Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.790
Peer-review started: August 17, 2021
First decision: November 8, 2021
Revised: November 17, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: January 21, 2022
Processing time: 151 Days and 4.2 Hours
Intrahepatic cholangiocarcinoma (ICC) is malignancies of the biliary duct system and constitutes approximately 10%-20% of all primary liver cancers. Tumor mutation burden (TMB) is a useful biomarker across many cancer types for the identification of patients who will benefit from immunotherapy. Despite the role of TMB in calculating the effectiveness and prognosis of immune checkpoint inhibitors has been confirmed in multiple human cancer types, the prognostic value of TMB in ICC patients is rare investigated.
To investigate the prognostic value of TMB in patients with ICC.
Data of 412 patients with ICC were included in the study. TMB was calculated as the total number of somatic non-silent protein-coding mutations divided by the coding region. The Kaplan-Meier method was used to analyze overall survival (OS), and relapse free survival (RFS). The cut-off value of TMB was determined by time-dependent receiver operating characteristic (ROC) curve. Cox regression was performed for multivariable analysis of OS. The nomogram and calibration curve were analyzed to construct and evaluate the prognostic model.
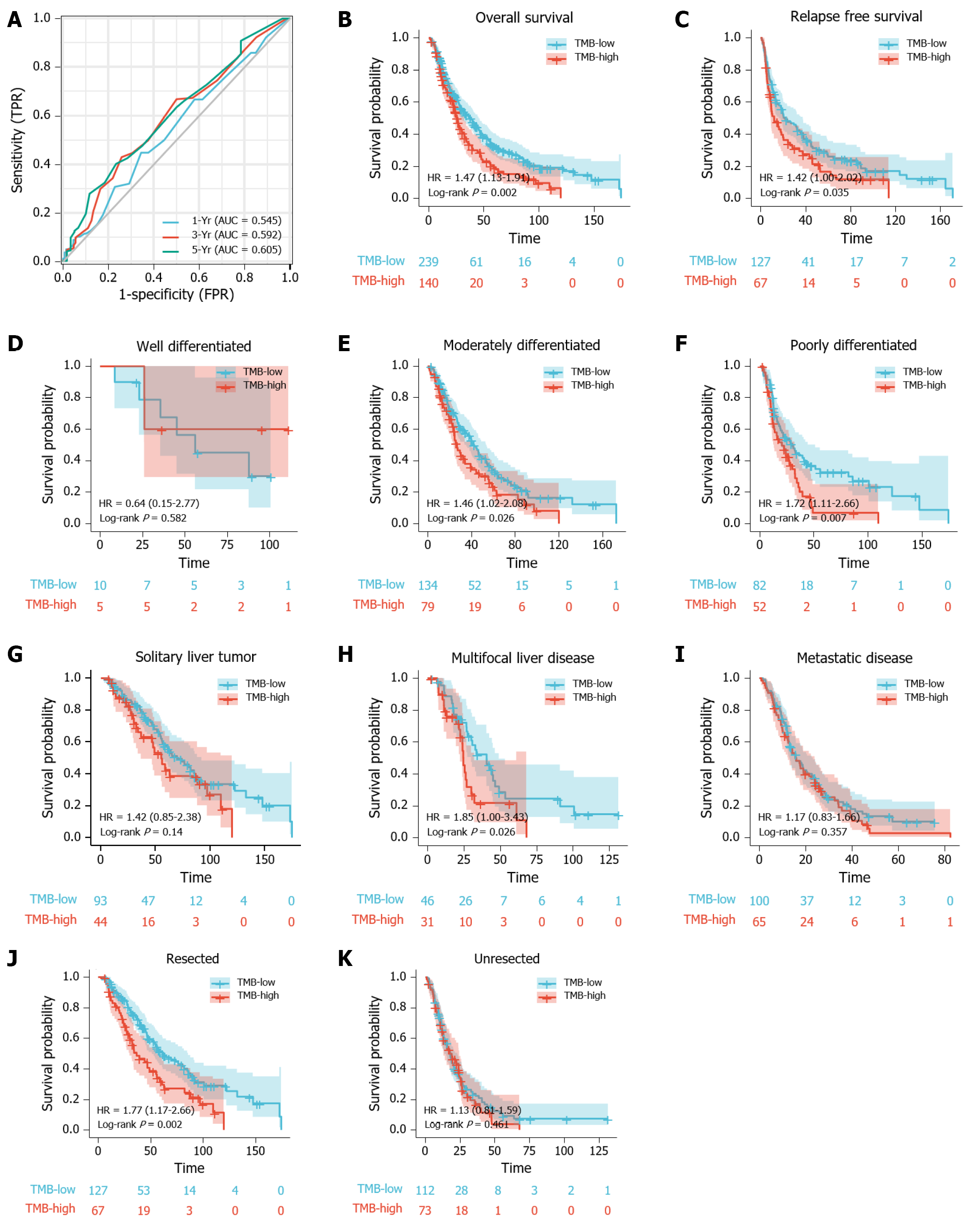
In the analysis of the time-dependent ROC curve, we defined 3.1 mut/Mb as the cut-off value of TMB. The Kaplan-Meier plot revealed that patients with high TMB had poor OS (HR = 1.47, P = 0.002) and RFS (HR = 1.42, P = 0.035). Cox regression analysis also demonstrated that TMB was an independent risk predictor for ICC (HR = 1.43, P = 0.0240). Furthermore, independent prognostic factors of ICC included CA19-9 (HR = 1.78, P = 0.0005), chronic viral hepatitis (HR = 1.72, P = 0.0468), tumor resection (HR = 2.58, P < 0.0001) and disease progression (metastatic disease vs. solitary liver tumor; HR = 2.55, P = 0.0002). The nomogram and calibration curve also indicated the effectiveness of the constructed prognostic model.
TMB was an independent prognostic biomarker in patients with ICC. Moreover, patients with ICC with high TMB had poor OS and RFS as compared to those with low TMB.
Core Tip: We analyzed the data of 412 patients with intrahepatic cholangiocarcinoma (ICC) from the Memorial Sloan Kettering Cancer Center cohort in the study. ICC patients with high tumor mutation burden (TMB) indicated a poor overall survival (OS) and relapse free survival compared with those with those with low TMB. Cox regression analysis of patient OS also demonstrated that TMB was an independent risk predictor for ICC. The nomogram and calibration curve also indicated the effectiveness of the constructed prognostic model.
- Citation: Song JP, Liu XZ, Chen Q, Liu YF. High tumor mutation burden indicates a poor prognosis in patients with intrahepatic cholangiocarcinoma. World J Clin Cases 2022; 10(3): 790-801
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/790.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.790
Cholangiocarcinomas are malignancies of the biliary duct system, classified as being either intrahepatic or extrahepatic in origin. Particularly, intrahepatic cholangiocarcinoma (ICC) constitutes approximately 10%-20% of all primary liver cancers[1]. Despite its increasing incidence rate worldwide, the etiology of ICC remains unclear[2]. Moreover, although surgery is the only potentially curative treatment for ICC, more than two-thirds of patients have been found to be unsuitable for surgery at the time of diagnosis, and more than 60% of patients who underwent surgery reported relapse of disease[3]. A previous study also showed that the 5-year survival rate and median survival time of patients with ICC (hereinafter, ICC patients) who underwent curative resection was approximately 30% and 28 mo, respectively[4]. Besides surgical resection, the standard treatment for ICC includes gemcitabine-based chemotherapy, liver transplantation, and local treatment, such as transarterial chemoembolization[5]. Of the several prognostic factors of ICC, radical resection (R0), number of tumors (single or multiple), vascular invasion, and lymph node metastasis have all been recognized as the most important independent prognostic predictors for ICC patients[6].
Multiple studies have also demonstrated that tumor mutation burden (TMB), defined as the total number of somatic coding errors, base substitutions, and indel mutations per million bases[7], can effectively estimate both overall mutational and neoantigen load[8]. Recent studies have shown that TMB is associated with immunotherapy response, since it reflects the overall neoantigen load[9-11]. Moreover, TMB can be used to predict immune checkpoint inhibitor (ICI) therapy, acting as a useful biomarker across many cancer types for the identification of patients who will benefit from immunotherapy[12,13]. In addition to the identification of patients viable for immunotherapy, TMB has also been shown to be an indicator of immunotherapy efficacy. Specifically, high TMB is associated with higher rates of treatment response and longer survival among patients who received treatment with ICIs[14-16]. However, among patients who did not receive ICI treatment, high TMB was generally associated with poorer overall survival in many cancer types[17]. Furthermore, despite the role of TMB in calculating the effectiveness and prognosis of ICIs has been confirmed in multiple human cancer types, the prognostic value of TMB in ICC patients is rare investigated.
Therefore, in this study, we used the ICC database from the Memorial Sloan Kettering (MSK) Cancer Center to investigate the impact of TMB on the prognosis of ICC patients in combination with other clinical features, confirming that TMB was an independent prognostic factor for ICC patients.
Data of 412 ICC patients from the MSK Cancer Center cohort (MSK cohort: http://www.cbioportal.org/study/summary?id=ihch_msk_2021) were included[18]. TMB was calculated as the total number of somatic, non-silent, protein-coding mutations divided by the coding region captured in each MSK-IMPACT panel (341 genes, 0.98 Mb; 410 genes, 1.06 Mb; 468 genes, 1.22 Mb). Ethics approval and patient consent were waived by the MSKCC Institutional Review Board and the need for informed consent has been waived by the MSKCC IRB per 45 CFR 46.116 and 45 CFR 164.512, since our data were retrieved from a public database. Clinicopathological information, including age, gender, BMI, TMB, CA19-9, chronic viral hepatitis, tumor resection, tumor grade, disease progression and smoking status, were all reviewed retrospectively.
Cox regression analysis was performed to examine the correlation between TMB and patient’s overall survival (OS). According to the time-dependent receiver operating characteristic (ROC) curve, patients were divided into either the high (TMB > 3.1 mut/Mb) or low TMB (TMB ≤ 3.1 mut/Mb) group. Kaplan-Meier method was used to construct the survival curves of patients. The time dependent specificity and sensitivity of survival were analyzed by deploying timeROC and survival in the R package. The log-rank test was used to examine the differences between the curves, and a P value < 0.05 was considered to be statistically significant. The nomogram model and calibration curve were also analyzed using the rms package in R.
Statistical analyses were performed using the SPSS version 25.0 (IBM Corp.) software. The Kaplan-Meier curve was analyzed using the survival package in R version 3.6.3, and the time dependent ROC curve was analyzed using the timeROC package, wherein the picture was generated by the ggplot2 package in R version 3.6.3. All reported P values were two-tailed, and P ≤ 0.05 was considered statistically significant for all analyses in this study.
In this study, the MSK-IMPACT cohort included a total of 412 ICC patients who were mainly compared using TMB as an independent prognostic factor. Most patients in this cohort were examined using the 341- (IMPACT341) and 410-gene (IMPACT410) panels. In comparison to the latest 468-gene panel (IMPACT468), the unsequenced genes in the earlier versions were assumed to be wild-type or non-mutated. Clinical data in this study included age (< 65, ≥ 65), gender (male, female), BMI (< 28, ≥ 28), TMB (≤ 3.1, > 3.1), CA19-9 (< 40 U/mL, ≥ 40 U/mL), chronic viral diseases (negative, positive), tumor resection (resected, unresected), tumor grade (well differentiated, moderately differentiated, poorly differentiated), disease progression (solitary liver tumor, multifocal liver disease, metastatic disease), and smoking status (never smoked, former smoker, current smoker). Baseline clinicopathological features of the study cohort are summarized in Table 1 (median age: 63 years, range: 18-88; 46.1% of patients were females; median: TMB 2.5 mut/MB, range: 0-51.6).
| Characteristics | All patients (n = 412) | |
| Number (n) | Percent (%) | |
| Age, yr | ||
| Median | 63 | |
| Range | 18-88 | |
| < 65 | 188 | 45.6 |
| ≥ 65 | 224 | 54.4 |
| Gender | ||
| Female | 190 | 46.1 |
| Male | 222 | 53.9 |
| BMI | ||
| Median | 27.5 | |
| Range | 17.6-59.8 | |
| < 28 | 191 | 46.4 |
| ≥ 28 | 217 | 52.7 |
| TMB, mut/Mb | ||
| Median | 2.5 | |
| Range | 0-51.6 | |
| ≤ 3.1 | 239 | 58.0 |
| > 3.1 | 140 | 34.0 |
| CA19-9 | ||
| < 40 U/mL | 121 | 29.4 |
| ≥ 40 U/mL | 192 | 46.6 |
| Chronic viral hepatitis | ||
| Negative | 379 | 92.0 |
| Positive | 33 | 8.0 |
| Tumor resection | ||
| Resected | 203 | 49.3 |
| Unresected | 209 | 50.7 |
| Tumor grade | ||
| Well differentiated | 15 | 3.6 |
| Moderately differentiated | 231 | 56.1 |
| Poorly differentiated | 146 | 35.4 |
| Disease progression | ||
| Solitary liver tumor | 148 | 35.9 |
| Multifocal liver disease | 86 | 20.9 |
| Metastatic disease | 178 | 43.2 |
| Smoking status | ||
| Never smoked | 202 | 49.0 |
| Former smoker | 166 | 40.3 |
| Current smoker | 41 | 10.0 |
First, we analyzed the utility of TMB in prognosis, calculating a median TMB of 2.5 mut/Mb (range: 0-51.6 mut/Mb). To analyzed the predictive performance of TMB relating to OS, we generated a time-dependent ROC curve which showed the area under the curve (AUC) for TMB involving 1-, 3-, and 5-year survival was 0.545, 0.592, and 0.605, respectively (Figure 1A). Afterwards, we used the 1-, 3-, and 5-year ROC curve analysis with the corresponding maximum Youden index to calculate the TMB threshold values. As a result, when the TMB cut-off value was 3.1, the maximal AUC value was achieved (1-year sensitivity: 0.448, specificity: 0.656; 3-year sensitivity: 0.430, specificity: 0.742; 5-year sensitivity: 0.402, specificity: 0.767). Therefore, we defined 3.1 mut/Mb as the cut-off value. Patients with a TMB > 3.1 mut/Mb were clarified as the high group (n = 140), and patients with a TMB ≤ 3.1 mut/Mb were clarified as the low group (n = 239).
Following TMB classification, the Kaplan-Meier plotter of survival analysis showed that high TMB patients had a poor OS (HR = 1.47, P = 0.002; Figure 1B) and RFS (HR = 1.42, P = 0.035; Figure 1C), as compared to low TMB patients. We then performed subgroup analysis of prognosis to assess the impact of TMB in different clinical subsets (Table 2). For tumor grade, high TMB patients had poor OS in moderately differentiated (HR = 1.46, P = 0.026; Figure 1E) and poorly differentiated subsets (HR = 1.72, P = 0.007; Figure 1F). In contrast, no definite results can be obtained in well differentiated subsets due to the small sample size (HR = 0.64, P = 0.582; Figure 1D).
| Median survival (mo) | Log-rank test | |||
| TMB-high | TMB-low | HR | P value | |
| Tumor grade | ||||
| Well differentiated | - (n = 5) | 56.1 (n = 10) | 0.64 (0.15-2.27) | 0.582 |
| Moderately differentiated | 26.5 (n = 79) | 42.5 (n = 134) | 1.46 (1.02-2.08) | 0.026 |
| Poorly differentiated | 20.2 (n = 52) | 29.8 (n = 82) | 1.72 (1.11-2.66) | 0.007 |
| Disease progression | ||||
| Solitary liver tumor | 55.1 (n = 44) | 69.4 (n = 93) | 1.42 (0.85-2.38) | 0.140 |
| Multifocal liver disease | 24.4 (n = 31) | 40.6 (n = 46) | 1.85 (1.00-3.43) | 0.026 |
| Metastatic disease | 15.5 (n = 65) | 15.8 (n = 100) | 1.17 (0.83-1.66) | 0.357 |
| Tumor resection | ||||
| Resected | 36.6 (n = 67) | 61.5 (n = 127) | 1.77 (1.17-2.66) | 0.002 |
| Unresected | 17.5 (n = 73) | 17.7 (n = 112) | 1.13 (0.81-1.59) | 0.461 |
For disease progression, high TMB indicated poor OS in patients with multifocal liver disease (HR = 1.85, P = 0.026; Figure 1H). However, no significant differences in survival between the high TMB and low TMB groups were found in patients with solitary liver tumor (HR = 1.42, P = 0.140; Figure 1G) and metastatic disease (HR = 1.17, P = 0.357; Figure 1I).
For tumor resection, high TMB indicated a shorter OS in patients who underwent tumor resection (HR = 1.77, P = 0.002; Figure 1J). Conversely, no differences in prognosis were observed between the high TMB and low TMB groups in patients without tumor resection (HR = 1.13, P = 0.461; Figure 1K).
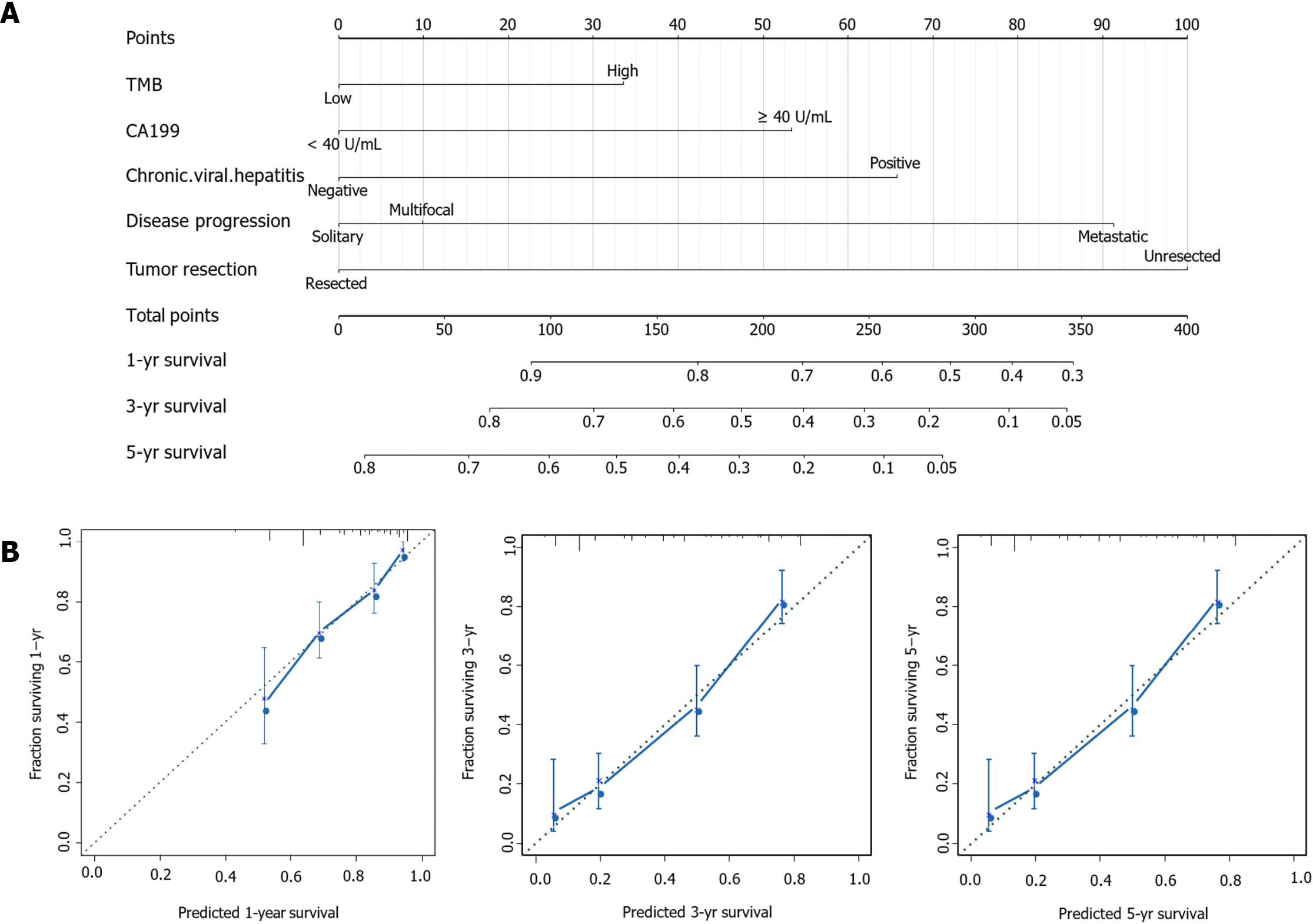
Finally, we would like to screen the independent prognostic factors and establish a prognostic model of ICC patients. Multivariate Cox regression analysis to was used to analyze the associations between OS and specific factors, including age, sex, and TMB. As a result, TMB was identified as an independent risk predictor for ICC patients [HR = 1.43 (1.05-1.96), P = 0.0240]. Additionally, independent prognostic factors of ICC included CA19-9 [HR = 1.78 (1.28-2.46), P = 0.0005], chronic viral hepatitis [HR = 1.72 (1.01-2.95), P = 0.0468], tumor resection [HR = 2.58 (1.72-3.88), P < 0.0001], and disease progression [metastatic disease vs solitary liver tumor HR = 2.55 (1.55-4.20), P = 0.0002] (Table 3). Following this, we constructed a predictive nomogram based on the Cox regression coefficients of selected variables, and the predictive accuracy of every nomogram was evaluated using calibration plots (Figure 2A). The total score for ICC patients can be calculated to predict the 1-, 3-, and 5-year survival rates, which would help clinicians assess the risk level of ICC patients in clinical practice. Notably, the calibration curve indicated that the observed and predicted values were consistent in predicting OS (Figure 2B).
| Univariable | Multivariable | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| < 65 | ||||||
| ≥ 65 | 0.96 | 0.76-1.21 | 0.7286 | 1.16 | 0.84-1.61 | 0.3662 |
| Gender | ||||||
| Female | ||||||
| Male | 1.27 | 1.00-1.61 | 0.0424 | 1.27 | 0.94-1.72 | 0.1145 |
| BMI | ||||||
| < 28 | ||||||
| ≥ 28 | 0.80 | 0.63-1.00 | 0.0536 | 0.84 | 0.62-1.132 | 0.2517 |
| TMB, mut/Mb | ||||||
| Low (≤ 3.1) | ||||||
| High (> 3.1) | 1.47 | 1.13-1.91 | 0.002 | 1.43 | 1.05-1.96 | 0.0240 |
| CA19-9 | ||||||
| < 40 U/mL | ||||||
| ≥ 40 U/mL | 1.79 | 1.37-2.33 | < 0.0001 | 1.78 | 1.28-2.46 | 0.0005 |
| Chronic viral hepatitis | ||||||
| Negative | ||||||
| Positive | 1.19 | 0.78-1.83 | 0.3667 | 1.72 | 1.01-2.95 | 0.0468 |
| Tumor resection | ||||||
| Resected | ||||||
| Unresected | 3.09 | 2.39-3.99 | < 0.0001 | 2.58 | 1.72-3.88 | < 0.0001 |
| Tumor grade | ||||||
| Well differentiated | ||||||
| Moderately differentiated | 2.01 | 1.19-3.39 | 0.0471 | 1.08 | 0.49-2.38 | 0.8489 |
| Poorly differentiated | 2.26 | 1.34-3.80 | 0.0211 | 1.15 | 0.52-2.57 | 0.7298 |
| Disease progression | ||||||
| Solitary liver tumor | ||||||
| Multifocal liver disease | 2.26 | 1.53-3.36 | < 0.0001 | 1.47 | 0.86-2.52 | 0.1587 |
| Metastatic disease | 3.79 | 2.87-5.01 | < 0.0001 | 2.55 | 1.55-4.20 | 0.0002 |
| Smoking status | ||||||
| Never smoked | ||||||
| Former smoker | 1.02 | 0.80-1.31 | 0.8591 | 0.94 | 0.69-1.29 | 0.7077 |
| Current smoker | 1.03 | 0.69-1.52 | 0.8971 | 1.64 | 0.98-2.73 | 0.0587 |
In this study, we investigated the role of TMB in predicting survival among patients with ICC. First, the clinical and mutation data of the 412 ICC patients were obtained from the MSK public database. Next, the best cut-off TMB value was determined using time-dependent ROC curve. Combined with other clinical features, univariate and multivariate Cox regression analyses were used to establish a risk model for prognosis prediction, showing that elevated TMB was associated with poor OS and RFS. In addition to TMB, CA19-9, chronic viral hepatitis, tumor resection, and disease progression (metastatic disease vs solitary liver tumor) were also found to be independent predictors of OS in ICC patients. Based on these risk factors, a reliable nomogram model was then constructed, demonstrating a satisfactory performance in predicting OS in ICC patients. Therefore, this study provided an effective indicator for the clinical prognostic evaluation of ICC patients, as well as contributed to the screening of high-risk ICC patients and the provision of individualized treatment.
Recently, TMB has become a novel predictive biomarker with the potential to predict the therapeutic effect of ICIs and screen suitable patients for immunotherapy[19]. At present, the research on TMB has mainly focused on its ability to predict the efficacy of ICIs, with numerous studies showing its association with the survival rate of cancer patients. In particular, Xie et al[20] found that papillary thyroid carcinoma patients with high TMB reported a worse prognosis. A study by Zhang et al[21] also indicated that low TMB resulted in a better prognosis in patients with head and neck squamous cell carcinoma. Similarly, a study of 318 ICC patients showed that high TMB indicated a worse prognosis [HR = 1.500 (1.085-2.073)][22]. In the present study, the data of 412 ICC patients published by the MSK Cancer Center in March 2021 were used to determine the utility of TMB in prognosis prediction. Notably, the original researchers investigated the relationship between the mutation gene, clinical characteristics, and the prognosis of ICC patients; however, they did not explore the role of TMB in prognosis. Analyzing the aforementioned data, we found that ICC patients with high TMB had a poor OS and RFS, which was consistent with the findings of previous studies.
Clinically, CEA and CA19-9 levels are commonly used prognostic indicators in ICC[23,24]. However, their prognostic thresholds vary widely across different reports, with a lack of a large meta-analysis to consolidate these values[25]. Moreover, some studies have reported on other prognostic indicators associated with poor prognosis in ICC patients, including elevated C-reactive protein, circulating osteopontin, as well as KRAS and TP53 mutations in tumor tissues[26-29]. With the wide application of immunotherapy, TMB has also become a common clinical index. In order to detect TMB, common mutations in ICC patients were detected, which reflected the overall mutation of tumor tissue. Therefore, TMB is a convenient and crucial prognostic value in clinical practice.
Medical nomograms use biologic and clinical variables, including tumor grade and patient age, to graphically depict a statistical prognostic model that generates a probability of a clinical event for a given individual, such as cancer recurrence or death. Furthermore, nomograms are user-friendly, can incorporate continuous variables and relevant disease determinants into prognosis, and are superior to clinician judgment in estimating disease course[30,31]. In this study, we constructed a predictive nomogram according to the Cox regression coefficients of selected variables to help clinicians evaluate the prognostic risk of ICC patients, calculate their survival rate, and make correct clinical decisions. Particularly, TMB and CA19-9 were combined to construct a nomogram model to predict the prognosis of ICC patients, which was helpful for its clinical application. To ensure the accuracy of this nomogram model, we used a calibration plot, as it allowed us to determine how close the nomogram estimated risk was to the observed risk.
In conclusion, we explored the prognostic role of TMB in ICC patients. Multivariate analysis indicated that TMB and CA19-9 were among the identified independent prognostic factors in ICC. Although our study confirmed the prognostic value of TMB, our study had several limitations. First, the clinical characteristics and TMB data of the cases analyzed in this study were all extracted from the MSK Cancer Center, of which some cases had missing data. As a result, this increased the analysis error in our study. Second, using a single data source also increases statistical error. Thus, further larger‐cohort studies are necessary to confirm the predictive value of TMB in the prognosis of ICC patients. For the benefit of future studies, we will continue to collect the clinical data of ICC patients and consolidate our conclusions by expanding the present study’s sample size.
Intrahepatic cholangiocarcinoma (ICC) is malignancies of the biliary duct system and constitutes approximately 10%-20% of all primary liver cancers. Tumor mutation burden (TMB) is a useful biomarker across many cancer types for the identification of patients who will benefit from immunotherapy. This study collected the ICC database from the Memorial Sloan Kettering Cancer Center to investigate the impact of TMB on the prognosis of ICC patients.
The prognosis of ICC patients is very poor. Previous studies suggest that TMB can used to be a prognostic factor in many types of cancer. It is critical to analyze the prognostic value of TMB in ICC to help individual clinical treatment.
This study aims to investigate the prognostic value of TMB in patients with intrahepatic cholangiocarcinoma ICC. In particular, we sought to confirm that TMB is an independent prognostic factor of ICC and construct a nomogram model to predict the prognosis of ICC patients, which was helpful for its clinical application.
This study is a retrospective cohort study of ICC patients. This is a study of large sample to investigate the prognostic value of TMB and other clinical characters in ICC.
TMB was an independent risk predictor for ICC. Furthermore, independent prognostic factors of ICC included CA19-9, chronic viral hepatitis, tumor resection and disease progression (metastatic disease vs solitary liver tumor). The clinical characteristics and TMB data of some cases had missing. which increased the analysis error in our study. Using a single data source also increases statistical error. Further larger–cohort studies are necessary to confirm the predictive value of TMB in the prognosis of ICC patients.
These findings suggest that TMB was an independent prognostic biomarker in patients with ICC. Moreover, patients with ICC with high TMB had poor overall survival and relapse free survival as compared to those with low TMB.
We will continue to collect the clinical data of ICC patients and consolidate our conclusions by expanding the present study’s sample size.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernández-Placencia RM, Suzuki H S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Cao J, Hu J, Liu S, Meric-Bernstam F, Abdel-Wahab R, Xu J, Li Q, Yan M, Feng Y, Lin J, Zhao S, Wang J, Kwong LN, Carapeto F, Borad MJ, Wang K, Javle M, Zhao H. Intrahepatic Cholangiocarcinoma: Genomic Heterogeneity Between Eastern and Western Patients. JCO Precis Oncol. 2020;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 3. | Yamamoto M, Takasaki K, Yoshikawa T. Lymph node metastasis in intrahepatic cholangiocarcinoma. Jpn J Clin Oncol. 1999;29:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 5. | Sui M, Li Y, Wang H, Luo Y, Wan T, Wang X, Hu B, Cheng Y, Lv X, Xin X, Xu Q, Wang G, Lu S. Two cases of intrahepatic cholangiocellular carcinoma with high insertion-deletion ratios that achieved a complete response following chemotherapy combined with PD-1 blockade. J Immunother Cancer. 2019;7:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 7. | Zhang C, Li Z, Qi F, Hu X, Luo J. Exploration of the relationships between tumor mutation burden with immune infiltrates in clear cell renal cell carcinoma. Ann Transl Med. 2019;7:648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1568] [Cited by in RCA: 1878] [Article Influence: 313.0] [Reference Citation Analysis (0)] |
| 9. | Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3101] [Cited by in RCA: 3388] [Article Influence: 308.0] [Reference Citation Analysis (0)] |
| 10. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6065] [Cited by in RCA: 6337] [Article Influence: 633.7] [Reference Citation Analysis (0)] |
| 11. | Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, Yang JJ, Yang XN, Lin JX, Yan HH, Zhai HR, Yan LX, Liao RQ, Wu SP, Wu YL. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017;23:3012-3024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 12. | Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 2353] [Article Influence: 261.4] [Reference Citation Analysis (0)] |
| 13. | Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA; CheckMate 026 Investigators. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 2017] [Article Influence: 252.1] [Reference Citation Analysis (0)] |
| 14. | Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D'Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2239] [Cited by in RCA: 2802] [Article Influence: 467.0] [Reference Citation Analysis (0)] |
| 15. | Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2065] [Cited by in RCA: 2167] [Article Influence: 216.7] [Reference Citation Analysis (0)] |
| 16. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4953] [Article Influence: 619.1] [Reference Citation Analysis (0)] |
| 17. | Valero C, Lee M, Hoen D, Wang J, Nadeem Z, Patel N, Postow MA, Shoushtari AN, Plitas G, Balachandran VP, Smith JJ, Crago AM, Long Roche KC, Kelly DW, Samstein RM, Rana S, Ganly I, Wong RJ, Hakimi AA, Berger MF, Zehir A, Solit DB, Ladanyi M, Riaz N, Chan TA, Seshan VE, Morris LGT. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat Genet. 2021;53:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 18. | Boerner T, Drill E, Pak LM, Nguyen B, Sigel CS, Doussot A, Shin P, Goldman DA, Gonen M, Allen PJ, Balachandran VP, Cercek A, Harding J, Solit DB, Schultz N, Kundra R, Walch H, D'Angelica MI, DeMatteo RP, Drebin J, Kemeny NE, Kingham TP, Simpson AL, Hechtman JF, Vakiani E, Lowery MA, Ijzermans JNM, Buettner S, Koerkamp BG, Doukas M, Chandwani R, Jarnagin WR. Genetic Determinants of Outcome in Intrahepatic Cholangiocarcinoma. Hepatology. 2021;74:1429-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 19. | Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1661] [Cited by in RCA: 2512] [Article Influence: 314.0] [Reference Citation Analysis (0)] |
| 20. | Xie Z, Li X, Lun Y, He Y, Wu S, Wang S, Sun J, Xin S, Zhang J. Papillary thyroid carcinoma with a high tumor mutation burden has a poor prognosis. Int Immunopharmacol. 2020;89:107090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Zhang L, Li B, Peng Y, Wu F, Li Q, Lin Z, Xie S, Xiao L, Lin X, Ou Z, Cai T, Rong H, Fan S, Li J. The prognostic value of TMB and the relationship between TMB and immune infiltration in head and neck squamous cell carcinoma: A gene expression-based study. Oral Oncol. 2020;110:104943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Zhang R, Li Q, Fu J, Jin Z, Su J, Zhang J, Chen C, Geng Z, Zhang D. Comprehensive analysis of genomic mutation signature and tumor mutation burden for prognosis of intrahepatic cholangiocarcinoma. BMC Cancer. 2021;21:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Loosen SH, Roderburg C, Kauertz KL, Koch A, Vucur M, Schneider AT, Binnebösel M, Ulmer TF, Lurje G, Schoening W, Tacke F, Trautwein C, Longerich T, Dejong CH, Neumann UP, Luedde T. CEA but not CA19-9 is an independent prognostic factor in patients undergoing resection of cholangiocarcinoma. Sci Rep. 2017;7:16975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Sasaki H, Sueda T. Elevated perioperative serum CA 19-9 Levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014;110:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Macias RIR, Kornek M, Rodrigues PM, Paiva NA, Castro RE, Urban S, Pereira SP, Cadamuro M, Rupp C, Loosen SH, Luedde T, Banales JM. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:108-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Lin ZY, Liang ZX, Zhuang PL, Chen JW, Cao Y, Yan LX, Yun JP, Xie D, Cai MY. Intrahepatic cholangiocarcinoma prognostic determination using pre-operative serum C-reactive protein levels. BMC Cancer. 2016;16:792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Loosen SH, Roderburg C, Kauertz KL, Pombeiro I, Leyh C, Benz F, Vucur M, Longerich T, Koch A, Braunschweig T, Ulmer TF, Heidenhain C, Tacke F, Binnebösel M, Schmeding M, Trautwein C, Neumann UP, Luedde T. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J Hepatol. 2017;67:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Nepal C, O'Rourke CJ, Oliveira DVNP, Taranta A, Shema S, Gautam P, Calderaro J, Barbour A, Raggi C, Wennerberg K, Wang XW, Lautem A, Roberts LR, Andersen JB. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma. Hepatology. 2018;68:949-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, Zhao X, Li Y, Li Q, Wang H, Hu J, Kong G, Wu M, Ding C, Chen N, Hu H. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 30. | Cho CS, Gonen M, Shia J, Kattan MW, Klimstra DS, Jarnagin WR, D'Angelica MI, Blumgart LH, DeMatteo RP. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Kattan MW, Yu C, Stephenson AJ, Sartor O, Tombal B. Clinicians vs nomogram: predicting future technetium-99m bone scan positivity in patients with rising prostate-specific antigen after radical prostatectomy for prostate cancer. Urology. 2013;81:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |