Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11381
Peer-review started: September 9, 2022
First decision: September 19, 2022
Revised: September 26, 2022
Accepted: October 9, 2022
Article in press: October 9, 2022
Published online: November 6, 2022
Processing time: 48 Days and 1.2 Hours
The relationship between C-reactive protein (CRP) levels and prolonged intensive care unit (ICU) length of stay (LoS) has not been well defined.
To explore the association between CRP levels at ICU admission and prolonged ICU LoS in gastrointestinal cancer (GC) patients after major surgery.
A retrospective study was performed to quantify serum CRP levels and to establish their association with prolonged ICU LoS (≥ 72 h) in GC patients admitted to the ICU. Univariate and multivariate regression analyses were conducted, and restricted cubic spline curves with four knots (5%, 35%, 65%, 95%) were used to explore non-linearity assumptions.
A total of 408 patients were enrolled. Among them, 83 (20.3%) patients had an ICU LoS longer than 72 h. CRP levels were independently associated with the risk of prolonged ICU LoS [odds ratio (OR) 1.47, 95% confidence interval (CI) 1.00–2.17]. Restricted cubic spline analysis revealed a non-linear relationship between CRP levels and OR for the prolonged ICU LoS (P = 0.035 for non-linearity). After the cut-off of 2.6 (log transformed mg/L), the OR for prolonged ICU LoS significantly increased with CRP levels. The adjusted regression coefficient was 0.70 (95%CI 0.31–1.57, P = 0.384) for CRP levels less than 2.6, whereas it was 2.43 (95%CI 1.39–4.24, P = 0.002) for CRP levels higher than 2.6.
Among the GC patients, CRP levels at ICU admission were non-linearly associated with prolonged ICU LoS in survivors. An admission CRP level > 2.6 (log transformed mg/L) was associated with increased risk of prolonged ICU LoS.
Core Tip: Among the gastric cancer patients, C-reactive protein (CRP) levels at intensive care unit (ICU) admission were non-linearly associated with prolonged ICU length of stay (LoS) in survivors. An admission CRP level > 2.6 (log transformed mg/L) was associated with increased risk for prolonged ICU LoS.
- Citation: Yan YM, Gao J, Jin PL, Lu JJ, Yu ZH, Hu Y. C-reactive protein as a non-linear predictor of prolonged length of intensive care unit stay after gastrointestinal cancer surgery. World J Clin Cases 2022; 10(31): 11381-11390
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11381.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11381
Gastrointestinal cancer (GC) surgery is associated with high postoperative complications, and requires prolonged intensive care unit (ICU) length of stay (LoS), especially for high-risk patients[1]. Prolonged ICU LoS can increase the consumption of healthcare resources and often leads to adverse immediate outcomes, which increases the short-term and long-term morbidity as well as mortality[2,3]. Therefore, it is important to develop strategies to predict ICU LoS thereby improve the management of beds, staff, and identify individual patients with unexpectedly long ICU LoS[4,5]. Although multiple models for predicting case-mix–adjusted ICU LoS have been published, Verburg et al[5] indicated that no model can satisfactorily satisfy the above requirements. Moreover, attributable factors may have occurred during ICU stay or patients’ responses to ICU-associated insults.
A potential risk factor for prolonged ICU LoS is the inflammatory status of patients. Surgical intervention is associated with direct mechanical tissue injury, and can also induce the activation of innate and adaptive immune components. Excessive inflammatory responses among ICU patients are associated with prolonged LoS and increased treatment costs[6]. Inflammatory markers regulate the complex network of inflammatory responses. Mean interleukin (IL)-6 Levels in coronavirus disease 2019 (COVID-19) patients admitted for less than 7 d were significantly suppressed relative to those hospitalized for more than 7 d[7]. In our previous study, critically ill patients exhibited high levels of IL-2, IL-6, IL-8, and TNF-α in the first 24 h post-operatively, and this was associated with poor clinical outcomes[8]. Adverse outcomes are correlated with prolonged ICU LoS[3]. Therefore, serum inflammatory biomarkers are potential predictors for ICU LoS and patient outcomes.
C-reactive protein (CRP) analysis is simple and reproducible. CRP levels are associated with ongoing organ dysfunction, and can act as a specific prognostic indicator for predicting the mortality of COVID-19 patients[9]. It may be an Acute Physiology and Chronic Health Evaluation (APACHE) independent risk factor for mortality in medical ICU populations[10]. However, the relationship between CRP levels at ICU admission and prolonged ICU LoS in GC patients after major surgery has not been well defined.
In this study, the association between CRP levels at ICU admission and prolonged ICU LoS were evaluated. Our hypothesis was that CRP levels are potential biomarkers for predicting ICU LoS in GC patients after major surgery.
This was a retrospective study involving adult patients subjected to gastrointestinal cancer surgery and admitted to a 28-bed surgical ICU ward between January 1, 2018 and June 30, 2021 in Zhongshan Hospital, Fudan University.
Gastrointestinal cancer patients who had been discharged from the surgical ICU ward alive were enrolled, and divided into two pre-defined groups according to their ICU LoS: Those with a prolonged ICU stay of 3 d or more and those with an ICU stay of less than 3 d[3,11]. For patients with 2 or more episodes of ICU admission, we only analyzed the first episode, and patients were divided according to the number of days spent in the ICU during admission. The exclusion criteria were: (1) Patients younger than 18 years of age; (2) Missing CRP data at ICU admission; and (3) Loss of follow-up during the ICU stay period.
Standard surgical management was performed by a team of surgeons, nurses, anesthetists in surgery center, and standard postoperative management was performed by a team of intensivists, nurses, rehabilitation therapists, and respiratory therapists in the surgical ICU ward. If the intensivist considered the disease condition to be stable enough and that the patient no longer requires ICU-specific treatment and care, the patient was transferred out of the ICU.
Blood samples were collected at the time of ICU admission and analyzed within 4 h. High-sensitivity CRP levels were analyzed using the latex-enhanced immunoturbidimetric method on a Cobas c702 analyzer (Roche Diagnostics). Normal ranges were set at 0-3 mg/L. All assays were performed in the same laboratory.
All patients’ data were extracted from the electronic medical records system, anonymized, and deidentified before analysis. The Ethical Committee of Human Experimentation of Zhongshan Hospital, Fudan University approved this study (No. B2020-107R).
The following data were collected on the first day after admission to the surgical ICU ward: Socio-demographic information (age, sex); type of surgery (elective or emergency surgery); cancer site; CRP levels at ICU admission, type and length of organ support [e.g., continuous renal replacement therapy (CRRT), mechanical ventilation therapy]; APACHE II scores, ICU readmission and clinical outcomes. Data were collected from admission to death or discharge from SICU by trained health providers.
For continuous variables, normally distributed data are presented as mean ± standard deviation (SD) and analyzed by the Student’s independent t-test whereas non-normally distributed data are presented as medians and interquartile ranges (IQR: 25th-75th percentile) and analyzed by the Wilcoxon rank-sum test (Mann-Whitney U test). Categorical variables are presented as numbers (percentages, %), and compared using the chi-square or Fisher’s exact tests, as appropriate. The average value was used to interpolate the missing data.
Logistic regression models were used to investigate the association between CRP levels and prolonged ICU LoS in both univariate and multivariate analyses. Unadjusted, moderately adjusted, and fully adjusted models were established. In the moderately adjusted model, CRP levels at ICU admission were individually entered and adjusted for age, gender, and laparoscopic surgery. In the fully adjusted model, age, gender, laparoscopic surgery, emergency surgery, cancer site, ICU readmission, tracheotomy, CRRT therapy, and APACHE II variables were adjusted.
Then, restricted cubic spline curves with four knots (5%, 35%, 65%, 95%) were used to assess linearity or non-linearity assumption between CRP levels and prolonged ICU LoS. A two-piecewise multivariable logistic regression model was used to assess the effects of CRP levels on prolonged ICU LoS. Using a tail and error approach, the cut-off level of CRP, at which level the relationship between CRP levels and prolonged ICU LoS began to change, was determined.
A predefined analysis was conducted according to the key subgroups, and the results are presented in a forest plot.
The SPSS version 26.0 (SPSS Inc., Chicago, IL, United States) and R version 3.5.1 (R Center for Statistical Computing, Vienna, Austria) software were used for analyses. All significance tests were two-sided and P < 0.05 indicated significance.
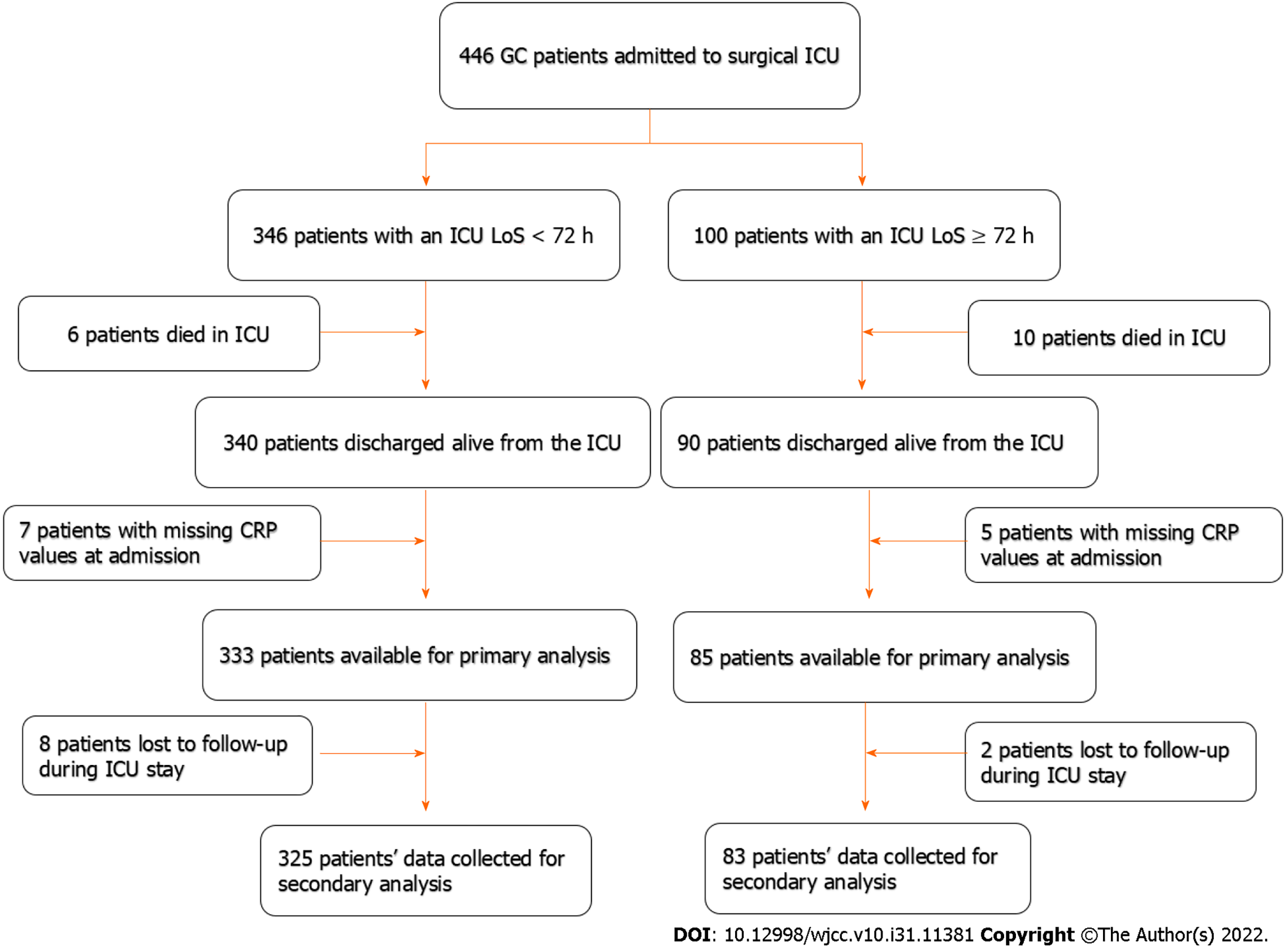
A total of 430 gastric cancer patients were discharged alive from the surgical ICU ward. The flowchart for this study is shown in Figure 1. After exclusion, 408 patients were included in this study. Among them, 325 (79.7%) patients had an ICU LoS shorter than 72 h, while 83 (20.3%) patients had an ICU LoS longer than 72 h. The demographic and clinical characteristics of patients are presented in Table 1. Patients with ICU LoS longer than 72 h were associated with higher rates of emergency surgery (28.92% vs 15.38%, P = 0.004), tracheotomy therapy (19.28% vs 0.62%, P < 0.001), mechanical ventilation (49.40% vs 11.08%, P < 0.001), APACHE II scores (12.34 ± 5.25 vs 9.77 ± 4.31, P < 0.001), and CRP values (4.67 ± 0.88 vs 3.95 ± 1.21, P < 0.001).
| Variables | ICU LoS < 72 h (n = 325), % | ICU LoS ≥ 72 h (n = 83), % | P value |
| Age (yr) | 73.38 ± 11.31 | 74.18 ± 10.80 | 0.56 |
| Sex | 0.363 | ||
| Male | 218 (67.08) | 60 (72.29) | |
| Female | 107 (32.92) | 23 (27.71) | |
| Laparoscopic surgery | 0.221 | ||
| No | 250 (76.92) | 69 (83.13) | |
| Yes | 75 (23.08) | 14 (16.87) | |
| Emergency surgery | 0.004 | ||
| No | 275 (84.62) | 59 (71.08) | |
| Yes | 50 (15.38) | 24 (28.92) | |
| Cancer site | 0.896 | ||
| Stomach | 162 (49.84) | 39 (46.99) | |
| Rectum | 41 (12.62) | 11 (13.25) | |
| Colon | 102 (31.38) | 26 (31.33) | |
| Duodenum | 20 (6.15) | 7 (8.43) | |
| ICU readmission | 0.775 | ||
| No | 308 (94.77) | 78 (93.98) | |
| Yes | 17 (5.23) | 5 (6.02) | |
| Tracheotomy | < 0.001 | ||
| No | 323 (99.38) | 67 (80.72) | |
| Yes | 2 (0.62) | 16 (19.28) | |
| Mechanical ventilation | < 0.001 | ||
| No | 289(88.92) | 42(50.60) | |
| Yes | 36(11.08) | 41(49.40) | |
| CRRT therapy | 0.442 | ||
| No | 318 (97.85) | 80 (96.39) | |
| Yes | 7 (2.15) | 3 (3.61) | |
| APACHE II scores | 9.77 ± 4.31 | 12.34 ± 5.25 | < 0.001 |
| CRP values | 3.95 ± 1.21 | 4.67 ± 0.88 | < 0.001 |
The multivariable regression analysis results shown in Table 2 indicate that CRP levels at ICU admission were significantly associated with prolonged ICU LoS. Strong correlation with OR [95% confidence interval (CI)] of 2.12 (1.52–2.98) was seen in the crude model. In the moderately adjusted model, the association was more evident after adjustment for age, gender, laparoscopic surgery with OR (95%CI) of 2.30 (1.56–3.38). A significant association with OR (95%CI) of 1.47 (1.00–2.17) remained after adjustments for age, gender, laparoscopic surgery, emergency surgery, cancer site, ICU readmission, tracheotomy, CRRT therapy, mechanical ventilation therapy, and APACHE II scores in the fully adjusted model. A significant trend (P Trend < 0.05) across tertiles was observed among the three models.
| Exposure variable | Crude model | Multivariable-adjusted model 1 | Multivariable-adjusted model 2 | |||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| CRP | 2.12 (1.52, 2.98) | < 0.001 | 2.30 (1.56, 3.38) | < 0.001 | 1.47 (1.00, 2.17) | 0.014 |
| Tertiles | ||||||
| T1 | 1.0 | -- | 1.0 | -- | 1.0 | -- |
| T2 | 2.19 (0.84, 5.74) | 0.109 | 2.29 (0.78, 6.72) | 0.130 | 2.43 (0.62, 9.47) | 0.200 |
| T3 | 8.13 (3.36, 19.66) | < 0.001 | 10.03 (3.61, 27.83) | < 0.001 | 6.51 (1.74, 24.34) | 0.005 |
| Trend analysis | 3.02 (1.96, 4.65) | < 0.001 | 3.42 (2.07, 5.63) | < 0.001 | 2.57 (1.35, 4.88) | 0.004 |
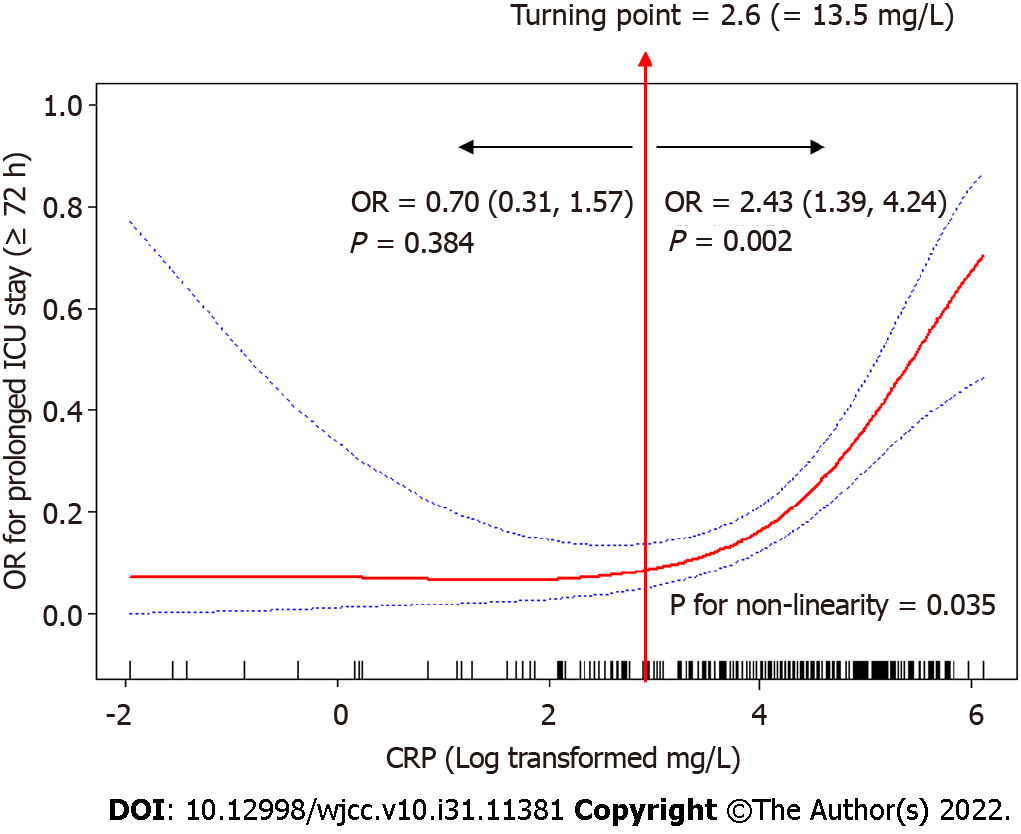
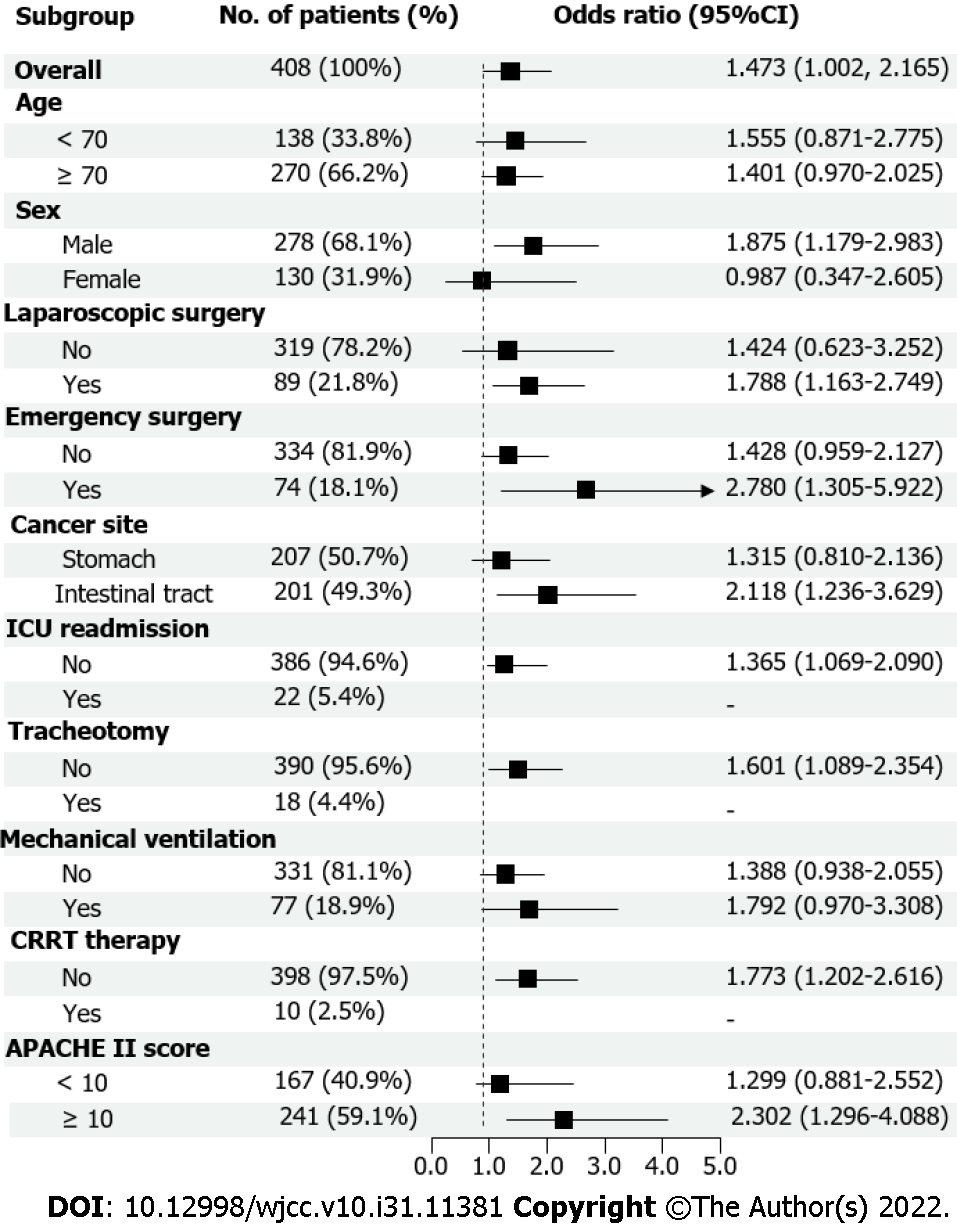
Restricted cubic spline curves analysis revealed a non-linear relationship between CRP levels and odd ratio (OR) for prolonged ICU LoS (P = 0.035 for non-linearity). The OR for prolonged ICU LoS significantly increased with the CRP levels after the 2.6 (log transformed mg/L) cut-off level. The adjusted regression coefficient was 0.70 (95%CI 0.31–1.57, P = 0.384) for CRP level less than 2.6 (log transformed mg/L), while it was 2.43 (95%CI 1.39–4.24, P = 0.002) for CRP level higher than 2.6 (log transformed mg/L) (Figure 2). Results of the comparisons of the prespecified subgroups such as age, sex, laparoscopic surgery, emergency surgery, cancer site, ICU readmission, tracheotomy, mechanical ventilation, CRRT therapy, APACHE II scores are shown in Figure 3.
In this study, we found prolonged ICU LoS in 20.3% of the patients, indicating that CRP levels at ICU admission in GC cancer patients after surgery is a strong predictor of prolonged ICU LoS. Moreover, CRP levels exhibited a non-linear relationship with prolonged ICU LoS, and the cut-off level was 2.6 (log transformed mg/L), which equals to 13.5 mg/L, implying that CRP levels higher than 13.5 mg/L are potential predictors of a higher risk of prolonged ICU LoS.
Prolonged ICU LoS refers to a period longer than 2 d (the day of surgery + 1 d) because based on the protocol in our center, a standard ICU stay is 1 or 2 d. A short ICU stay was associated with shorter overall hospital admission time and did not negatively impact short-term surgical outcomes[12]. Prolonged ICU LoS is a risk factor for mortality[2]. We found a higher mortality rate for the prolonged ICU LoS (≥ 72 h) compared with the ICU LoS shorter than 72 h (10.0% vs 1.7%). Moreover, patients with ICU LoS ≥ 72 h had a higher rate of emergency surgery, tracheotomy therapy, mechanical ventilation, APACHE II score, and CRP values.
Patients with a prolonged ICU LoS have higher incidences of infections caused by multi-drug resistant microorganisms[13]. As a major acute phase protein, severe inflammatory induces the expression of CRP[14]. The ability of CRP to predict mortality in ICU patients has been widely investigated. In a prospective study, elevated CRP level at ICU admission was associated with increased risks of organ failure and mortality, and persistently high levels were associated with poor clinical outcomes[15]. In sepsis patients older than 75 years, CRP was found to be an independent predictor of mortality, and the additional effects of CRP to APACHE II score can significantly improve prognostication[16]. In surgical critically ill patients, APACHE II, Sequential Organ Failure Assessment (SOFA), and Simplified Acute Physiology Scores scores showed a better predictive performance with regards to mortality outcomes, compared to CRP[17]. However, in B-cell lymphoma patients treated with axicabtagene ciloleucel, there was no correlation between CRP levels at ICU admission and length of ICU stay[18].
We found that a CRP level of 2.6 (log transformed mg/L), which equals to 13.5 mg/L, could stratify the association between CRP levels and prolonged ICU LoS. The non-linear relationship showed that OR of prolonged ICU LoS increased with increasing CRP levels after the 13.5 mg/L cut-off level. Therefore, attention should be paid to patients with elevated CRP levels, especially those higher than 13.5 mg/L. Previous studies have also evaluated the clinical cut-off levels of CRP. In older population, elevated CRP level (> 3.0 mg/L) was associated with a 1.45-fold increased 10-year risk of coronary heart disease[19]. In a retrospective cohort study, elevated CRP (> 75 mg/L) at ICU discharge served as a moderate risk factor and is not recommended for individual clinical decision-making[20]. In a Swedish multicenter study, higher CRP levels (> 100 mg/L) at ICU admission were associated with increased ICU mortality and prolonged ICU LoS (> 3 d)[21]. Differences in outcomes may be attributed to the nature of the study population and test systems used. These findings imply that CRP is a potential predictor of patients at a high risk of mortality and prolonged ICU LoS.
The most important concern when assessing the relationship between CRP levels and prolonged ICU LoS is the reverse causality related to mortality. For instance, patients who died in ICU had higher levels of biomarkers (including CRP) at admission[22], and they especially died within 48 h after admission, thus, they may have falsely been included in the non-prolonged ICU LoS group because of the short ICU stay. To minimize bias, patients who died in ICU were excluded from this study. Another concern is that confounding variables may influence the association between CRP levels and prolonged ICU LoS, for example, APACHE scores. Thus, we adjusted for potential confounders in the fully adjusted model, and the results also showed a statistical difference.
In this study, we found a non-linear relationship between CRP levels and prolonged ICU LoS using restricted cubic spline curves. However, there are several limitations that should be explored. First, this was a retrospective and single-center study focused on GC cancer. It is not clear whether our findings can be generalized to other populations or ICU settings. Second, CRP levels were measured only at ICU admission, therefore, we could not investigate the effect of dynamic CRP level changes on prolonged ICU LoS. Third, although we confounded many factors through multivariate logistic regression analysis, CRP levels may still be a predictor for other unknown processes. Models based on combinations of risk factors and biomarkers may be more effective in predicting prolonged ICU LoS or mortality. Future studies should explore this aspect.
Among GC cancer patients, CRP levels at ICU admission are non-linearly associated with prolonged ICU LoS in survivors. An admission CRP level > 2.6 (log transformed mg/L) is associated with increased risk of prolonged ICU LoS. Thus, intervention trials should be performed to confirm whether low CRP levels can decrease the risk of prolonged ICU LoS.
C-reactive protein (CRP) levels are associated with ongoing organ dysfunction. It may be an Acute Physiology and Chronic Health Evaluation (APACHE) independent risk factor for mortality in medical intensive care unit (ICU) populations. However, the relationship between CRP levels at ICU admission and prolonged ICU length of stay (LoS) in gastric cancer patients after surgery has not been well defined.
In this study, our hypothesis was that CRP levels are potential biomarkers for predicting ICU LoS in GC patients after surgery. The findings were important to develop strategies to predict ICU LoS thereby improve the management of beds, staff, and identify individual patients with unexpectedly long ICU LoS.
In this study, the association between CRP levels at ICU admission and prolonged ICU LoS were evaluated.
A retrospective study was performed to quantify serum CRP levels and to establish their association with prolonged ICU LoS (longer than 72 h) in GC patients admitted to the ICU. Univariate and multivariate regression analyses were conducted, and restricted cubic spline curves with four knots (5%, 35%, 65%, 95%) were used to explore non-linearity assumptions.
A total of 408 patients were enrolled. Among them, 83 (20.3%) patients had an ICU LoS longer than 72 h. CRP levels were independently associated with the risk of prolonged ICU LoS [odds ratio (OR) 1.47, 95% confidence interval (CI) 1.00–2.17]. Restricted cubic spline analysis revealed a non-linear relationship between CRP levels and OR for the prolonged ICU LoS (P = 0.035 for non-linearity). After the cut-off of 2.6 (log transformed mg/L), the OR for prolonged ICU LoS significantly increased with CRP levels. The adjusted regression coefficient was 0.70 (95%CI 0.31–1.57, P = 0.384) for CRP levels less than 2.6, whereas it was 2.43 (95%CI 1.39–4.24, P = 0.002) for CRP levels higher than 2.6.
Among the GC patients, CRP levels at ICU admission were non-linearly associated with prolonged ICU LoS in survivors. An admission CRP level > 2.6 (log transformed mg/L) was associated with increased risk of prolonged ICU LoS.
CRP levels may still be a predictor for other unknown processes, models based on combinations of risk factors and biomarkers may be more effective in predicting prolonged ICU LoS or mortality. Future studies should explore this aspect.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iglesias J, United States; Jiraviriyakul A, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Jakobson T, Karjagin J, Vipp L, Padar M, Parik AH, Starkopf L, Kern H, Tammik O, Starkopf J. Postoperative complications and mortality after major gastrointestinal surgery. Medicina (Kaunas). 2014;50:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Chaudhary MA, Schoenfeld AJ, Koehlmoos TP, Cooper Z, Haider AH. Prolonged ICU stay and its association with 1-year trauma mortality: An analysis of 19,000 American patients. Am J Surg. 2019;218:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Mahesh B, Choong CK, Goldsmith K, Gerrard C, Nashef SA, Vuylsteke A. Prolonged stay in intensive care unit is a powerful predictor of adverse outcomes after cardiac operations. Ann Thorac Surg. 2012;94:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Rapoport J, Teres D, Zhao Y, Lemeshow S. Length of stay data as a guide to hospital economic performance for ICU patients. Med Care. 2003;41:386-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Verburg IW, Atashi A, Eslami S, Holman R, Abu-Hanna A, de Jonge E, Peek N, de Keizer NF. Which Models Can I Use to Predict Adult ICU Length of Stay? Crit Care Med. 2017;45:e222-e231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Nassar AP Jr, Caruso P. ICU physicians are unable to accurately predict length of stay at admission: a prospective study. Int J Qual Health Care. 2016;28:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Taher Al Barzin RMG, Ghafour Raheem S, Khudhur PK, Abdulkarimi R, Mohammadnejad E, Tabatabaee A. Interleukin-6 role in the severity of COVID-19 and intensive care unit stay length. Cell Mol Biol (Noisy-le-grand). 2020;66:15-18. [PubMed] |
| 8. | Yan Y, Hu Y, Wang X, Yu Z, Tang Y, Zhang Y, Pan W. The predictive prognostic values of serum interleukin-2, interleukin-6, interleukin-8, tumor necrosis factor-α, and procalcitonin in surgical intensive care unit patients. Ann Transl Med. 2021;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ali A, Noman M, Guo Y, Liu X, Zhang R, Zhou J, Zheng Y, Zhang XE, Qi Y, Chen X, Men D. Myoglobin and C-reactive protein are efficient and reliable early predictors of COVID-19 associated mortality. Sci Rep. 2021;11:5975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Wang F, Pan W, Pan S, Wang S, Ge Q, Ge J. Usefulness of N-terminal pro-brain natriuretic peptide and C-reactive protein to predict ICU mortality in unselected medical ICU patients: a prospective, observational study. Crit Care. 2011;15:R42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kongsayreepong S, Lomarat N, Thamtanavit S, Sodapak C, Vongvises T, Kueaphet S, Saeheng S, Komoltri C. Predictors of Prolonged Length of Stay in General Surgical Intensive Care Unit. J Med Assoc Thai. 2016;99 Suppl 6:S47-S54. [PubMed] |
| 12. | Voeten DM, van der Werf LR, Gisbertz SS, Ruurda JP, van Berge Henegouwen MI, van Hillegersberg R; Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. Postoperative intensive care unit stay after minimally invasive esophagectomy shows large hospital variation. Results from the Dutch Upper Gastrointestinal Cancer Audit. Eur J Surg Oncol. 2021;47:1961-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Soares M, Salluh JIF, Torres VBL, Leal JVR, Spector N. Short- and long-term outcomes of critically ill patients with cancer and prolonged ICU length of stay. Chest. 2008;134:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Pathak A, Agrawal A. Evolution of C-Reactive Protein. Front Immunol. 2019;10:943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Lobo SM, Lobo FR, Bota DP, Lopes-Ferreira F, Soliman HM, Mélot C, Vincent JL. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest. 2003;123:2043-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Li H, Shan-Shan Z, Jian-Qiang K, Ling Y, Fang L. Predictive value of C-reactive protein and NT-pro-BNP levels in sepsis patients older than 75 years: a prospective, observational study. Aging Clin Exp Res. 2020;32:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Basile-Filho A, Lago AF, Menegueti MG, Nicolini EA, Rodrigues LAB, Nunes RS, Auxiliadora-Martins M, Ferez MA. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: A retrospective cohort study. Medicine (Baltimore). 2019;98:e16204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Melody M, Rahman ZA, Saunders H, Diaz PL, Gannon N, Rosenthal A, Ayala E, Tun HW, Murthy H, Roy V, Foran J, Castro JE, Guru P, Kharfan-Dabaja MA. C-reactive protein and ferritin levels and length of intensive care unit stay in patients with B-cell lymphomas treated with axicabtagene ciloleucel. Hematol Oncol Stem Cell Ther. 2021;14:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Gülcher SS, Bruins NA, Kingma WP, Boerma EC. Elevated C-reactive protein levels at ICU discharge as a predictor of ICU outcome: a retrospective cohort study. Ann Intensive Care. 2016;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Koozi H, Lengquist M, Frigyesi A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J Crit Care. 2020;56:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Yan Y, Yu Z, Lu J, Jin P, Tang Z, Hu Y. Predictive values profiling of interleukin-2, interleukin-8, tumor necrosis factor-α, procalcitonin, and C-reactive protein in critical gastrointestinal cancer patients. J Gastrointest Oncol. 2021;12:1398-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |