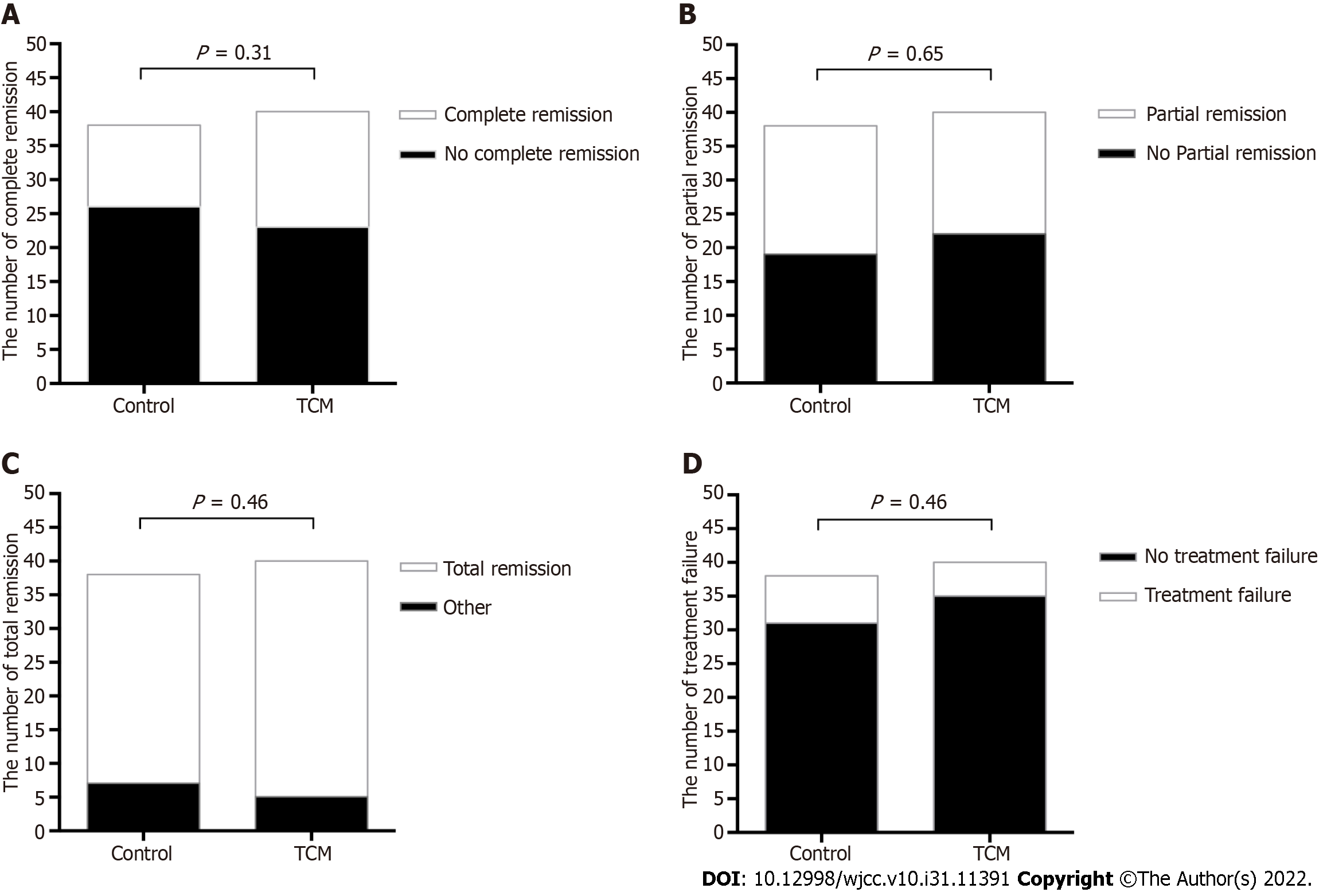
As shown in Figure 2A, 17 out of 40 (42.5%) patients in the TCM group and 12 out of 38 (31.5%) patients in the control group achieved complete remission at 24 wk (P = 0.31). Partial remission occurred in 18 (45%) patients in the TCM group and 19 (50%) in the control group (P = 0.65, Figure 2B). Overall, 35 (87.5%) patients in the TCM group and 31 (81.6%) in the control group achieved either complete or partial remission (P = 0.46, Figure 2C). The treatment failure was similar in both groups: Five out of 40 (12.5%) patients in the TCM group vs 7 out of 38 (18%) patients in the control group (P = 0.46, Figure 2D).
Renal parameters throughout the study are shown in Table 3. The baseline urinary protein excretion levels in the control and TCM groups were 1.29 g/24 h ± 1.81 g/24 h and 1.32 g/24 h ± 1.11 g/24 h, respectively. Serum albumin levels were 29.23 g/L ± 5.99 g/L and 30.92 g/L ± 4.61 g/L, respectively. Proteinuria levels decreased significantly from the baseline after only 4 wk of treatment in the TCM and control groups, and proteinuria levels decreased significantly in the TCM group compared with the control group at 4, 12, and 24 wk (P < 0.05). Furthermore, t urine red blood cell count in the TCM group was significantly lower than that of the control group at weeks 4, 12, and 24 (P < 0.05). Serum albumin levels increased significantly from the baseline in both groups. At 24 wk, serum albumin appeared to be higher in the TCM group; but no significant difference was observed between the two groups. The levels of complements C3 and C4 improved significantly from the baseline in the two groups at 24 wk. No significant difference was found between the two groups for complements C3 and C4, serum creatinine, and blood urea nitrogen. The percentage of positive dsDNA antibodies decreased significantly from the baseline after 24 wk of therapy, with no significant differences between the two groups. The levels of changes in SLEDAI improved significantly at 24 wk (P < 0.05), but there were no statistically significant differences between the two groups (Table 3).
| Characteristic | Follow up (wk) | Control group (n = 38) | TCM group (n = 40) | P value |
| Urine protein (g/24 h) | 4 | 1.11 ± 1.54 | 0.73 ± 1.48 | 0.008 |
| 12 | 0.67 ± 0.91 | 0.34 ± 0.82 | 0.0159 | |
| 24 | 0.30 ± 0.29 | 0.09 ± 0.14 | < 0.0001 | |
| Urine RBC count/HPF | 4 | 17.63 ± 15.48 | 9.02 ± 8.78 | 0.001 |
| 12 | 10.55 ± 9.73 | 4.35 ± 8.54 | 0.0007 | |
| 24 | 10.47 ± 8.43 | 2.82 ± 5.11 | < 0.0001 | |
| Serum albumin (g/L) | 4 | 34.60 ± 6.57 | 36.36 ± 4.91 | 0.324 |
| 8 | 37.27 ± 6.64 | 39.35 ± 3.82 | 0.428 | |
| 12 | 39.65 ± 6.73 | 41.16 ± 3.99 | 0.701 | |
| 16 | 40.26 ± 6.45 | 41.17 ± 5.02 | 0.879 | |
| 20 | 41.50 ± 5.16 | 41.08 ± 7.00 | 0.821 | |
| 24 | 40.30 ± 6.28 | 41.15 ± 5.14 | 0.808 | |
| Serum C3 (g/L) | 4 | 0.61 ± 0.14 | 0.53 ± 0.19 | 0.112 |
| 8 | 0.73 ± 0.16 | 0.69 ± 0.25 | 0.205 | |
| 12 | 0.80 ± 0.19 | 0.73 ± 0.0.21 | 0.143 | |
| 16 | 0.82 ± 0.15 | 0.77 ± 0.20 | 0.452 | |
| 20 | 0.82 ± 0.15 | 0.85 ± 0.14 | 0.874 | |
| 24 | 0.88 ± 0.17 | 0.86 ± 0.22 | 0.990 | |
| Serum C4 (g/L) | 4 | 0.10 ± 0.04 | 0.08 ± 0.05 | 0.800 |
| 8 | 0.12 ± 0.05 | 0.11 ± 0.05 | 0.730 | |
| 12 | 0.14 ± 0.07 | 0.10 ± 0.05 | 0.122 | |
| 16 | 0.15 ± 0.06 | 0.13 ± 0.05 | 0.235 | |
| 20 | 0.16 ± 0.06 | 0.14 ± 0.06 | 0.874 | |
| 24 | 0.18 ± 0.07 | 0.18 ± 0.23 | 0.624 | |
| SCR (μmol/L) | 4 | 44.75 ± 19.10 | 42.65 ± 12.86 | 0.768 |
| 8 | 44.18 ± 19.10 | 44.02 ± 13.08 | 0.794 | |
| 12 | 59.08 ± 76.32 | 45.17 ± 14.43 | 0.244 | |
| 16 | 46.53 ± 11.70 | 48.81 ± 14.68 | 0.922 | |
| 20 | 49.46 ± 12.47 | 49.38 ± 14.09 | 0.939 | |
| 24 | 50.11 ± 9.58 | 45.40 ± 10.32 | 0.060 | |
| BUN (IU/mL) | 4 | 6.93 ± 2.60 | 6.08 ± 2.01 | 0.135 |
| 8 | 6.24 ± 3.13 | 5.46 ± 2.04 | 0.492 | |
| 12 | 5.20 ± 1.86 | 5.39 ± 1.61 | 0.654 | |
| 16 | 5.04 ± 1.38 | 5.04 ± 1.60 | 0.975 | |
| 20 | 4.93 ± 1.42 | 4.67 ± 1.43 | 0.469 | |
| 24 | 4.88 ± 1.24 | 4.34 ± 1.38 | 0.051 | |
| Dosage of GCs | 0 | 55.05 ± 7.44 | 52.70 ± 10.94 | 0.683 |
| 4 | 52.94 ± 6.38 | 46.79 ± 12.72 | 0.098 | |
| 8 | 47.67 ± 6.44 | 40.78 ± 12.11 | 0.011 | |
| 12 | 43.58 ± 6.32 | 35.14 ± 12.77 | < 0.0001 | |
| 16 | 39.14 ± 5.38 | 30.62 ± 9.87 | 0.0001 | |
| 20 | 34.64 ± 5.04 | 25.74 ± 8.84 | 0.060 | |
| 24 | 31.44 ± 4.85 | 17.61 ± 7.45 | < 0.0001 | |
| ds-DNA positive, n (%) | 24 | 10 (26.3) | 9 (22.5) | 0.694 |
| SLEDAI | 4 | 11.00 ± 5.21 | 9.02 ± 4.02 | 0.073 |
| 12 | 7.16 ± 3.52 | 6.58 ± 3.64 | 0.625 | |
| 24 | 5.67 ± 4.27 | 5.12 ± 3.48 | 0.990 |
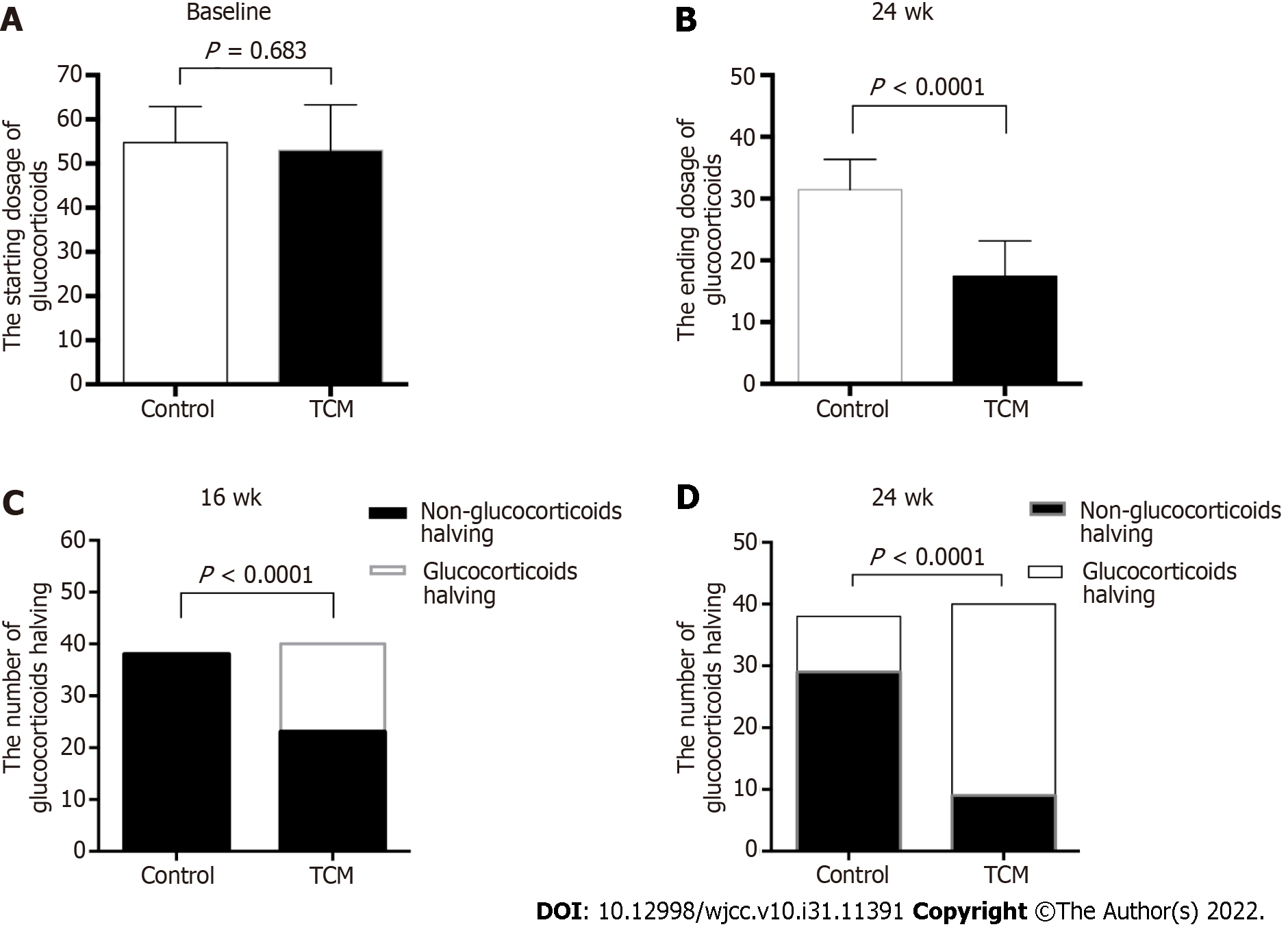
As shown in Figure 3, the starting GC dose was similar between the two treatment groups (mean ± SD, 52.70 mg/d ± 10.94 mg/d in the TCM group vs 55.05 mg/d ± 7.44 mg/d in the control group, P = 0.683). However, the ending GC dose was significantly different between the two groups at 24 wk (mean ± SD, 17.61 mg/d ± 7.45 mg/d in the TCM group vs 31.45 mg/d ± 4.85 mg/d in the control group, P < 0.0001). In the TCM group, 17 of 40 (42.5%) patients achieved GC halving at 16 wk, whereas no patient (0/38, 0%) achieved GC halving in the control group (P < 0.0001) (Figure 3). During the 8-24 wk of follow-up, the GC dosage of the TCM group was significantly lower than that of the control group (P < 0.05). Overall, it was possible to achieve gradual GC reduction in the TCM group, whereas the routine reduction in GCs according to the disease condition could not be achieved in the control group.
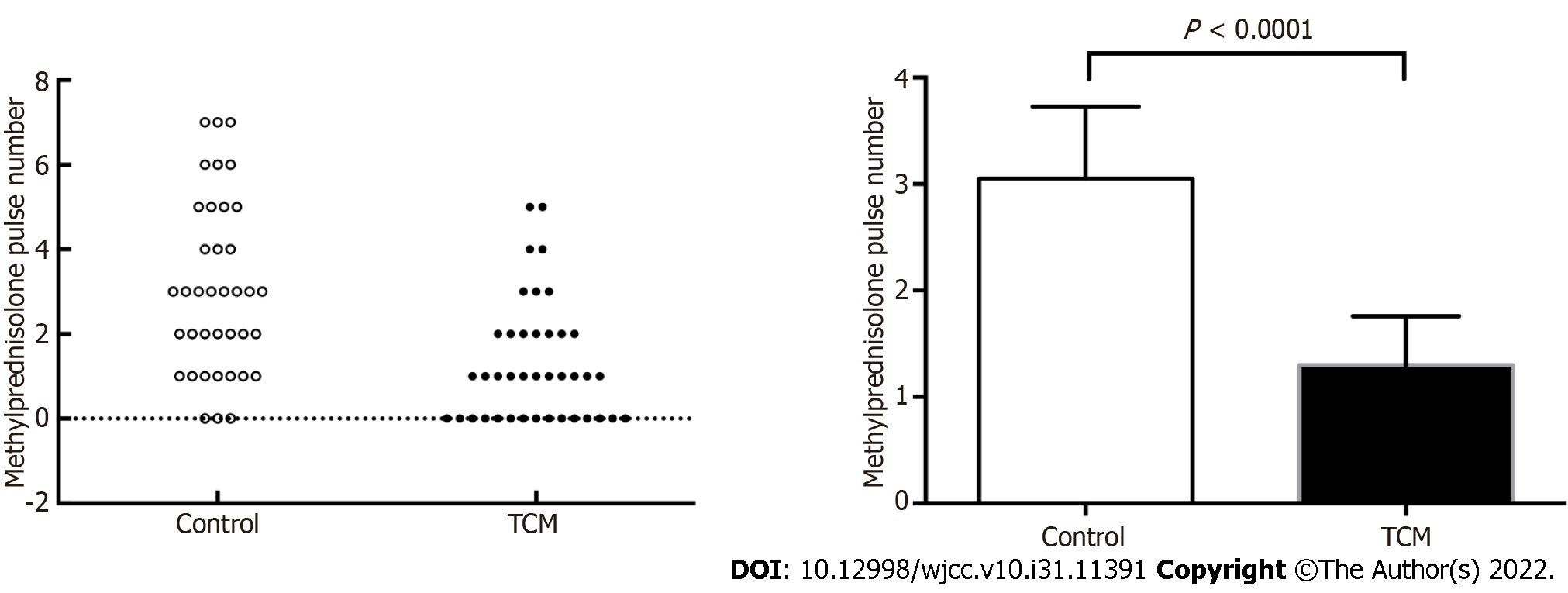
If patients responded satisfactorily and demonstrated decreasing proteinuria or improving renal function, the number of bolus intravenous methylprednisolone pulses was appropriately reduced. Patients in the TCM group had a lower proportion of methylprednisolone pulses than those in the control group (1.30 ± 1.41 vs 3.05 ± 2.02, P < 0.0001) (Figure 4).
There were no severe AEs that required physician’s withdrawal or hospitalization during the 24-wk trial. Gastroenteritis, anemia, upper respiratory tract infection, hepatic function damage, leukopenia, phlebothrombosis, urinary infection, hypothyroidism, and hypertension were the most common AEs experienced by the patients (Table 4). The TCM group had a significantly lower incidence of AEs (25/40, 62.5%) than the control group (32/38, 84.2%, P = 0.04). These AEs, however, did not necessitate hospitalization. As shown in Table 3, hepatic function damage was observed in both groups, with a rate of 42.1% (16/38) in the control group and 22.5% (9/40) in the TCM group (P < 0.05). Gastroenteritis was more common in the control group than in the TCM group (24/38, 63.1% vs 12/40, 30%, P = 0.006). Moreover, hypertension was observed more often in the control group, and there was a significant difference compared with the TCM group (10/38, 26.3% vs 3/40, 7.5%, P = 0.02).
| Item | Control group | TCM group | P value | ||
| n = 38 | Percent (%) | n = 40 | Percent (%) | ||
| Gastroenteritis | 24 | 63.1 | 12 | 30.0 | 0.006 |
| Anemia | 5 | 13.1 | 2 | 5.0 | 0.970 |
| Hepatic function damage | 16 | 42.1 | 9 | 22.5 | 0.030 |
| Leukopenia | 3 | 7.8 | 0 | 0 | 0.060 |
| Upper respiratory infection | 5 | 13.1 | 7 | 17.5 | 0.590 |
| Phlebothrombosis | 2 | 5.3 | 0 | 0 | 0.140 |
| Urinary infection | 1 | 2.6 | 0 | 0 | 0.300 |
| Hypothyroidism | 3 | 7.9 | 1 | 2.5 | 0.280 |
| Hypertension | 10 | 26.3 | 3 | 7.5 | 0.020 |
This pilot study assessed the clinical efficacy of the following treatments for the treatment of LN: DBXF combined with GCs and cyclophosphamide (TCM group) or GCs and cyclophosphamide (CYC; control group). Previous studies have demonstrated the therapeutic efficacy of GCs and CYC. However, the well-recognized treatment with GCs and other immunosuppressants has rarely resulted in complete remission[15]. A significant correlation has been noted between complete remission and favored outcome in LN[16]. Renal remission has been reported to occur with GCs and CYC in 45%-74% of the treated patients, but over 75% of the patients with LN in the trial were Classes III, IV, and/or V[17]. Complete remission is most likely to occur in patients with stable renal function and without aggravating renal damage, although there is significant individual variability[18]. The renal remission associated with DBXF containing combination treamtent for patients with active LN in the present study was 87.5% at 6 mo; this was higher than that reported in a recent clinical research.
It has been shown that DBXF is effective in treating LN and that it plays a considerable role in symptom relief and improving the quality of life of patients with LN in China[19-22]. Understanding the benefits and risks of long-term maintenance of DBXF is an important consideration in the care of LN. Our data asserted that proteinuria levels and urine red blood cell count were significantly decreased in the TCM group compared with the control group at 4, 12, and 24 wk (P < 0.05). These results indicate that TCM can improve the clinical efficacy of routine treatment, with a faster resolution of proteinuria and hematuria.
There is a general agreement on the toxicity of the long-term administration of high-dose GCs in patients. Finding an alternative treatment with equivalent efficacy but with a lower dosage of GCs is important to improve the quality of life of the patients. In the present study, DBXF showed promising effects when combined with GCs and CYC, with a lower ending GC dose (mean ± SD, 17.61 mg/d ± 7.45 mg/d in the TCM group vs 31.45 mg/d ± 4.85 mg/d in the control group, P < 0.0001) and lower number of methylprednisolone pulses (1.3 ± 1.41 in the TCM group vs 3.05 ± 2.02 in the control group, P < 0.0001). These data support the clinical efficacy of DBXF containing combination treamtent, with smoother GC reduction and a lower dosage of GCs for disease induction in a patient with active LN. Indeed, the assisting function of DBXF could make the patient’s condition more stabilized, thereby resulting in smoother GC reduction and a lower number of methylprednisolone pulses.
The data presented in this study showed that the complete response rate according to the renal activity index was dramatically increased in the TCM group over a period of 24 wk, although it was not statistically significant. It is possible to make a significant difference by expanding the sample size as it requires further large-sample research.
GCs and CYC are commonly considered the standard therapeutic regimes for remission induction[23]. They were selected as the additional treatments to be combined with TCM in our trial because their efficacies in LN have been proven in clinical treatment. The reason for adding DBXF to GCs and CYC instead of using only GCs and CYC was that according to previous studies and clinical experience, an increased incidence of adverse effects was detected in long-term treatment with GCs and CYC. In the present study, although patients in the TCM group also experienced many AEs, the total AE incidence was significantly lower in the TCM group (25/40, 62.5% in the TCM group vs 32/38, 84.2% in the control group, P = 0.04). Hepatic function damage, gastroenteritis, and hypertension were observed more often in the control group, and there were statistically significant differences between the two groups. Together, our findings imply that DBXF containing combination treatment could significantly reduce the incidence of AEs in patients with active LN.
There are several limitations to this study. First, we did not have a large sample in the two groups because of the rarity of the disease. Evidence from multicenter large-sample double-blind randomized controlled trial studies supporting the efficacy and safety of combined DBXF in LN is still lacking. Second, the short period of follow-up did not allow discerning the long-term outcome and determining whether a completely GC-free condition can maintain the therapeutic efficacy. A further 2-year study would be appropriate to confirm the long-term outcome. Therefore, we intend to cooperate with the Rheumatism ward to carry out the control group. However, this situation is more aligned with the clinical practice in the real world. Moreover, uniform TCM diagnosis, treatment, and efficacy criteria should be established, which would be helpful in demonstrating the efficacy, mechanism, and safety of DBXF treatment in patients with LN.
In conclusion, despite some limitations, our study demonstrates that DBXF containing combination treatment is a comparatively safe and effective interventional measure that obviously improves the effectiveness and lowers the adverse reactions in patients with LN. Our data indicate that DBXF containing combination treatment provides a good treatment response, along with a faster resolution of proteinuria and hematuria, smoother GC reduction, fewer methylprednisolone pulses, and fewer AEs than GCs and CYC in active LN. Thus, DBXF might help optimize the therapeutic schemes in LN. We hypothesize that DBXF will be an effective and safe way to treat patients with LN. However, further controlled trials with larger sample sizes are required to confirm our observations.
Systemic lupus erythematosus (SLE) diagnosed in childhood or adolescence is more aggressive, and up to 80% of SLE children (also referred to as patients below) have lupus nephritis (LN). Glucocorticoids (GCs) and immunosuppressants were considered routine treatments for patients with LN. However, there is a widespread consensus regarding the toxicity of immunosuppressive agents and the necessity of preventing children from taking these medications over an extended period. Accordingly, there is an increasing need for holistic and optimal therapy that results in a higher rate of remission and fewer side effects.
We aimed to prove that Dan Bai Xiao Formula (DBXF) treatment is effective and safe as a supplementary therapy for LN and that it is superior to routine glucocorticoids (GCs) and cyclophosphamide (CYC) therapy.
To assess the efficacy and safety of DBXF for the resolution of proteinuria and hematuria in children with LN.
A 24-wk pilot study was conducted.
The urine red blood cell count and urine protein level were significantly lower in the traditional Chinese medicine (TCM) group than in the control group at weeks 4, 12, and 24 (P < 0.05). Furthermore, patients in the TCM group had a lower proportion of methylprednisolone pulses than those in the control group (1.3 ± 1.41 vs 3.05 ± 2.02, P < 0.0001). The ending GC dose was significantly lower in the TCM group than in the control group (P < 0.001). Moreover, more hepatic function damage, gastrointestinal adverse effects, and hypertension were observed in the control group than in the TCM group (P < 0.05).
The findings suggest that DBXF treatment is effective and safe as a supplementary therapy for LN; moreover, this treatment is superior to routine GC and CYC therapies. DBXF containing combination treatment may lead to faster proteinuria and hematuria resolution, smoother GC reduction, fewer methylprednisolone pulses, and fewer adverse events.
The integrated treatment of TCM has been indicated to be beneficial for LN. To increase effectiveness and reduce side effects during the active phase of LN, immunosuppression can be used in conjunction with TCM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bacharaki D, Greece; Cheng TH, Taiwan S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic Inflammation and Its Therapeutic Targeting in Systemic Lupus Erythematosus. Front Immunol. 2015;6:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Hersh AO, von Scheven E, Yazdany J, Panopalis P, Trupin L, Julian L, Katz P, Criswell LA, Yelin E. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2009;61:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Kulkarni OP, Anders HJ. Lupus nephritis. How latest insights into its pathogenesis promote novel therapies. Curr Opin Rheumatol. 2012;24:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Yang Z, Xie RF, Zhong MH, Xie GQ, Fan YS, Zhao T. Potential Molecular Mechanisms of Zhibai Dihuang Wan in Systemic Lupus Erythematosus Based on Network Biology. Evid Based Complement Alternat Med. 2020;2020:7842179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Ren HJ, Sun YL, Yuan B. Chinese patent medicine Bailing capsule for treating lupus nephritis: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e17041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Tu J, Guo Y, Hong W, Fang Y, Han D, Zhang P, Wang X, Körner H, Wei W. The Regulatory Effects of Paeoniflorin and Its Derivative Paeoniflorin-6'-O-Benzene Sulfonate CP-25 on Inflammation and Immune Diseases. Front Pharmacol. 2019;10:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Zhao Y, Mu Z, Cai L, Liu X, Jia J, Zhang J. Tetra-arsenic tetra-sulfide ameliorates lupus syndromes by inhibiting IL-17 producing double negative T cells. Dermatol Ther. 2019;32:e12849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Zhou W, Wu Y, Pan M, Liu D, Liu B. Proliferation and Migration of Lung Cancer Could be Inhibited by Oxymatrine through the Regulation for miR-520/VEGF. Am J Chin Med. 2019;47:865-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Jung YY, Shanmugam MK, Narula AS, Kim C, Lee JH, Namjoshi OA, Blough BE, Sethi G, Ahn KS. Oxymatrine Attenuates Tumor Growth and Deactivates STAT5 Signaling in a Lung Cancer Xenograft Model. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 10. | Shibeshi W, Makonnen E, Zerihun L, Debella A. Effect of Achyranthes aspera L. on fetal abortion, uterine and pituitary weights, serum lipids and hormones. Afr Health Sci. 2006;6:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | A brief introduction to Beijing Children's Hospital. Pediatr Investig. 2017;1:5-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2816] [Cited by in RCA: 3539] [Article Influence: 272.2] [Reference Citation Analysis (0)] |
| 13. | Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288-291. [PubMed] |
| 14. | An Y, Zhou Y, Bi L, Liu B, Wang H, Lin J, Xu D, Wang M, Zhang J, Wang Y, An Y, Zhu P, Xie R, Zhang Z, Mei Y, Liu X, Deng X, Yao Z, Xiao W, Shen H, Yang X, Xu H, Yu F, Wang G, Lu X, Li Y, Zuo X, Liu Y, Zhao Y, Guo J, Sun L, Zhao M, Li Z. Combined immunosuppressive treatment (CIST) in lupus nephritis: a multicenter, randomized controlled study. Clin Rheumatol. 2019;38:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 16. | Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ; Collaborative Study Group. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Chan TM. Treatment of severe lupus nephritis: the new horizon. Nat Rev Nephrol. 2015;11:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Arıcı ZS, Batu ED, Ozen S. Reviewing the recommendations for lupus in children. Curr Rheumatol Rep. 2015;17:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Lin TJ, Wu CY, Tsai PY, Hsu WH, Hua KF, Chu CL, Lee YC, Chen A, Lee SL, Lin YJ, Hsieh CY, Yang SR, Liu FC, Ka SM. Accelerated and Severe Lupus Nephritis Benefits From M1, an Active Metabolite of Ginsenoside, by Regulating NLRP3 Inflammasome and T Cell Functions in Mice. Front Immunol. 2019;10:1951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Wu PW, Shih PH, Kung YY, Chen FP, Chang CM. Integrated therapy improve urinary total protein in patients with lupus nephritis: A case report. Complement Ther Med. 2018;39:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Chang CM, Wu PC, Chiang JH, Wei YH, Chen FP, Chen TJ, Pan TL, Yen HR, Chang HH. Integrative therapy decreases the risk of lupus nephritis in patients with systemic lupus erythematosus: A population-based retrospective cohort study. J Ethnopharmacol. 2017;196:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Ka SM, Lin JC, Lin TJ, Liu FC, Chao LK, Ho CL, Yeh LT, Sytwu HK, Hua KF, Chen A. Citral alleviates an accelerated and severe lupus nephritis model by inhibiting the activation signal of NLRP3 inflammasome and enhancing Nrf2 activation. Arthritis Res Ther. 2015;17:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, Frangou E, Houssiau FA, Hollis J, Karras A, Marchiori F, Marks SD, Moroni G, Mosca M, Parodis I, Praga M, Schneider M, Smolen JS, Tesar V, Trachana M, van Vollenhoven RF, Voskuyl AE, Teng YKO, van Leew B, Bertsias G, Jayne D, Boumpas DT. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |