Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1106
Peer-review started: July 24, 2021
First decision: October 22, 2021
Revised: October 28, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 21, 2022
Processing time: 174 Days and 21.1 Hours
Metastasis to the thyroid gland (TM) from primary breast cancer is uncommon and usually presents as thyroid nodules; however, diffuse goiter without thyroid nodules is the first sign of TM in rare cases. Skip metastases (SMs) to the lymph nodes in breast cancer, defined as discontiguous higher-level metastases in the absence of lower levels of contiguous metastases, have been reported in the contralateral cervical area of the primary tumor site in rare cases.
A 49-year-old previously healthy Chinese woman was diagnosed with right lateral invasive ductal carcinoma and underwent neoadjuvant chemotherapy treatment and bilateral mastectomy with axillary lymph node dissection. No malignancy of the left breast or axillary or distant metastases were identified preoperatively. However, enlarged left cervical lymph nodes were detected 36 mo after surgery, and rapidly enlarging thyroid glands without nodules were detected 42 mo after surgery. Fine-needle aspiration cytology was performed on the left cervical lymph nodes and left lobe of the thyroid, which were both revealed to contain metastases from the primary breast cancer. Additionally, the immunostaining profiles changed in the process of metastases. The patient was discharged with the NP (vinorelbine and cisplatin) regimen for subsequent treatment, and stable disease was determined when the curative effect was evaluated.
Diffuse goiter may be the first sign of TM, and enlarged lymph nodes in the contralateral cervical area may be SMs of primary breast cancer.
Core Tip: This is a case report of metastasis to the thyroid gland (TM) from primary breast cancer presenting as diffuse goiter associated with skip metastases (SMs) to the contralateral cervical lymph nodes. The patient presented with a cervical mass and progressive neck swelling that were found to be metastases with altered immunostaining profiles upon fine-needle aspiration cytology. These findings indicate that rapidly occurring diffuse goiter without nodules may be the first sign of TM and that enlarged lymph nodes in the contralateral cervical area may be SMs of breast cancer. Raising awareness of these clinical presentations is helpful for the early detection of metastatic disease.
- Citation: Wen W, Jiang H, Wen HY, Peng YL. Metastasis to the thyroid gland from primary breast cancer presenting as diffuse goiter: A case report and review of literature. World J Clin Cases 2022; 10(3): 1106-1115
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1106.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1106
Metastasis to the thyroid gland (TM) is uncommon, accounting for approximately 1.4% to 3% of all thyroid malignancies[1]. It was reported that metastases mostly arise from the lung (21.8%), followed by the gastrointestinal tract (18.2%), breast (14.5%), and kidney (12.7%), in a recent Chinese study[2]. Patients with TM usually present with symptoms of thyroid nodules, thyroiditis or neck swelling, dysphagia, dysphonia, and cough[3]. Diffuse goiter without thyroid nodules is rarely seen as the first manifestation of TM. Skip metastases (SMs) of breast cancer to the lymph nodes, defined as discontiguous higher-level lymphadenopathy in the absence of lower levels of contiguous lymphadenopathy, have rarely been reported. Chung et al[4] reported that SMs occurred in 2.6% of 1300 newly diagnosed invasive breast cancers, and only 6% of these SMs occurred in the contralateral lymph nodes. Here, we report a rare case of TM first presenting as rapidly occurring diffuse goiter without thyroid nodules associated with SMs to the contralateral cervical lymph nodes in a primary breast cancer patient.
A 53-year-old woman with a 4-year breast cancer history presented to the clinic with a cervical mass and progressive neck swelling without pain or airway pressure symptoms.
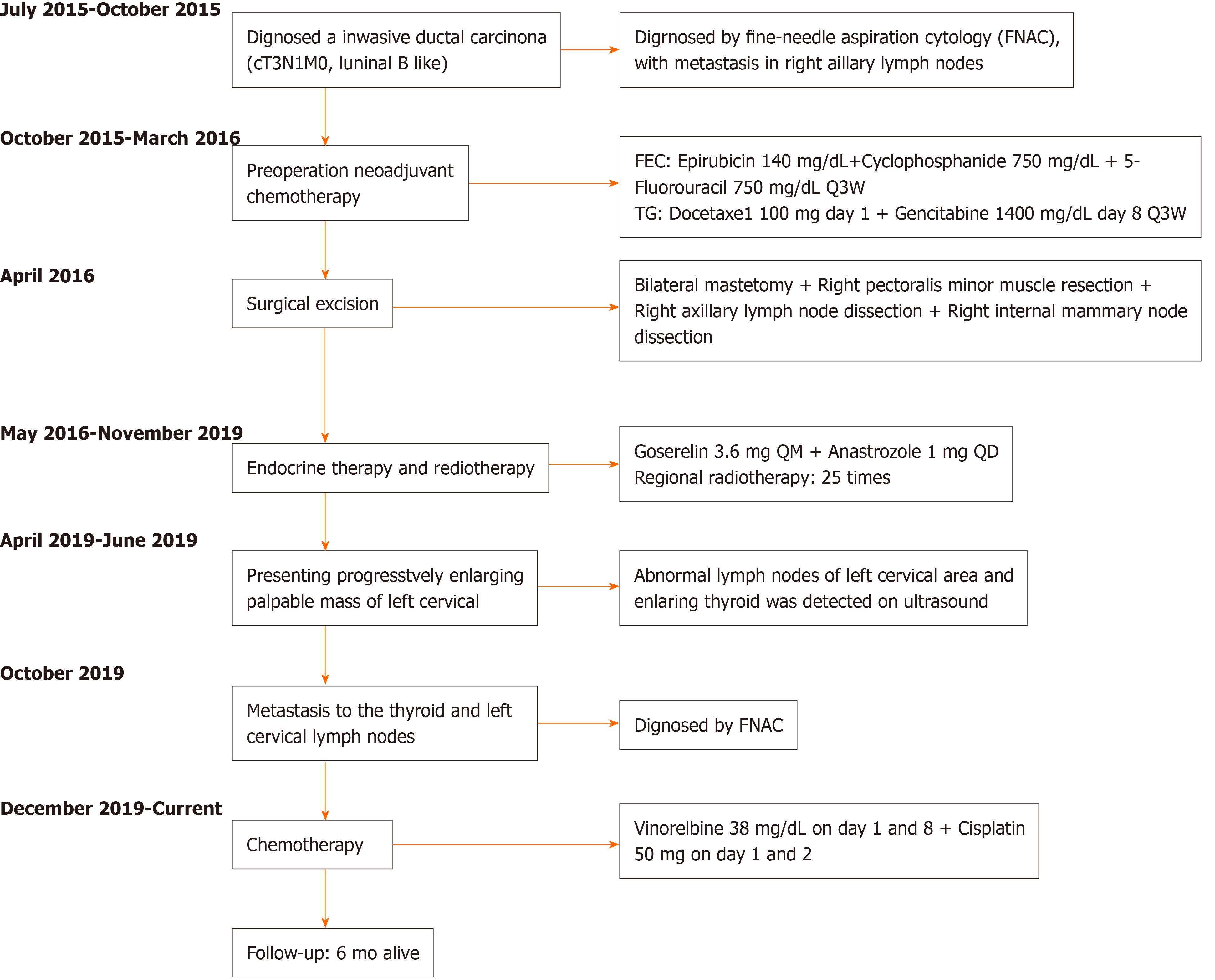
A 49-year-old Chinese woman was diagnosed with invasive ductal carcinoma (IDC) of the right breast with a chief complaint of palpable masses and right nipple discharge in October 2015 (Figure 1). The right axillary lymph nodes were also found to contain poorly differentiated metastatic breast carcinoma cells by fine-needle aspiration cytology (FNAC). No mass or enlarged lymph nodes were seen on the left side upon computed tomography (CT) scan or by ultrasound. The patient underwent preoperative neoadjuvant chemotherapy, with 3 cycles of the FEC (5-fluorouracil + epirubicin + cyclophosphamide) regimen and 3 cycles of the TG (vinorelbine + cisplatin) regimen (Figure 1). She was evaluated as having achieved partial remission after finishing chemotherapy. Subsequently, bilateral mastectomy was performed at the request of the patient in April 2016 (Figure 1). Surgical specimens showed T2N3 (12/27) M0 grade 3 IDC with ductal carcinoma in situ on the right side based on hematoxylin and eosin (HE) staining and immunohistochemistry (IHC). Right axillary and intercostal lymph node metastases were also observed microscopically. No carcinoma was found in the left breast or axillary lymph nodes. IHC staining suggested estrogen receptor (ER, strong positive), progesterone receptor (PR, moderate positive) and human epidermal growth factor receptor 2 (HER-2, 2+) positivity, and the monoclonal antibody Ki-67 index was 60%. After surgery, the patient received endocrine therapy with anastrozole and goserelin. Radiation therapy was administered in 25 fractions to the right breast.
In April 2019, the patient presented at the clinic with a cervical mass and intermittent neck discomfort without pain and airway pressure symptoms. CT revealed lymphadenopathy in the left cervical area and posterior mediastinum. Observation and follow-up were recommended. Two months later, the size of the mass had increased. Further investigation was performed by ultrasound, and levels IV and V left cervical nodal disease was confirmed. On CT with contrast, the patient was found to have left cervical and posterior mediastinum lymph node enlargement and suspected right scapula metastasis. No treatment was initiated per the patient’s decision, and she was scheduled to return to the clinic in 3 mo. In October 2019, the patient presented at her outpatient visit with progressive neck swelling that had persisted for 3 mo.
The patient had no history of any previous disease.
The patient had no personal or familial risk factors for thyroid malignancies.
On physical examination, a hard fixed palpable nontender left lateral neck mass and enlarged thyroid gland were palpated.
Tumor indicators revealed that the carcinoembryonic antigen was elevated at 3.92 μg/L and carbohydrate antigen 15-3 was elevated at 22.30 kU/L. The thyroid function analysis revealed mild hypothyroidism, with a thyroid stimulating hormone level of 5.61 mU/L and a free thyroxine level of 11.62 pmol/L. Blood analysis and inflammatory indicators were normal.
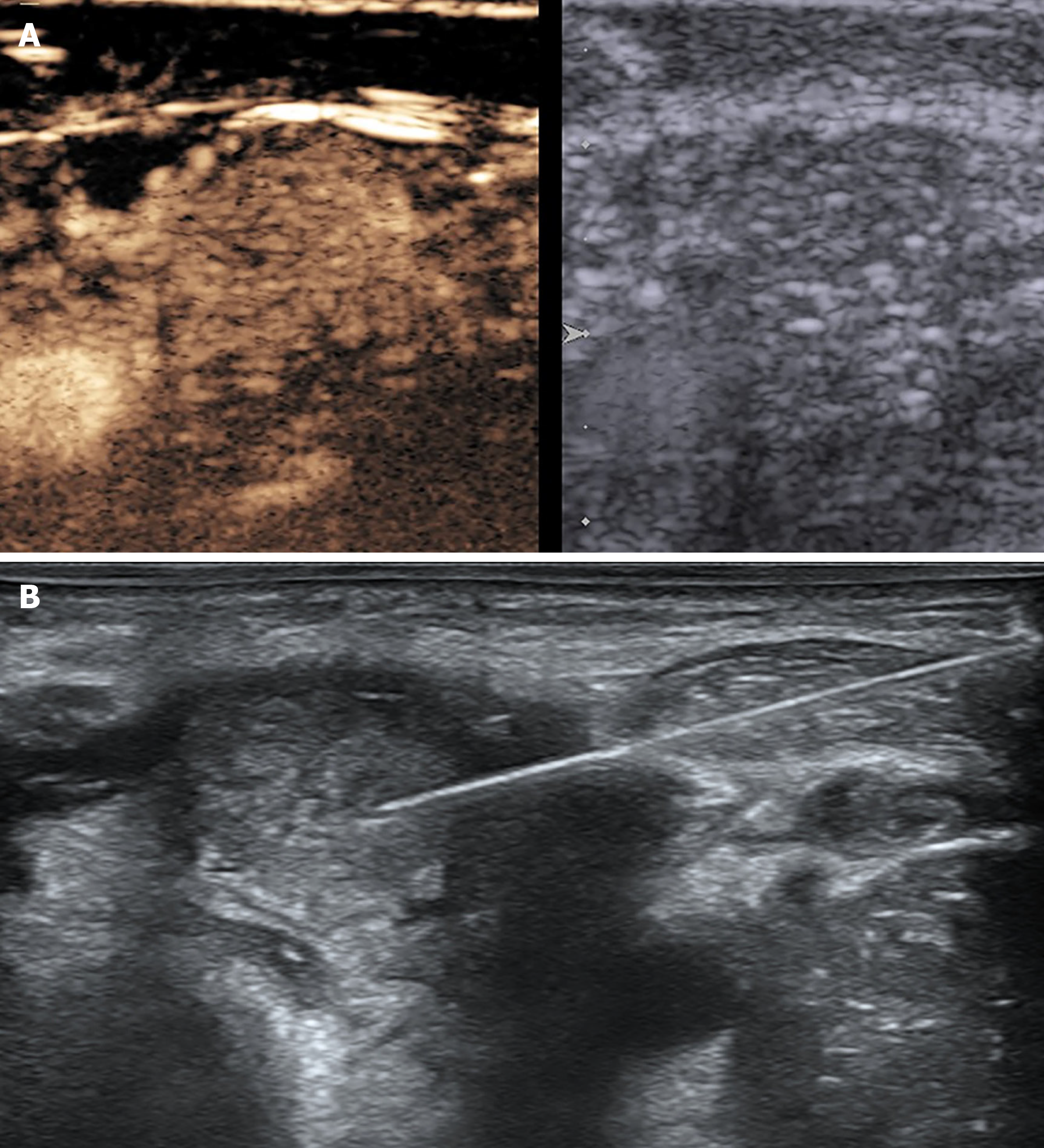
Multimodality ultrasound was performed to evaluate neck swelling, revealing homogeneous enlargement of the thyroid gland without nodules (measuring 2.5 cm × 5.0 cm × 2.2 cm in the right lobe, 2.5 cm x 5.0 cm x 2.0 cm in the left lobe, and 0.8 cm in the isthmus) and level II-VI left abnormally enlarged cervical lymph nodes (the largest measuring 2.7 cm x 2.8 cm x 2.3 cm with microcalcifications and heterogeneous high enhancement (Figure 2A). Fine-needle aspiration cytology (FNAC) of the enlarged thyroid and lymph nodes was conducted for diagnosis (Figure 2B).
Hashimoto’s thyroiditis, Grave’s disease and primary/secondary thyroid malignancy were our initial differential diagnoses to explain the rapid growth of the thyroid gland.
The patient was ultimately diagnosed with TM from primary breast cancer and SMs to the contralateral cervical lymph nodes (Figure 3).
Given the suspected bone metastasis and financial issues, the patient was discharged on the NP (vinorelbine and cisplatin) regimen (Figure 1).
To date, after 6 cycles of chemotherapy, the patient has remained clinically stable, and no recurrence at other sites has been detected or confirmed.
We performed a comprehensive literature search of the PubMed and Medline databases to identify studies of TM metastasis from breast cancer published from 2000 to 2020, and twenty-five articles were found. Detailed information about thyroid metastatic breast cancer was extracted from these articles (Table 1), and only descriptive analyses and literature reviews were found, given the low incidence of TM.
| Ref. | Study year | No of patients | Sex | Age | Primary tumor | Other recurrence | Time interval (mo) | Presentation | Treatment | Response | Follow-up (mo) |
| Wang et al [8], 2020 | - | 1 | F | 58 | Mucinous | Nil | 156 | Neck swelling | Thyroidectomy | Stable | 9 alive |
| Durmo et al[10], 2019 | - | 1 | F | 72 | Ductal | - | - | Abnormality of 18F-FDG PET/CT | - | - | - |
| Pensabene et al[11], 2018 | 2011-2015 | 1 | F | 64 | Lobular | Nil | 6 | Multinodular goiter | Hemithyroidectomy | Recurrence in bone | 32 |
| Zhou et al[12], 2017 | 2005-2015 | 8 | F | 48 | Poorly differentiated | Chest wall | 84 | - | Chemotherapy | PR | 14 alive |
| 59 | Invasive | Chest wall | 24 | Chemotherapy | CR | 5 alive | |||||
| 57 | Invasive | LN, lung | 108 | Chemotherapy | CR | 21 alive | |||||
| 67 | Ductal | Nil | 74 | Chemotherapy | CR | 4 alive | |||||
| 48 | Ductal | Lung | 120 | Total thyroidectomy | CR | 15 alive | |||||
| 52 | Ductal | Nil | 6 | Hemithyroidectomy | CR | 45 alive | |||||
| 69 | Poorly differentiated | Nil | 60 | Total thyroidectomy | CR | 38 alive | |||||
| 43 | Medullary | LN | 84 | Chemotherapy | PR | 30 alive | |||||
| Plonczak et al[13], 2017 | 2004-2017 | 1 | F | 62 | Ductal | Lung, bone | 144 | Neck swelling | Total thyroidectomy | Stable | 14 alive |
| Magers et al[14], 2016 | - | 1 | F | 37 | Ductal | Brain, bone | 72 | - | - | - | - |
| Liu et al[15], 2014 | 2007-2009 | 1 | F | 47 | Ductal | Nil | 24 | Enlarged thyroid with diffuse microcalcification | - | - | - |
| Nguyen et al[16], 2013 | - | 1 | F | 67 | Lobular | Nil | 48 | - | - | - | - |
| Lacka et al[17], 2012 | - | 1 | F | 54 | Ductal + lobular | Bone, suprarenal gland | 168 | Multinodular goiter | Total thyroidectomy | - | 36 alive |
| Kolarevic et al[18], 2012 | 2002-2011 | 1 | F | 54 | Ductal + lobular | Nil | 84 | Palpable thyroid nodules | Chemotherapy and hemithyroidectomy | PR | 24 alive |
| Leboeuf et al[20], 2006 | 1989-2005 | 1 | F | 59 | Ductal | Mediastinal, lung, LN, adrenal | 168 | Unpalpable thyroid nodules | Total thyroidectomy | 12 | |
| Skowronska Jozwiak et al[19], 2010 | - | 2 | F | 49 | Lobular | Nil | 0 | Palpable thyroid nodules | Total thyroidectomy | - | - |
| 65 | - | lung | 48 | Palpable thyroid nodules | Disqualified | - | - | ||||
| Peteiro et al [23], 2005 | - | 1 | F | 42 | Ductal | Nil | 0 | Palpable thyroid nodules | Hemithyroidectomy | - | - |
| Garrido et al [21], 2006 | 2003-2005 | 1 | F | 43 | - | Nil | 24 | Palpable thyroid nodules, hoarseness, dysphonia, dysphagia | Total thyroidectomy and chemotherapy | Carcinomatous lymphangitis | 1 |
| Cichoń et al[22], 2006 | 1993-2005 | 1 | F | 50 | - | Nil | 120 | Multinodular goiter | Total thyroidectomy | Stable | 24 alive |
| Owens et al[24], 2005 | - | 1 | F | 64 | Invasive | Nil | 60 | Neck swelling and pain | Chemotherapy | - | - |
| Kim et al[7], 2005 | 1997-2003 | 5 | F | 36 | Ductal | LN, lung | 18 | Palpable thyroid nodules | Chemotherapy | Stable | 6 alive |
| 34 | Ductal | Lung, scalp | 25 | Multinodular goiter | Chemotherapy | Stable | 17 alive | ||||
| 44 | Ductal | Nil | 37 | Palpable thyroid nodules | Chemotherapy | Stable | 4 alive | ||||
| 55 | Ductal | Lung, parotid gland | 68 | Multinodular goiter | Chemotherapy | PD | 26 | ||||
| 45 | Ductal | Neck LN, lung, bone | 85 | Palpable thyroid nodules | Chemotherapy | Stable | 8 alive | ||||
| Wood et al[26], 2004 | 1985–2002 | 1 | F | 72 | Invasive | Nil | 180 | - | Total thyroidectomy | Stable | 36 alive |
| Mistelou et al[9], 2019 | 1998-2013 | 3 | F | 62 | Ductal | Pleura, chest wall, lung, heart, liver | - | - | - | - | - |
| 76 | Ductal | Pleura, bone, chest wall, lung, adrenal | - | - | - | - | - | ||||
| 76 | Lobular | Chest wall, pleura, lung, bone, liver | - | - | - | - | - | ||||
| Ridder et al[28], 2003 | - | 1 | F | - | Lobular | - | - | - | Hemithyroidectomy | - | 19 |
| Chung et al[30], 2001 | 1995-2000 | 6 | F | 49 | Lung, bone | ||||||
| 61 | Lung | ||||||||||
| 51 | Lung, bone, liver | ||||||||||
| 32 | Lung, liver | ||||||||||
| 22 | Bone, peritoneum | ||||||||||
| 33 | Lung | ||||||||||
| Bult et al[31], 2000 | - | 1 | F | 64 | Invasive | Nil | 144 | Palpable thyroid nodules | Chemotherapy + radiation | No response | 10 |
| Loo et al[29], 2003 | - | 1 | F | 52 | Ductal | bone | 96 | Palpable thyroid nodules | Chemotherapy | Stable | 24 alive |
| Gong et al[25], 2005 | - | 1 | F | 57 | Metaplastic | Nil | 24 | Palpable thyroid nodules, hoarseness, dysphasia | - | - | - |
| Jimenez et al[27], 2004 | - | 1 | F | 37 | - | Nil | 36 | Acute thyroiditis | Total thyroidectomy | Stable | 7 alive |
| Current study | 2015-2020 | 1 | F | 49 | Ductal | neck LN | 36 | Enlarged homogeneous thyroid | Chemotherapy | Stable | 6 alive |
Metastasis to the thyroid gland is rare due to its rich blood supply; however, reports of TM have increased in recent years as a result of more sophisticated diagnostic methods, such as FNAC and proton emission tomography[5,6]. The characteristics of TM from breast cancer are listed in Table 1. We collected information regarding age, sex, histology of the primary tumor, other sites of recurrence, the time interval between primary diagnosis and TM, presentation of TM, treatment of metastasis, response to the treatment and follow-up for 45 women with TM of breast cancer from 2000 to 2020[6-30]. The development of TM does not seem to be age-related and mostly occurs in women. The time interval between primary and metastatic disease and the prognosis of TM varies among the reports. In two patients, TMs were detected synchronously with the diagnosis of the primary cancer[17,21].
As shown in Table 1, TM has various clinical presentations. On physical examination, TM usually appears as a palpable mass or neck swelling, with or without dysphagia, hoarseness, dysphonia, pain and other symptoms, when thyroid metastasis is the first presentation of recurrent disease. In the reports that presented clinical information, most patients (90.1%) had thyroid nodules confirmed with ultrasound or CT, except one patient who had an enlarged thyroid with diffuse calcification[14] and one patient who presented with acute thyroiditis[25]. Only 5 out of 97 patients presented with diffuse goiter out of all primary cancer origins in a study at the Mayo Clinic[1]. Here, we report a rare case of TM presenting as diffuse goiter without thyroid nodules that had metastasized from primary breast carcinoma. This case report provides valuable information for clinicians, indicating that rapidly occurring diffuse goiter without other symptoms may be the first sign of TM in patients with malignant disease.
Thyroid metastasis usually occurs in patients with widespread metastatic disease, and the other sites of metastasis are primarily the bone and lung, according to the data shown in Table 1. Not all previously published studies contained information on other recurrence sites, and in 18 patients (41.9%, Table 1), the thyroid was the first and only site of recurrence. In the studies that contained histological information, primary breast cancer was mostly referred to as "invasive" carcinoma (85.7%), indicating that invasive carcinoma might be the most prevalent type of cancer to result in TM (Table 1). Among those reports, two patients had poorly differentiated adenocarcinoma as the primary disease[11], one patient had medullary carcinoma[11], one had metaplastic carcinoma[23], and one had mucinous carcinoma[7].
Patients with TMs generally have a poor prognosis[31,32]. Therapeutic choices for TM vary among reports, depending on the primary cancer origin, recurrence at other sites and the symptoms caused by TM. Surgical excision is considered the first choice for thyroid metastasis, and it has been reported that thyroidectomy improves the prognosis of patients[33]. Patients with multisite metastases are usually recommended for treatment with chemotherapeutic and endocrine approaches according to studies of metastatic breast cancer, but research on the effects of chemotherapy for thyroid metastasis is limited[34]. Among 30 patients, 16 were treated with chemotherapy, and 75% of them were clinically stable during follow-up (Table 1). It is believed that the biological behavior of primary cancer might be the primary influence on the prognosis of patients with TM[2]. Thus, therapeutic choices for TM patients should be determined individually and with multidisciplinary board discussion.
Another notable fact in this case report is that the patient had SMs in the contralateral cervical area of the primary tumor site, and no malignancy was previously found in the left breast or axillary region. SM to the lymph nodes in breast cancer is an important phenomenon, and it is critical to make the correct choice of surgical resection techniques and chemotherapies. It has been reported that only 6% of SMs occur on the contralateral side of the primary tumor site[4], with SMs accounting for 3.5% to 34.6% of metastatic lymph nodes[4,35,36]. Enlarged lymph nodes were detected before diffuse goiter. Aron et al[37] reported that the vast majority of metastases are able to remain dormant for a long period of time, referred to as metastatic dormancy. This suggests that thyroid metastasis probably occurred before the cervical lymph node changes and remained indolent and silent for a long period of time. It remains unknown whether the metastasis to the contralateral cervical lymph nodes originated from the thyroid metastasis or from the breast directly.
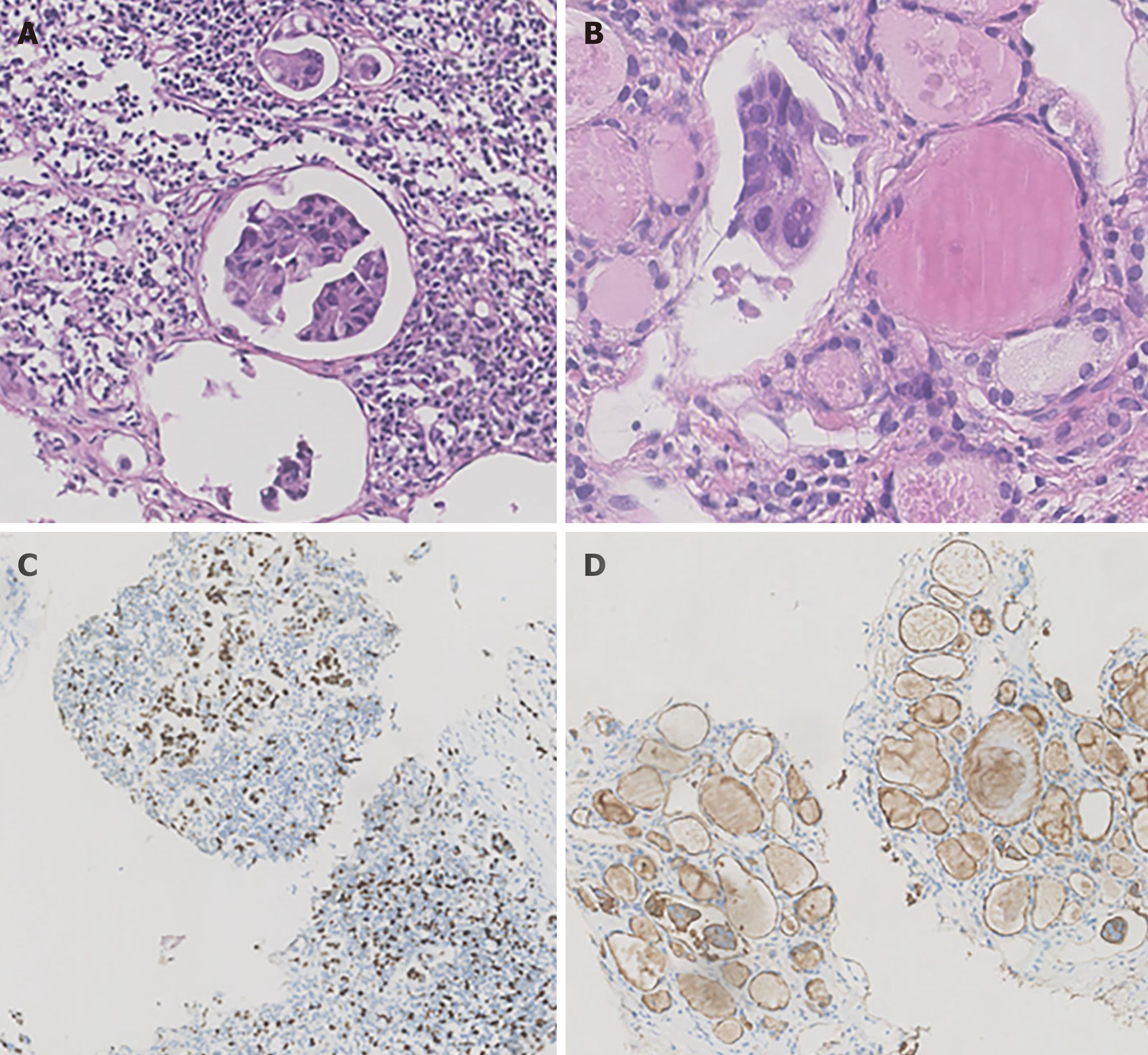
The IHC profiles of the patient changed during the process of cancer management. The immunostaining profiles of the core-tissue needle biopsy before NAC were ER (+++), PR (+++) and HER-2 (-), which changed to ER (+++), PR (++) and HER-2 (2+) in the surgical pathology results; finally, the cytology of enlarged lymph nodes and thyroid indicated triple negative breast cancer. Several studies have demonstrated that hormone receptor (ER and PR) status changes between initial core-tissue needle biopsy and surgical specimens obtained after chemotherapy and endocrine treatment. Tacca et al[38] reported that the positivity rate of HER-2 decreased from 42.0% to 32.1% after neoadjuvant chemotherapy, which could explain the conversion of HER-2 status between FNAC and surgery pathology results. A Chinese nationwide multicenter study showed that 37.7% of breast cancer patients have hormone receptor conversion in metastatic lesions, and patients with PR conversion had shorter overall survival times than patients whose PR remained positive (P = 0.016)[39]. This reveals that IHC profiles may change in the process of metastasis, which offers more information for making precise individual treatment decisions.
Few studies on diffuse goiter without thyroid nodules and SMs in the contralateral cervical area have been published. This report illustrates that rapidly occurring diffuse goiter without thyroid nodules may be the first sign of TM and that enlarged lymph nodes in the contralateral cervical area may indicate SMs of primary breast cancer. This finding raises awareness of these clinical presentations, which would be helpful for the early detection of metastatic breast cancer. In addition, IHC profiles may change during the process of metastasis, which indicates that biomarker testing for metastatic disease may be crucial for clinical decision-making.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alvarez-Bañuelos MT S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Hegerova L, Griebeler ML, Reynolds JP, Henry MR, Gharib H. Metastasis to the thyroid gland: report of a large series from the Mayo Clinic. Am J Clin Oncol. 2015;38:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Wu Y, Huang K, Zheng X, Gao M, Liu H. Tumor Biology is King: Secondary Tumors of the Thyroid From 2 Medical Centers in China. Cancer Control. 2020;27:1073274820945984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Rossi ED, Martini M, Straccia P, Gerhard R, Evangelista A, Pontecorvi A, Fadda G, Maria Larocca L, Schmitt F. Is thyroid gland only a "land" for primary malignancies? Diagn Cytopathol. 2015;43:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Chung HL, Sun J, Leung JWT. Breast Cancer Skip Metastases: Frequency, Associated Tumor Characteristics, and Role of Staging Nodal Ultrasound in Detection. AJR Am J Roentgenol. 2021;217:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Chung AY, Tran TB, Brumund KT, Weisman RA, Bouvet M. Metastases to the thyroid: a review of the literature from the last decade. Thyroid. 2012;22:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Kim TY, Kim WB, Gong G, Hong SJ, Shong YK. Metastasis to the thyroid diagnosed by fine-needle aspiration biopsy. Clin Endocrinol (Oxf). 2005;62:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Wang M, Liu X, Wei B, Liu N, Li Q, Su X. Mucinous breast carcinoma metastatic to thyroid gland: Report of a case diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2020;48:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Mistelou A, Papadatos SS, Kousi C, Lampri E, Mitsis M, Vougiouklakis T, Galani V. Thyroid Gland as a Target of Secondary Malignancies - an Autopsy Study and Review Data. Folia Med (Plovdiv). 2019;61:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Durmo R, Albano D, Giubbini R. Thyroid metastasis from breast cancer detected by 18F-FDG PET/CT. Endocrine. 2019;64:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Pensabene M, Stanzione B, Cerillo I, Ciancia G, Cozzolino I, Ruocco R, Condello C, Di Lorenzo G, Giuliano M, Forestieri V, Arpino G, De Placido S, Lauria R. It is no longer the time to disregard thyroid metastases from breast cancer: a case report and review of the literature. BMC Cancer. 2018;18:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Zhou L, Chen L, Xu D, Shao Q, Guo Z, Ge M. Breast cancer metastasis to thyroid: a retrospective analysis. Afr Health Sci. 2017;17:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Plonczak AM, DiMarco AN, Dina R, Gujral DM, Palazzo FF. Breast cancer metastases to the thyroid gland - an uncommon sentinel for diffuse metastatic disease: a case report and review of the literature. J Med Case Rep. 2017;11:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Magers MJ, Dueber JC, Lew M, Pang JC, Davenport RD. Metastatic ductal carcinoma of the breast to the thyroid gland diagnosed with fine needle aspiration: A case report with emphasis on morphologic and immunophenotypic features. Diagn Cytopathol. 2016;44:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Liu YP, Tiu CM, Chou YH, Hsu CY, King KL, Lai YC, Wang HK, Chiou HJ, Chang CY. Thyroid metastasis from breast cancer presenting with diffuse microcalcifications on sonography: a case report. J Clin Ultrasound. 2014;42:430-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Lacka K, Breborowicz D, Uliasz A, Teresiak M. Thyroid metastases from a breast cancer diagnosed by fine-needle aspiration biopsy. Case report and overview of the literature. Exp Oncol. 2012;34:129-133. [PubMed] |
| 16. | Kolarević D, Tomasević Z, Marković I, Zegarac M, Pupić G. Rare localisation of breast cancer metastasis to thyroid gland. Vojnosanit Pregl. 2012;69:1106-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 17. | Skowrońska-Jóźwiak E, Krawczyk-Rusiecka K, Adamczewski Z, Sporny S, Zadrożny M, Dedecjus M, Brzeziński J, Lewiński A. Metastases of breast cancer to the thyroid gland in two patients - a case report. Endokrynol Pol. 2010;61:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 18. | Leboeuf R, Bénard F, Langlois MF. Thyroid cancer presenting as a PET incidentaloma in a patient with concomitant breast cancer metastases to the thyroid. Clin Nucl Med. 2006;31:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Molina Garrido MJ, Guillén Ponce C, Maciá Escalante S, Martínez Y Sevila C, Carrato Mena A. Dysphagia and dysphonia in a woman with a previous breast cancer. Clin Transl Oncol. 2006;8:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Cichoń S, Anielski R, Konturek A, Barczyński M, Cichoń W. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbecks Arch Surg. 2006;391:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Peteiro A, Duarte AM, Honavar M. Breast carcinoma metastatic to follicular adenoma of the thyroid gland. Histopathology. 2005;46:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 22. | Owens CL, Basaria S, Nicol TL. Metastatic breast carcinoma involving the thyroid gland diagnosed by fine-needle aspiration: a case report. Diagn Cytopathol. 2005;33:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Gong Y, Jalali M, Staerkel G. Fine needle aspiration cytology of a thyroid metastasis of metaplastic breast carcinoma: a case report. Acta Cytol. 2005;49:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Wood K, Vini L, Harmer C. Metastases to the thyroid gland: the Royal Marsden experience. Eur J Surg Oncol. 2004;30:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Jiménez-Heffernan JA, Perez F, Hornedo J, Perna C, Lapuente F. Massive thyroid tumoral embolism from a breast carcinoma presenting as acute thyroiditis. Arch Pathol Lab Med. 2004;128:804-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | De Ridder M, Sermeus AB, Urbain D, Storme GA. Metastases to the thyroid gland-a report of six cases. Eur J Intern Med. 2003;14:377-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Loo CK, Burchett IJ. Fine needle aspiration biopsy of neuroendocrine breast carcinoma metastatic to the thyroid. A case report. Acta Cytol. 2003;47:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Chung SY, Kim EK, Kim JH, Oh KK, Kim DJ, Lee YH, An HJ, Kim JS. Sonographic findings of metastatic disease to the thyroid. Yonsei Med J. 2001;42:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Bult P, Verwiel JM, Wobbes T, Kooy-Smits MM, Biert J, Holland R. Malignant adenomyoepithelioma of the breast with metastasis in the thyroid gland 12 years after excision of the primary tumor. Case report and review of the literature. Virchows Arch. 2000;436:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Nguyen MS, Ginat DT, Giampoli EJ, O'Connell AM. Breast metastases to thyroid gland. Ultrasound Q. 2013;29:327-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Chen JY, Chen IW, Hsueh C, Chao TC, Gao BR, Lin JD. Synchronous diagnosis of metastatic cancer to the thyroid is associated with poor prognosis. Endocr Pathol. 2015;26:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Miraglia A, Carani C, Pontecorvi A. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf). 2007;66:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Romero Arenas MA, Ryu H, Lee S, Morris LF, Grubbs EG, Lee JE, Perrier ND. The role of thyroidectomy in metastatic disease to the thyroid gland. Ann Surg Oncol. 2014;21:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397:1750-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 927] [Article Influence: 231.8] [Reference Citation Analysis (0)] |
| 35. | Kim WG, Lee J. Axillary Skip Metastases and the False-Negative Rate of Sentinel Lymph Node Biopsy in Patients With Breast Cancer Are Related to Negative ALDH-1 Expression and Ki-67 Expression. Int J Surg Pathol. 2017;25:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Kwon Y, Ro J, Kang HS, Kim SK, Hong EK, Khang SK, Gong G, Ro JY. Clinicopathological parameters and biological markers predicting non-sentinel node metastasis in sentinel node-positive breast cancer patients. Oncol Rep. 2011;25:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Aron M, Kapila K, Verma K. Role of fine-needle aspiration cytology in the diagnosis of secondary tumors of the thyroid-twenty years' experience. Diagn Cytopathol. 2006;34:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Tacca O, Penault-Llorca F, Abrial C, Mouret-Reynier MA, Raoelfils I, Durando X, Achard JL, Gimbergues P, Curé H, Chollet P. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. 2007;12:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Peng JH, Zhang X, Song JL, Ran L, Luo R, Li HY, Wang YH. Neoadjuvant chemotherapy reduces the expression rates of ER, PR, HER2, Ki67, and P53 of invasive ductal carcinoma. Medicine (Baltimore). 2019;98:e13554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |