Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.762
Peer-review started: April 27, 2021
First decision: June 15, 2021
Revised: June 15, 2021
Accepted: December 21, 2021
Article in press: December 21, 2021
Published online: January 21, 2022
Processing time: 262 Days and 19.6 Hours
Severe acute respiratory syndrome coronavirus 2 infection affects not only the lungs, but also the cardiovascular system, having a major impact on patients’ outcomes. Myocardial injury (MI) occurs in the context of coronavirus infectious disease 2019 (COVID-19) and is associated with a higher risk of severe clinical outcome and mortality. COVID-19-related MI can have various clinical manifestations, of which the main ones are myocarditis, stress cardiomyopathy, acute coronary syndrome, and pulmonary embolism. The exact mechanisms of how MI occurs in these patients are not yet fully known. Direct injury, through direct viral myocardial invasion, and indirect injury, through interaction with angiotensin I converting enzyme 2, increased inflammation, and thrombocyte and endothelial dysfunction, could be involved in acute MI in patients with COVID-19. A better understanding of these multiple potential mechanisms may help to develop new targeted therapeutic strategies. The purpose of this review is to provide the current understanding of the potential mechanisms involved in MI induced by COVID-19 and to discuss the current progress in the therapeutic strategies.
Core Tip: Myocardial injury (MI) has been described in coronavirus infectious disease 2019 patients and is associated with a higher risk of severe clinical outcome and mortality, but the exact mechanisms involved are not completely elucidated. Multiple potential mechanisms have been proposed, such as direct viral infection and indirect injury through inflammation, angiotensin I converting enzyme 2 interaction and hemostatic anomalies. Understanding the mechanisms underlying MI is needed to guide effective therapeutic strategies in these patients.
- Citation: Rusu I, Turlacu M, Micheu MM. Acute myocardial injury in patients with COVID-19: Possible mechanisms and clinical implications. World J Clin Cases 2022; 10(3): 762-776
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/762.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.762
Since December 2019, coronavirus infectious disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus (SARS-CoV)-2 has quickly become a global health issue that is having a major impact on the healthcare system worldwide. High infectivity and rapid transmission of the virus have led to an international public health crisis. A wide range of symptoms had been reported, with most infected patients developing respiratory tract disease with different severity level. Not only the lungs are affected, and other organs are involved, with COVID-19 affecting multiple organs and systems, with different cardiovascular implications. Also, cardiovascular comorbidities have an important impact on the severity of COVID-19 and they seem to be linked with severe clinical outcomes and higher risk of death. Clinical studies have reported that COVID-19 can significantly affect the heart, causing acute myocardial injury (MI)[1-3], in patients with and without pre-existing cardiovascular disease[4]. MI is defined as an elevation of at least one cardiac troponin (cTn) concentration above the 99th percentile upper reference limit[5]. COVID-19-related MI can have various clinical manifestations, of which the main ones are myocarditis[6-8], stress cardiomyopathy[9,10], acute coronary syndrome[11-13], and pulmonary embolism[14-16]. In this review, we aim to provide an overview of the potential mechanism involved in MI induced by COVID-19, and the progress in the therapeutic strategies addressing it.
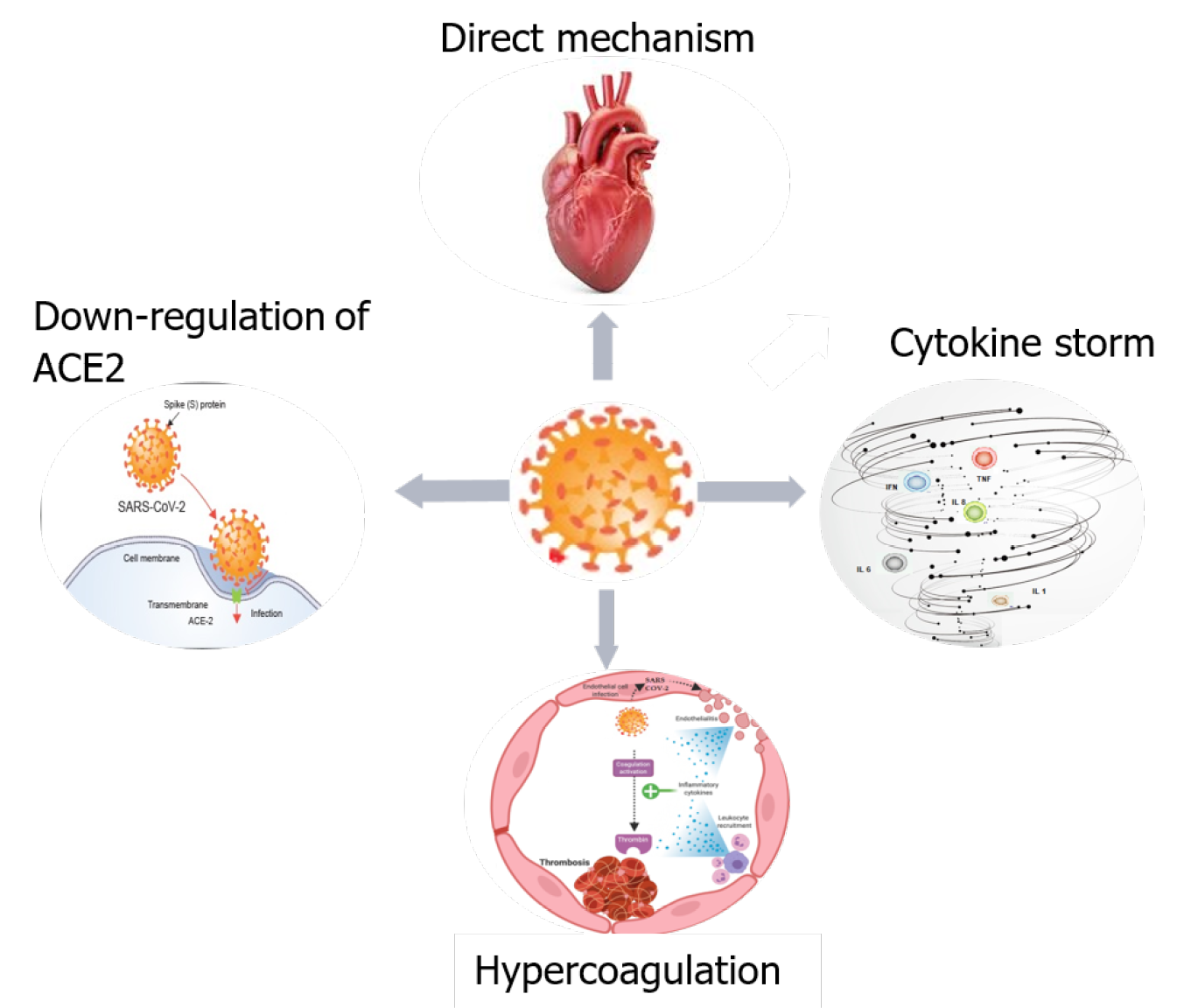
COVID-19 may cause MI via various mechanisms, either directly, or indirectly. The first mechanism might be a direct injury to myocardial cells due to a viral invasion of endothelial cells and cardiomyocytes via angiotensin I converting enzyme (ACE)2. Other possible mechanisms are: downregulation of ACE2, cytokine storm/cytokine releasing syndrome, and hypercoagulation (Figure 1).
SARS-CoV-2 is an RNA virus with a high affinity for ACE2. For virus attachment to the receptor, SARS-CoV-2 uses the S protein and the transmembrane protease serine 2 (TMPRSS2) to cleave the S protein and facilitate infection[18,19]. The receptors of ACE2 are located in the lung, heart, endothelial cells and immune cells[20]. These locations could explain intracellular viral replication in the myocardium and other tissues, resulting in degeneration, necrosis and dysfunction. Recently, it has been showed that ACE2 and other mediators of SARS-CoV-2 entry (such as cathepsin B and cathepsin L) are preferentially enriched in cardiomyocytes, explaining at least in part the cardiac susceptibility to COVID-19[21].
Only a few case reports have demonstrated the presence of the genome of SARS-CoV-2 in cardiac samples[4,5]. The COVID-19 virus was detected in the interstitial and endothelial cells and not necessarily in the myocytes, which emphasized the presence of lymphocyte and monocyte infiltration, and a particularly high level of monocytes causes myocardial ischemia[24,25]. Varga et al[27] have suggested that viral attack determines endothelium injury. This issue causes endotheliitis with the recruitment of inflammatory cells, apoptosis and pyroptosis, and subsequent microcirculatory distress[26,27]. Hence, the latest Position Statement[28] issued by the Working Group on Atherosclerosis and Vascular Biology, together with the Council of Basic Cardiovascular Science of the European Society of Cardiology acknowledges the key role of the endothelium in COVID-19-associated cardiovascular pathophysiology, and recommend that endothelial biomarkers and tests of function to be considered for early detection of cardiovascular complications.
Recognition of ACE2 as the primary human receptor for the SARS-CoV-2 was the first step to identify the virus tropism and pathogenicity[29,30]. The literature shows that ACE2 is expressed in type II alveolar epithelial cells, myocardial cells, vascular endothelium, esophageal and bladder epithelium cells, and renal cells[31,32]. The virus uses S protein for binding to the ACE2 receptor of target cells, and the cellular serine protease TMPRSS2 cleaves the S protein into two functional domains, S1 that binds to ACE2 and S2 designed for membrane fusion[30,33-37]. The cleavage can be produced near a fusion peptide located within the S2 domain[33,38]. This mechanism helps the virus priming and entry into the cells and promotes virus infectivity[30,33,39,40]. Lai et al[39] have demonstrated that SARS-CoV fusion depends on calcium level, so a low level of calcium decreases infectivity. It is known that ACE2 and ACE are linked to the renin–angiotensin–aldosterone system, which promotes angiotensin I maturation, and has a crucial effect on the cardiovascular system[41].
Angiotensin I hydrolyzation produced by ACE2 yields angiotensin 1-9 peptide, on which ACE acts to produce angiotensin 1-7 (Ang 1-7)[17]. Ang 1-7 is the ligand for the G-protein Mas receptor that provides cardioprotective effects as vasodilatory, antiproliferative and antioxidative effects[17]. ACE2 has a direct effect on angiotensin II, producing Ang 1-7, but also acts on bradykinin ligand receptor, Des-arg9-bradykinin, thereby inactivating an inflammatory response[17,41,42].
In SARS-CoV 2 infection, decreased ACE2 expression causes lower levels of Ang 1–7 and an increase in angiotensin II level[17,41,43]. This effect results in vasoconstriction, inflammation, proliferation, fibrosis, apoptosis, and de novo heart injury or aggravation of pre-existing cardiovascular problems[43].
Ang II activates both mitogen-activated protein kinase and ADAM-17 phosphory
Downregulation of ACE2 causes an increased level of angiotensin II, which induces production of inflammatory cytokines such as interferon-γ, interleukin (IL)-6, and the chemokine monocyte chemoattractant protein (MCP)-1, promoting inflammation[45-47]. MCP-1 can be an ROS source, promoting negative remodeling after MI[44,46].
Many severe infectious and noninfectious diseases, including COVID-19, are associated with cytokine overproduction, activating lots of signals and communication pathways[48,49]. The inflammation starts in the lungs via ACE2 receptor, which is localized in the pneumocytes, local pulmonary macrophages, and dendritic cells, and it spreads through the circulation to organs expressing ACE2, with significant effects on the cardiovascular system[50].
Oudit et al[51] have shown that an increased level of Ang II determines infiltration and activation of neutrophils in the myocardium, which release inflammatory cytokines (IL-6, IL-1β and MCP-1) and are a source of ROS, with a negative inotropic effect on murine myocardial contraction[51].
SARS-CoV-2 activates the innate immune system and triggers the JAK–STAT pathway via the pattern of recognition receptor, with overproduction of IFNs[38,52]. IFN type I increases the inflammatory factors and activates the cytokine storm[38,52,53]. Rapid replication of the virus determines the activation and differentiation of T helper (Th)1 cells, producing cytokines such as IL-6, granulocyte–macrophage colony-stimulating factor and IFN-γ, and increases the number of Th1 and Th2 cells, macrophages and natural killer cells[54]. The virus has developed new mechanisms through nonstructural protein to avoid the immune system, and suppresses the effects of IFNs, which lead to virus dissemination and promotion of cytokine realizing syndrome[50,54-57]. The first cytokines produced in the early phase of the infection are IL-6, tumor necrosis factor (TNF)-α, IL-1, IL-8 and MCP-1[56,58].
In the severe form of COVID-19, chemokines CCL3, CXCL8, CXCL9 and CXCL10 are released into the blood circulation, as well as proinflammatory cytokines TNF-α, IFN-γ, IFN-α, IL-12, IL-1β, IL-6, IL-33, IL-18 and transforming growth factor β, leading to an important inflammatory response[18,53,58,59]. Latest studies indicate that a higher level of inflammatory biomarkers such as IL-6, IL-8 and TNF-α determine MI and correlate with high mortality[60,61].
IL-6 plays the main role in inflammation. There are two mechanisms through the JAK–STAT3 signaling pathway for activating and promoting inflammation[59]. The first mechanism of action is the cis-signaling pathway that uses membrane IL-6 receptor, which activates the innate and acquired immune system[62,63]. The second mechanism is through the trans-signaling pathway, which uses soluble IL-6 receptors for activating cells without IL-6 membrane receptors, such as endothelial cells[62-64].
The IL-6 mechanism results in oversecretion of vascular endothelial growth factor, MCP-1, IL-8 and IL-6, and decrease of E-cadherin on endothelial cells, promoting apoptosis of cardiac cells and left ventricular remodeling[49,62,65]. Del Turco et al[62] have shown that hyperinflammation promotes vascular permeability, leakage, endothelial dysfunction, and hypercoagulation with a significant impact on the cardiovascular system. Also, the production of matrix metalloproteinase by the monocytes/macrophages increases the risk of atherosclerotic plaque rupture and the probability of MI[62].
Endothelial dysfunction, hyperinflammation, and hypoxia induced by SARS-CoV-2 contribute to a procoagulant status with majors effects on the cardiovascular system[66]. Inflammation and coagulation play a bidirectional role in vascular disease[67]. The inflammation causes endothelial dysfunction, which activates coagulation and together with the coagulation factors, increases cytokine production by the endothelial and mononuclear cells[67]. Endothelial dysfunction by activating the complement system, causes a hypercoagulant state and promotes inflammation[68].
The central role in thrombogenesis is played by tissue factor (TF)[50,68]. TF is a transmembrane protein expressed on the surface of macrophages, cardiomyocytes and smooth muscle cells[48,49]. The monocytes in atherosclerotic plaques tend to express more TF than the circulating ones, which stimulates cytokines such as IL-6, platelet-derived growth factor and MCP-1, and leads to thrombus formation. In severe infection, cytokines, especially IL-6, determine TF exposure and systemic activation of coagulation[68-70]. TF binds to factor VIIα, leading to thrombin formation, which converts fibrinogen into fibrin and determines coagulation[70]. Thrombin also binds to another class of specific receptors, protease-activating cell receptors (PARs), which are expressed in many cell types, including endothelial cells, monocytes, platelets, smooth muscle cells, and fibroblasts. Their activation is a key promoter of both coagulation and inflammation[70]. Four PAR types are identified; type 2 determines overproduction of ROS with negative inotropic action and adhesion molecules by macro
Neutrophil-derived extracellular traps are an extracellular web of chromatin and antimicrobials produced by neutrophils as an innate mechanism to combat pathogens. They can trigger the processes of inflammation and thrombosis by activating endothelial cells and platelets[72-74].
Severe hypoxia activated in SARS-CoV-2 infection leads to multiple effects such as endothelial inflammation with metabolic changes that affect ATP production and an increase in mitochondrial ROS, which causes platelet hyperactivation and apoptosis with release of proinflammatory and procoagulant factors[75,76].
Hypoxia promotes thrombogenesis through a direct mechanism via early growth response factor 1 induction, but also through and an indirect mechanism mediated by inflammatory cytokines (TNF-α and IL-1)[75-78]. Hypoxia also activates hypoxia-inducible transcription factors that promote coagulation targeting factors such as plasminogen activator inhibitor 1, but also the pyrin domain containing 3 inflammasome pathway with an increase of IL-1β, which causes venous thromboembolism (VTE)[79].
Platelets play a crucial role in coagulation and are the first blood cells that respond to endothelial damage. Coronavirus disease causes platelet hyperactivation due to P-selectin increased membrane expression, which interacts with its counter-receptors on neutrophils or other inflammatory cells, thereby activating thrombogenesis.[80]. After autopsy of patients with acute MI, many megakaryocytes and inflammatory cells are found in the microvascular system, along with venous thrombosis and platelet-rich thrombi[81]. Recent studies have shown that the antiphospholipid antibodies interact with complement factors, platelets, and endothelial cells, promoting coagulation; ongoing and future research will validate the role of antiphospholipid syndrome in COVID-19[82].
Various potential therapeutic strategies addressing specific pathophysiological mechanisms are currently used to prevent and/or alleviate the MI caused by COVID-19. Some pharmacological agents target mechanisms with definite evidence of causing cardiovascular damage, hence being part of standard of care therapy and recommended by existing guidelines, while others address hypothetical mechanisms, hence being under study.
Considering that one potential mechanism of acute MI is mediated by ACE2[83], the question arises whether therapy with ACE inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) should be continued or stopped. Existing evidence-based consensus and position statements[84-87] issued by prominent cardiovascular and hypertension societies recommend against modifying this therapy if it is already underway, and to prescribe it for newly diagnosed patients as usual, given the absence of consistent evidence regarding their potential risk[88,89]. A randomized clinical study of 659 patients hospitalized with mild to moderate COVID-19 and ACEIs or ARBs therapy prior to hospitalization has shown that there was no significant difference in the mean number of days alive and out of the hospital between the patients assigned to discontinue or continue this therapy[90]. Also, a large meta-analysis of > 28000 hypertensive patients with COVID-19 on ACEIs or ARBs has found a beneficial effect of using renin–angiotensin–aldosterone system inhibitors in these patients[91]. Nevertheless, additional studies are warranted to evaluate the role of ACE2 poly
IL-6 receptor antagonists such as tocilizumab and sarilumab may represent an interesting alternative for patients with significantly elevated IL-6, ferritin, D-dimer and high-sensitivity troponin I (TnI) levels[89]. A Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) has investigated the effectiveness of tocilizumab and sarilumab on survival and organ support in critically ill COVID-19 patients and it has shown improved outcomes and survival[93]. Other clinical trials are underway[94-96].
Another potential therapeutic is colchicine, due to its anti-inflammatory effect through inhibition of cytokine production and neutrophil activity, and it does not have an immunosuppressive effect compared with tocilizumab and sarilumab[97]. Several small randomized controlled trials have already shown a positive impact of adding colchicine to the standard treatment in COVID-19 patients[98-100]. Randomized trials with larger populations are in progress[101-103]. Given the high prevalence of thromboembolic anomalies and coagulopathy in patients with COVID-19, use of thromboprophylaxis may be necessary.
In nonhospitalized patients with mild COVID-19, anticoagulants and antiplatelet therapy are not recommended routinely[104], but should be considered depending on risk assessment[105]. For those with confirmed VTE, the CHEST guidelines recommend a direct oral anticoagulant (DOAC) with apixaban, rivaroxaban, dabigatran or edoxaban (before dabigatran and edoxaban an initial parenteral anticoagulation is needed). When a DOAC is not used, vitamin K antagonists are recommended over low-molecular-weight heparin (LMWH)[106] (Table 1).
| COVID-19 patients | Prevention | Treatment | Refs. |
| Outpatient | Thromboprophylaxis is not routinely recommended | DOAC (apixaban, rivaroxaban, dabigatran or edoxaban) | NIH COVID-19 Treatment Guidelines[104], CHEST Guideline[106] |
| Acutely ill hospitalized patient | LMWH or fondaparinux standard dose | Initial anticoagulation with LMWH or IV UFH or DOAC (apixaban, rivaroxaban) | CHEST Guideline[106] |
| Critically ill COVID-19 patient | LMWH or UFH standard or intermediate dose | LMWH or fondaparinux | The Royal College of Physicians[105], CHEST Guideline[106], ASH guidelines[107], ISTH interim guidance[108] |
In acutely ill hospitalized patients with COVID-19, anticoagulant thromboprophylaxis is recommended. The CHEST guidelines are in favor of anticoagulation with LMWH or fondaparinux over unfractionated heparin (UFH) or DOAC[106]. UFH is not preferred, in order to limit staff exposure, and DOAC is not recommended as a primary prevention strategy due to possible risk of interactions between therapies for COVID-19 and oral anticoagulants[106]. The American Society of Hematology guidelines do not recommend any specific anticoagulant to be used as first-choice treatment[107]. There is no recommendation to increase intensity of anticoagulation thromboprophylaxis, and the current standard dose should be used over intermediate or full treatment dosing[106-109]. However, the Italian Society on Thrombosis and Haemostasis suggests that the use of intermediate dose of LMWH should be considered in patients with multiple risk factors for VTE[110]. Also, the Royal College of Physicians suggests that a higher dose of LMWH may be considered in these patients[105].
In acutely ill hospitalized patients with COVID-19 with confirmed VTE, the CHEST guidelines recommend initial parenteral anticoagulation with LMWH or IV UFH or initial direct oral anticoagulation with apixaban or rivaroxaban (dabigatran and edoxaban can be used after initial parenteral anticoagulation)[106].
In critically ill patients with COVID-19 anticoagulant thromboprophylaxis is recommended. The CHEST guidelines are in favor of anticoagulation with LMWH or UFH over fondaparinux or a DOAC[106]. If there is any contraindication to pharmacological thromboprophylaxis, mechanical thromboprophylaxis may be considered, but it is not recommended to add it to pharmacological treatment[106].
Most guidelines recommend the use of current standard dose over intermediate or full treatment dosing due to insufficient data regarding intensified treatment[106-108]. Nevertheless, the Anticoagulation Forum suggests, based on expert opinion, that an increased dose of anticoagulant thromboprophylaxis such as enoxaparin 40 mg or 0.5 mg/kg subcutaneous twice daily, UFH 7500 U subcutaneous three times daily or low-intensity heparin infusion, should be considered for these patients[109]. The Royal College of Physicians also suggests intermediate dose of LMWH[105]. In critically ill COVID-19 patients with confirmed VTE, the CHEST guidelines recommend parenteral anticoagulation with LMWH or fondaparinux over UFH[106]. The CHEST and the Royal College of Physicians guidelines recommend a minimum duration of 3 mo of anticoagulation therapy for COVID-19 patients with confirmed VTE[105,106].
In COVID-19 patients discharged from hospital, we may consider extending thromboprophylaxis for those with increased postdischarge risk of VTE and low bleeding risk[105,106,109]. The Royal College of Physicians recommends a duration of 14–28 d of thromboprophylaxis with LMWH[105]. The Anticoagulation Forum suggests using anticoagulants such as betrixaban maximum 35–42 d, rivaroxaban maximum 31–39 d or enoxaparin maximum 6–14 d[109].
In patients with recurrent VTE and COVID-19 despite anticoagulation with DOAC or vitamin K antagonist therapy, the CHEST guidelines recommend switching treatment to LMWH. In patients with recurrent VTE despite anticoagulation with LMWH they suggest increasing the dose of LMWH by 25%–30%[106].
Regarding antiplatelet therapy for COVID-19 patients, there are no data that would suggest any benefit of using antiplatelet agents to prevent thrombosis and we should consider the risk associated with the use of them given that a thrombocytopenic status may exist in patients with COVID-19[66,67]. Furthermore, the CHEST guidelines recommend against the use of antiplatelet agents for VTE prevention[106].
Many studies have shown that there is a high prevalence of arterial and venous thromboembolism in hospitalized patients with COVID-19 despite standard thromboprophylaxis[14]. Hence, is it possible that a higher dose of anticoagulant might be necessary? The recommendation of the intensity of anticoagulant thromboprophylaxis is not based on direct evidence of the effects of intermediate or therapeutic dose in primary prevention because of the lack of well-designed randomized clinical studies. A collaboration between three randomized clinical trial platforms ATTACC (Antithrombotic Therapy to Ameliorate Complications of COVID-19), REMAP-CAP (Randomized Embedded Multi-factorial, Adaptive Platform Trial) and ACTIV-4a (Accelerating COVID-19 Therapeutic Interventions and Vaccines) is ongoing in order to clarify this issue[113].
As mentioned before, currently there is no specific recommendation for using antiplatelet agents in COVID-19 patients. However, according to the present understanding of the mechanisms of thrombocytopathy and endotheliopathy, targeting therapeutics to both endothelium and platelets may be effective. Considering the effects of aspirin such as antithrombotic and anti-inflammatory actions and inhibition of virus replication[114], clinical trials on the protective effect of aspirin in COVID-19 patients are underway[115,116].
In addition to this, antithrombotic agents with vasodilatory action on vascular smooth muscle cells and anti-inflammatory action, such as prostacyclin and NO, may become a potential therapeutic alternative in patients with thrombocytopathy and endotheliopathy[80]. Clinical trials on administration of prostacyclin or NO in COVID-19 patients are in progress[117,118]. Similarly, dipyridamole, a phosphodiesterase 3 inhibitor with antiplatelet and anti-inflammatory action, could have beneficial effects in COVID-19 patients[119]. The potential therapeutic benefits are being investigated[120,121].
A recent systematic review and meta-analysis has shown that the use of statins in patients with COVID-19 has a beneficial effect on improving clinical outcomes[122]. However, we must consider that elevated liver enzymes are common in patients with moderate to severe COVID-19, even though its impacts is still unknown and statin therapy should be discontinued in these patients[123,124]. Multiple clinical trials on using statins in COVID-19 patients are ongoing[116,125-127].
Various pharmacological agents aiming to limit viral entry into cells are currently under study. Previous data[128-130] have endorsed recombinant human ACE2 as an attractive therapeutic target for the current COVID-19; the molecule acting as a decoy receptor, hence curbing viral entry[83]. The efficacy of recombinant ACE2 is being investigated in a small pilot trial including patients with severe COVID-19 (Clinicaltrials.gov NCT04287686).
An alternative way of blocking SARS-CoV-2 cell invasion is inhibition of TMPRSS2 activity. Some potential therapeutic strategies targeting TMPRSS2 are already tackling COVID-19 clinically, while others are just being tested in the laboratory[131]. The former includes serine protease inhibitors such as camostat mesylate[19], which is presently considered for off-label treatment of SARS-CoV-2-infected patients (Clinicaltrials.gov NCT04321096).
MI is an important cardiovascular manifestation in COVID-19 patients associated with increased severity and high risk of mortality. At this point, the pathophysiology underlying COVID-19-related MI is not fully understood, but clinical evidence has shown that not only a direct mechanism is involved, but also SARS-CoV-2 might affect the cardiovascular system in an indirect manner through interaction with ACE2, production of cytokines, thrombocyte and endothelium dysfunction, and hypercoagulation. Elucidating the mechanisms underlying MI could help develop effective therapeutic strategies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Dai HL S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Wu YXJ
| 1. | Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 2. | Raad M, Dabbagh M, Gorgis S, Yan J, Chehab O, Dagher C, Jamoor K, Hussein IH, Cook B, Van Harn M, Singh G, McCord J, Parikh S. Cardiac Injury Patterns and Inpatient Outcomes Among Patients Admitted With COVID-19. Am J Cardiol. 2020;133:154-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3009] [Article Influence: 601.8] [Reference Citation Analysis (1)] |
| 4. | López-Otero D, López-Pais J, Antúnez-Muiños PJ, Cacho-Antonio C, González-Ferrero T, González-Juanatey JR. [Association between myocardial injury and prognosis of COVID-19 hospitalized patients, with or without heart disease. CARDIOVID registry]. Rev Esp Cardiol. 2021;74:105-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 2494] [Article Influence: 356.3] [Reference Citation Analysis (1)] |
| 6. | Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 7. | Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ, Xie XJ, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 384] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 8. | Mele D, Flamigni F, Rapezzi C, Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med. 2021;16:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Singh S, Desai R, Gandhi Z, Fong HK, Doreswamy S, Desai V, Chockalingam A, Mehta PK, Sachdeva R, Kumar G. Takotsubo Syndrome in Patients with COVID-19: a Systematic Review of Published Cases. SN Compr Clin Med. 2020;1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Szarpak L, Filipiak KJ, Gasecka A, Pruc M, Drozd A, Jaguszewski MJ. Correlation between takotsubo cardiomyopathy and SARS-CoV-2 infection. Med Hypotheses. 2021;146:110454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yolcu M, Gunesdogdu F, Bektas M, Bayirli DT, Serefhanoglu K. Coronavirus disease 2019 (COVID-19) and simultaneous acute anteroseptal and inferior ST-segment elevation myocardial infarction. Cardiovasc J Afr. 2020;31:335-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Tedeschi D, Rizzi A, Biscaglia S, Tumscitz C. Acute myocardial infarction and large coronary thrombosis in a patient with COVID-19. Catheter Cardiovasc Interv. 2021;97:272-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Koutsoukis A, Delmas C, Roubille F, Bonello L, Schurtz G, Manzo-Silberman S, Puymirat E, Elbaz M, Bouisset F, Meunier PA, Huet F, Paganelli F, Laine M, Lemesle G, Lamblin N, Henry P, Tea V, Gallet R, Teiger E, Huguet R, Fard D, Lim P. Acute Coronary Syndrome in the Era of SARS-CoV-2 Infection: A Registry of the French Group of Acute Cardiac Care. CJC Open. 2021;3:311-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, Morrison RB, Leiva O, Fanikos J, Nauffal V, Almarzooq Z, Goldhaber SZ. Registry of Arterial and Venous Thromboembolic Complications in Patients With COVID-19. J Am Coll Cardiol. 2020;76:2060-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 15. | Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, Gervaise A, Poissy J, Susen S, Hékimian G, Artifoni M, Periard D, Contou D, Delaloye J, Sanchez B, Fang C, Garzillo G, Robbie H, Yoon SH. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology. 2021;298:E70-E80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 319] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 16. | Roncon L, Zuin M, Barco S, Valerio L, Zuliani G, Zonzin P, Konstantinides SV. Incidence of acute pulmonary embolism in COVID-19 patients: Systematic review and meta-analysis. Eur J Intern Med. 2020;82:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82-C97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1463] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 18. | Bakhshandeh B, Sorboni SG, Javanmard AR, Mottaghi SS, Mehrabi MR, Sorouri F, Abbasi A, Jahanafrooz Z. Variants in ACE2; potential influences on virus infection and COVID-19 severity. Infect Genet Evol. 2021;90:104773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14273] [Article Influence: 2854.6] [Reference Citation Analysis (0)] |
| 20. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 740] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 21. | Yang J, Chen T, Zhou Y. Mediators of SARS-CoV-2 entry are preferentially enriched in cardiomyocytes. Hereditas. 2021;158:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 745] [Article Influence: 149.0] [Reference Citation Analysis (0)] |
| 23. | Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss HP. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7:2440-2447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 24. | Gauchotte G, Venard V, Segondy M, Cadoz C, Esposito-Fava A, Barraud D, Louis G. SARS-Cov-2 fulminant myocarditis: an autopsy and histopathological case study. Int J Legal Med. 2021;135:577-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Püschel K, Westermann D. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5:1281-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 596] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 26. | Maccio U, Zinkernagel AS, Shambat SM, Zeng X, Cathomas G, Ruschitzka F, Schuepbach RA, Moch H, Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63:103182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 27. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4583] [Article Influence: 916.6] [Reference Citation Analysis (0)] |
| 28. | Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, Weber C, Bochaton-Piallat ML, Bäck M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 29. | Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281-292.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4743] [Cited by in RCA: 6159] [Article Influence: 1231.8] [Reference Citation Analysis (0)] |
| 30. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4603] [Article Influence: 209.2] [Reference Citation Analysis (0)] |
| 31. | Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1709] [Cited by in RCA: 1732] [Article Influence: 346.4] [Reference Citation Analysis (0)] |
| 32. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1530] [Article Influence: 306.0] [Reference Citation Analysis (0)] |
| 33. | Straus MR, Tang T, Lai AL, Flegel A, Bidon M, Freed JH, Daniel S, Whittaker GR. Ca2+ Ions Promote Fusion of Middle East Respiratory Syndrome Coronavirus with Host Cells and Increase Infectivity. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1252] [Cited by in RCA: 1316] [Article Influence: 263.2] [Reference Citation Analysis (0)] |
| 35. | Neri Serneri GG, Boddi M, Modesti PA, Coppo M, Cecioni I, Toscano T, Papa ML, Bandinelli M, Lisi GF, Chiavarelli M. Cardiac angiotensin II participates in coronary microvessel inflammation of unstable angina and strengthens the immunomediated component. Circ Res. 2004;94:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Zipeto D, Palmeira JDF, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front Immunol. 2020;11:576745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 37. | Liu Z, Xiao X, Wei X, Li J, Yang J, Tan H, Zhu J, Zhang Q, Wu J, Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92:595-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 443] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 38. | Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, Nie X, Zhou L, Liu Z, Ren Y, Yuan L, Zhang Y, Zhang J, Liang L, Chen X, Liu X, Wang P, Han X, Weng X, Chen Y, Yu T, Zhang X, Cai J, Chen R, Shi ZL, Bian XW. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57:102833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 39. | Lai AL, Millet JK, Daniel S, Freed JH, Whittaker GR. The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner. J Mol Biol. 2017;429:3875-3892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Millet JK, Whittaker GR. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 656] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 41. | Warner FJ, Guy JL, Lambert DW, Hooper NM, Turner AJ. Angiotensin converting enzyme-2 (ACE2) and its possible roles in hypertension, diabetes and cardiac function. Lett Pept Sci. 2003;10:377-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB Jr, Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17-L31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 43. | Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2333] [Cited by in RCA: 2077] [Article Influence: 415.4] [Reference Citation Analysis (0)] |
| 44. | Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 45. | Gragnano F, Sperlongano S, Golia E, Natale F, Bianchi R, Crisci M, Fimiani F, Pariggiano I, Diana V, Carbone A, Cesaro A, Concilio C, Limongelli G, Russo M, Calabrò P. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediators Inflamm. 2017;2017:5620314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 46. | Wang M, Shah AM. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J Mol Cell Cardiol. 2015;83:101-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 48. | Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75:185-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 586] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 49. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3341] [Article Influence: 668.2] [Reference Citation Analysis (0)] |
| 50. | Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 240] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 51. | Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW, Khokha R, Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Haji Abdolvahab M, Moradi-Kalbolandi S, Zarei M, Bose D, Majidzadeh-A K, Farahmand L. Potential role of interferons in treating COVID-19 patients. Int Immunopharmacol. 2021;90:107171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 53. | Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 871] [Cited by in RCA: 959] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 54. | Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 1165] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 55. | Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 56. | Totura AL, Baric RS. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2:264-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 57. | Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, Rosain J, Bilguvar K, Ye J, Bolze A, Bigio B, Yang R, Arias AA, Zhou Q, Zhang Y, Onodi F, Korniotis S, Karpf L, Philippot Q, Chbihi M, Bonnet-Madin L, Dorgham K, Smith N, Schneider WM, Razooky BS, Hoffmann HH, Michailidis E, Moens L, Han JE, Lorenzo L, Bizien L, Meade P, Neehus AL, Ugurbil AC, Corneau A, Kerner G, Zhang P, Rapaport F, Seeleuthner Y, Manry J, Masson C, Schmitt Y, Schlüter A, Le Voyer T, Khan T, Li J, Fellay J, Roussel L, Shahrooei M, Alosaimi MF, Mansouri D, Al-Saud H, Al-Mulla F, Almourfi F, Al-Muhsen SZ, Alsohime F, Al Turki S, Hasanato R, van de Beek D, Biondi A, Bettini LR, D'Angio' M, Bonfanti P, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Oler AJ, Tompkins MF, Alba C, Vandernoot I, Goffard JC, Smits G, Migeotte I, Haerynck F, Soler-Palacin P, Martin-Nalda A, Colobran R, Morange PE, Keles S, Çölkesen F, Ozcelik T, Yasar KK, Senoglu S, Karabela ŞN, Rodríguez-Gallego C, Novelli G, Hraiech S, Tandjaoui-Lambiotte Y, Duval X, Laouénan C; COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group, Snow AL, Dalgard CL, Milner JD, Vinh DC, Mogensen TH, Marr N, Spaan AN, Boisson B, Boisson-Dupuis S, Bustamante J, Puel A, Ciancanelli MJ, Meyts I, Maniatis T, Soumelis V, Amara A, Nussenzweig M, García-Sastre A, Krammer F, Pujol A, Duffy D, Lifton RP, Zhang SY, Gorochov G, Béziat V, Jouanguy E, Sancho-Shimizu V, Rice CM, Abel L, Notarangelo LD, Cobat A, Su HC, Casanova JL. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1635] [Article Influence: 327.0] [Reference Citation Analysis (0)] |
| 58. | Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1324] [Article Influence: 101.8] [Reference Citation Analysis (1)] |
| 59. | Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, Rojas-Villarraga A, Ramírez-Santana C, Díaz-Coronado JC, Manrique R, Mantilla RD, Shoenfeld Y, Anaya JM. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev. 2020;19:102554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 60. | Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 1798] [Article Influence: 359.6] [Reference Citation Analysis (0)] |
| 61. | Song Y, Gao P, Ran T, Qian H, Guo F, Chang L, Wu W, Zhang S. High Inflammatory Burden: A Potential Cause of Myocardial Injury in Critically Ill Patients With COVID-19. Front Cardiovasc Med. 2020;7:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Del Turco S, Vianello A, Ragusa R, Caselli C, Basta G. COVID-19 and cardiovascular consequences: Is the endothelial dysfunction the hardest challenge? Thromb Res. 2020;196:143-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 63. | Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1228] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 64. | Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin-6 Signaling in Clinic. Immunity. 2019;50:1007-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 568] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 65. | Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 594] [Reference Citation Analysis (0)] |
| 66. | Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 926] [Article Influence: 185.2] [Reference Citation Analysis (0)] |
| 67. | Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 637] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 68. | Levi M, Scully M, Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018;16:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 69. | Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108:1447-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 70. | Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 453] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 71. | Heuberger DM, Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. 2019;17:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 72. | Döring Y, Soehnlein O, Weber C. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ Res. 2017;120:736-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 73. | Zuo Y, Zuo M, Yalavarthi S, Gockman K, Madison JA, Shi H, Woodard W, Lezak SP, Lugogo NL, Knight JS, Kanthi Y. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. 2021;51:446-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 200] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 74. | Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 950] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 75. | Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023-H2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 76. | Koziel A, Jarmuszkiewicz W. Hypoxia and aerobic metabolism adaptations of human endothelial cells. Pflugers Arch. 2017;469:815-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Liao H, Hyman MC, Lawrence DA, Pinsky DJ. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 2007;21:935-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | van der Poll T, Levi M. Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr Vasc Pharmacol. 2012;10:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114:4763-4768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 80. | Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI, Halene S, Martin KA, Chun HJ, Hwa J. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 81. | Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 82. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1731] [Cited by in RCA: 1606] [Article Influence: 321.2] [Reference Citation Analysis (0)] |
| 83. | Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1712] [Article Influence: 342.4] [Reference Citation Analysis (0)] |
| 84. | European Society of Cardiology Council on Hypertension. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. 2020. [cited 27 April 2021]. Available from: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang. |
| 85. | The International Society of Hypertension. A statement from the International Society of Hypertension on COVID-19. [cited 27 April 2021]. Available from: https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/. |
| 86. | European Society of Hypertension. ESH statement on COVID-19. [cited 27 April 2021]. Available from: https://www.eshonline.org/spotlights/esh-statement-covid-19/. |
| 87. | Heart Failure Society of America; American College of Cardiology; American Heart Association. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19 – American College of Cardiology. [cited 27 April 2021]. Available from: https://www.acc.org/Latest-in-cardiology/artic. |
| 88. | Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, Henry BM, Lippi G. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin Proc. 2020;95:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 89. | Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1220] [Article Influence: 244.0] [Reference Citation Analysis (0)] |
| 90. | Lopes RD, Macedo AVS, de Barros E Silva PGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, Feldman A, D'Andréa Saba Arruda G, de Albuquerque DC, Camiletti AS, de Sousa AS, de Paula TC, Giusti KGD, Domiciano RAM, Noya-Rabelo MM, Hamilton AM, Loures VA, Dionísio RM, Furquim TAB, De Luca FA, Dos Santos Sousa ÍB, Bandeira BS, Zukowski CN, de Oliveira RGG, Ribeiro NB, de Moraes JL, Petriz JLF, Pimentel AM, Miranda JS, de Jesus Abufaiad BE, Gibson CM, Granger CB, Alexander JH, de Souza OF; BRACE CORONA Investigators. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA. 2021;325:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 275] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 91. | Baral R, White M, Vassiliou VS. Effect of Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19: a Systematic Review and Meta-analysis of 28,872 Patients. Curr Atheroscler Rep. 2020;22:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 92. | Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2021;7:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 93. | REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1321] [Article Influence: 330.3] [Reference Citation Analysis (0)] |
| 94. | Caballero Bermejo AF, Ruiz-Antorán B, Fernández Cruz A, Diago Sempere E, Callejas Díaz A, Múñez Rubio E, Avendaño-Solá C, Ramos Martínez A, Sancho López A; Puerta de Hierro COVID-19 Study Group. Sarilumab vs standard of care for the early treatment of COVID-19 pneumonia in hospitalized patients: SARTRE: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Tocilizumab for SARS-CoV2 (COVID-19) Severe Pneumonitis [Internet]. [cited 27 April 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04315480. |
| 96. | Perrone F, Piccirillo MC, Ascierto PA, Salvarani C, Parrella R, Marata AM, Popoli P, Ferraris L, Marrocco-Trischitta MM, Ripamonti D, Binda F, Bonfanti P, Squillace N, Castelli F, Muiesan ML, Lichtner M, Calzetti C, Salerno ND, Atripaldi L, Cascella M, Costantini M, Dolci G, Facciolongo NC, Fraganza F, Massari M, Montesarchio V, Mussini C, Negri EA, Botti G, Cardone C, Gargiulo P, Gravina A, Schettino C, Arenare L, Chiodini P, Gallo C; TOCIVID-19 investigators, Italy. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J Transl Med. 2020;18:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 97. | Reyes AZ, Hu KA, Teperman J, Wampler Muskardin TL, Tardif JC, Shah B, Pillinger MH. Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 98. | Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, Dib SM, Gigante SL, Benatti MN, Rezek UC, Emrich-Filho LL, Sousa BAA, Almeida SCL, Luppino Assad R, Veras FP, Schneider A, Rodrigues TS, Leiria LOS, Cunha LD, Alves-Filho JC, Cunha TM, Arruda E, Miranda CH, Pazin-Filho A, Auxiliadora-Martins M, Borges MC, Fonseca BAL, Bollela VR, Del-Ben CM, Cunha FQ, Zamboni DS, Santana RC, Vilar FC, Louzada-Junior P, Oliveira RDR. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 99. | Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, Metallidis S, Sianos G, Baltagiannis S, Panagopoulos P, Dolianitis K, Randou E, Syrigos K, Kotanidou A, Koulouris NG, Milionis H, Sipsas N, Gogos C, Tsoukalas G, Olympios CD, Tsagalou E, Migdalis I, Gerakari S, Angelidis C, Alexopoulos D, Davlouros P, Hahalis G, Kanonidis I, Katritsis D, Kolettis T, Manolis AS, Michalis L, Naka KK, Pyrgakis VN, Toutouzas KP, Triposkiadis F, Tsioufis K, Vavouranakis E, Martinèz-Dolz L, Reimers B, Stefanini GG, Cleman M, Goudevenos J, Tsiodras S, Tousoulis D, Iliodromitis E, Mehran R, Dangas G, Stefanadis C; GRECCO-19 investigators. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3:e2013136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 100. | Scarsi M, Piantoni S, Colombo E, Airó P, Richini D, Miclini M, Bertasi V, Bianchi M, Bottone D, Civelli P, Cotelli MS, Damiolini E, Galbassini G, Gatta D, Ghirardelli ML, Magri R, Malamani P, Mendeni M, Molinari S, Morotti A, Salada L, Turla M, Vender A, Tincani A, Brucato A, Franceschini F, Furloni R, Andreoli L. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79:1286-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 101. | Whitlock R, Belley-Cote E, Eikelboom J. Anti-Coronavirus therapies to prevent progression of Coronavirus Disease 2019 (COVID-19) trial (ACTCOVID19). In: ClinicalTrials.gov [Internet]. [cited 30 March 2021]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04324463. |
| 102. | Tardif JC. Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA) (COVID-19). In: ClinicalTrials.gov [Internet]. [cited 30 March 2021]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04322682. |
| 103. | Diaz R. The ECLA PHRI COLCOVID Trial. Effects of Colchicine on Moderate/High-risk Hospitalized COVID-19 Patients. (COLCOVID). In: ClinicalTrials.gov [Internet]. [cited 30 March 2021]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04328480. |
| 104. | NIH. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [cited 30 March 2021]. Available from: https://covid19treatmentguidelines.nih.gov/. |
| 105. | Faculty of Intensive Care Medicine. Clinical guide for the prevention, detection and management of thromboembolic disease in patients with COVID-19. 2020; 1–6. [cited 30 March 2021]. Available from: https://icmanaesthesiacovid-19.org/s/VTE-Patients-with-COVID19.pdf. |
| 106. | Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, Jimenez D, Le Gal G, Rali P, Wells P. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 469] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 107. | Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, Davila J, DeSancho MT, Diuguid D, Griffin DO, Kahn SR, Klok FA, Lee AI, Neumann I, Pai A, Pai M, Righini M, Sanfilippo KM, Siegal D, Skara M, Touri K, Akl EA, Bou Akl I, Boulos M, Brignardello-Petersen R, Charide R, Chan M, Dearness K, Darzi AJ, Kolb P, Colunga-Lozano LE, Mansour R, Morgano GP, Morsi RZ, Noori A, Piggott T, Qiu Y, Roldan Y, Schünemann F, Stevens A, Solo K, Ventresca M, Wiercioch W, Mustafa RA, Schünemann HJ. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 296] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 108. | Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1289] [Cited by in RCA: 1312] [Article Influence: 262.4] [Reference Citation Analysis (0)] |
| 109. | Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, Dager WE, Deitelzweig SB, Ellsworth S, Garcia D, Kaatz S, Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (1)] |
| 110. | Marietta M, Ageno W, Artoni A, De Candia E, Gresele P, Marchetti M, Marcucci R, Tripodi A. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020;18:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 163] [Reference Citation Analysis (0)] |
| 111. | Wang Y, Ao G, Nasr B, Qi X. Effect of antiplatelet treatments on patients with COVID-19 infection: A systematic review and meta-analysis. Am J Emerg Med. 2021;43:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 112. | Sahai A, Bhandari R, Koupenova M, Freedman J, Godwin M, McIntyre T, Chung M, Iskandar JP, Kamran H, Aggarwal A, Kalra A, Bartholomew J, McCrae K, Elbadawi A, Svensson L, Kapadia S, Hariri E, Cameron S. SARS-CoV-2 Receptors are Expressed on Human Platelets and the Effect of Aspirin on Clinical Outcomes in COVID-19 Patients. Res Sq. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 113. | NIH. NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID-19 patients [Internet]. [cited 30 March 2021]. Available from: https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients. |
| 114. | Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19? Drugs. 2020;80:1383-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 115. | Protective effect of aspirin on COVID-19 Patients (PEAC) [Internet]. [cited 30 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04365309. |
| 116. | Mukonzo J. Safety & Efficacy of Low Dose Aspirin / Ivermectin Combination Therapy for Treatment of Covid-19 Patients (IVCOM). In: ClinicalTrials.gov [Internet]. [cited 25 September 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04768179. |
| 117. | Johansson PI, Bestle M, Søe-Jensen P, Kristiansen KT, Stensballe J, Clausen NE, Perner A. The effect of prostacyclin (Iloprost) infusion at a dose of 1 ng/kg/min for 72 hours compared to placebo in mechanically ventilated patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Lei C, Su B, Dong H, Fakhr BS, Grassi LG, Di Fenza R, Gianni S, Pinciroli R, Vassena E, Morais CCA, Bellavia A, Spina S, Kacmarek R, Berra L. Protocol for a randomized controlled trial testing inhaled nitric oxide therapy in spontaneously breathing patients with COVID-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Aliter KF, Al-Horani RA. Potential Therapeutic Benefits of Dipyridamole in COVID-19 Patients. Curr Pharm Des. 2021;27:866-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 120. | Liang B. Trial of Open Label Dipyridamole- In Hospitalized Patients With COVID-19 (TOLD). In: ClinicalTrials.gov [Internet]. [cited 30 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04424901. |
| 121. | Knight J. Dipyridamole to Prevent Coronavirus Exacerbation of Respiratory Status (DICER) in COVID-19 (DICER). In: ClinicalTrials.gov [Internet]. [cited 30 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04391179. |
| 122. | Pal R, Banerjee M, Yadav U, Bhattacharjee S. Statin use and clinical outcomes in patients with COVID-19: An updated systematic review and meta-analysis. Postgrad Med J. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 123. | Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020;19:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 124. | Subir R, Jagat J M, Kalyan K G. Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19). Diabetes Metab Syndr. 2020;14:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Masana L. Statin therapy and COVID-19 infection (STACOV) [Internet]. [cited 30 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04407273. |
| 126. | Lansky A. Colchicine/Statins for the prevention of COVID-19 complications (COLSTAT) trial (COLSTAT). [Internet]. [cited 30 March 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04472611. |
| 127. | Bikdeli B, Talasaz AH, Rashidi F, Sharif-Kashani B, Farrokhpour M, Bakhshandeh H, Sezavar H, Dabbagh A, Beigmohammadi MT, Payandemehr P, Yadollahzadeh M, Riahi T, Khalili H, Jamalkhani S, Rezaeifar P, Abedini A, Lookzadeh S, Shahmirzaei S, Tahamtan O, Matin S, Amin A, Parhizgar SE, Jimenez D, Gupta A, Madhavan MV, Parikh SA, Monreal M, Hadavand N, Hajighasemi A, Maleki M, Sadeghian S, Mohebbi B, Piazza G, Kirtane AJ, Lip GYH, Krumholz HM, Goldhaber SZ, Sadeghipour P. Intermediate vs standard-dose prophylactic anticoagulation and statin therapy vs placebo in critically-ill patients with COVID-19: Rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb Res. 2020;196:382-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 128. | Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J, Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 129. | Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, Hardes K, Powley WM, Wright TJ, Siederer SK, Fairman DA, Lipson DA, Bayliffe AI, Lazaar AL. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 130. | Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 131. | Matarese A, Gambardella J, Sardu C, Santulli G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |