Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1032
Peer-review started: June 3, 2021
First decision: June 25, 2021
Revised: July 7, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 21, 2022
Processing time: 226 Days and 7.5 Hours
Multiple endocrine neoplasia type 1 (MEN1) is a rare hereditary tumor syndrome inherited in an autosomal dominant manner and presents mostly as parathyroid, endocrine pancreas (such as gastrinoma) and anterior pituitary tumors. At present, papillary thyroid carcinoma (PTC) and nodular goiter are not regarded as components of MEN1.
A 35-year-old woman presented with MEN1 accompanied by coinstantaneous PTC and nodular goiter. The pathological diagnosis was PTC with cervical lymph node metastasis, nodular goiter, parathyroid cyst and adenomatoid hyperplasia. Genetic testing was performed and a MEN1 gene mutation was detected. The patient underwent unilateral lobectomy of the thyroid gland and surgical removal of the parathyroid tumors. At 18 mo of follow-up, ultrasonic examination of the neck showed no abnormality. Serum calcium and parathyroid hormone levels were normal. No new MEN1-associated tumors were detected.
The role of inactivating mutations of MEN1 gene in tumorigenesis of PTC and/or nodular goiter remains to be determined by more case reports and further research.
Core Tip: Multiple endocrine neoplasia type 1 (MEN1) is a rare hereditary tumor syndrome inherited in an autosomal dominant manner and presents mostly as parathyroid, endocrine pancreas and anterior pituitary tumors. We here report a case of MEN1 combined with papillary thyroid carcinoma (PTC) and nodular goiter, and review the literature. The role of inactivating mutations of MEN1 gene in tumorigenesis of PTC and/or nodular goiter is still controversial. There may be a potential correlation between MEN1 syndrome and papillary thyroid carcinoma/ nodular goiter.
- Citation: Xu JL, Dong S, Sun LL, Zhu JX, Liu J. Multiple endocrine neoplasia type 1 combined with thyroid neoplasm: A case report and review of literatures. World J Clin Cases 2022; 10(3): 1032-1040
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1032.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1032
Multiple endocrine neoplasia type 1 (MEN1) is a rare hereditary tumor syndrome inherited in an autosomal dominant manner and characterized by a predisposition to a multitude of endocrine neoplasms, mostly of the parathyroid, endocrine pancreas and anterior pituitary tumors. Other endocrine tumors in MEN1 include gastroenteropancreatic neuroendocrine tumors, adrenocortical tumors, and rarely pheochromocytoma[1-3]. MEN1 is caused by inactivating mutations of MEN1 gene. The incidence is 1/10000–1/100000. MEN1 is a tumor suppressor gene and is located on human chro
It has been reported that expression of menin is preserved in human normal thyroid tissue and thyroid tumors, but it can be decreased or absent in certain types of thyroid tumors[11-14]. Currently, little is known about the prevalence of papillary thyroid carcinoma (PTC) and nodular goiter in MEN1 patients, and it is unclear whether tumorigenesis of these thyroid tumors is MEN1 related. The role of menin protein deficiency in tumorigenesis of PTC and/or nodular goiter is still controversial. Here, we present a patient with MEN1 accompanied by coinstantaneous PTC and nodular goiter and review the related literature.
A 35-year-old woman presented with a neck mass on physical examination, but without abnormal feelings.
The patient immediately came to our hospital after discovery of the neck mass.
The patient underwent partial resection of the pancreas and stomach for pancreatic and gastroduodenal neuroendocrine tumor 4 years ago. The patient had a history of pituitary microadenoma for 2 years, which was not treated but under observation.
Her father had a history of stomach surgery, but the details were unknown since he died 20 years ago. Other relatives of the patient had no symptoms of MEN1 syndrome.
Her father had a history of stomach surgery, but the details were unknown since he died 20 years ago. Other relatives of the patient had no symptoms of MEN1 syndrome.
There was an anterior neck mass which was movable due to breathing.
The results of biochemical tests were as follows: serum calcium 2.82 mmol/L (reference range: 2.11–2.52 mmol/L); albumin 47.7 g/L (reference range: 40–55 g/L); serum intact parathyroid hormone (iPTH) elevated to 676.3 pg/mL (reference range: 12–88 pg/mL); gastrin 17: 0.8 pmol/L (reference range: 1–15 pmol/L); thyroid function was normal; thyroid peroxidase antibody was 23.98 IU/mL (reference range: < 35 IU/mL).
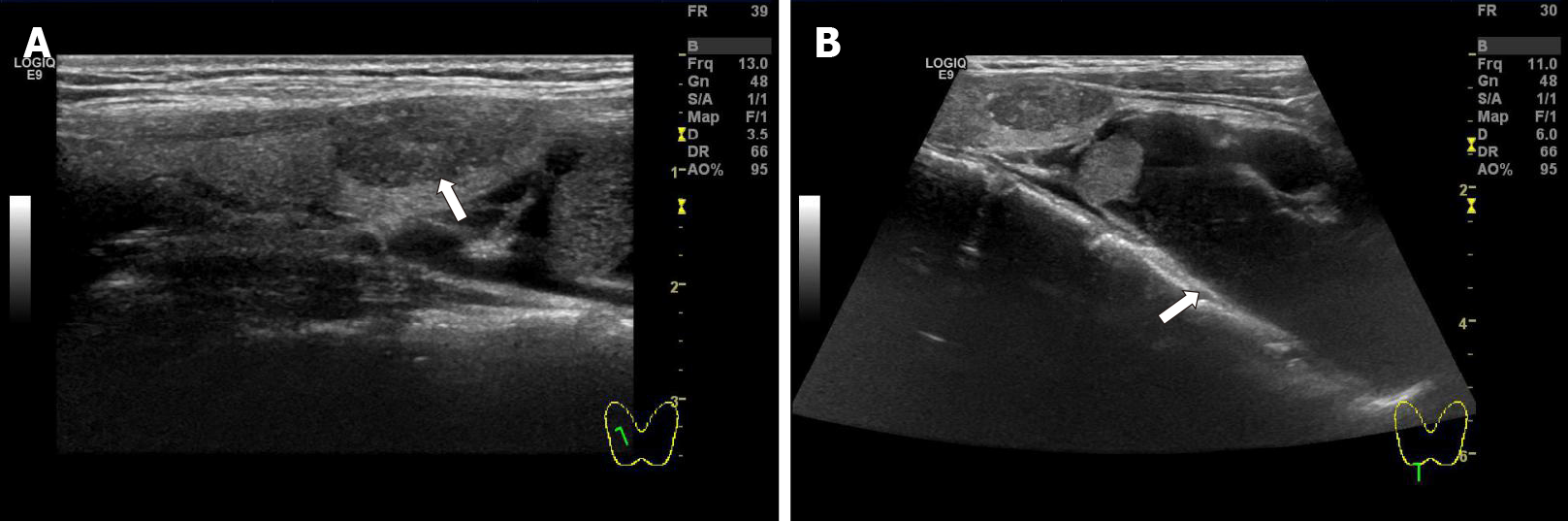
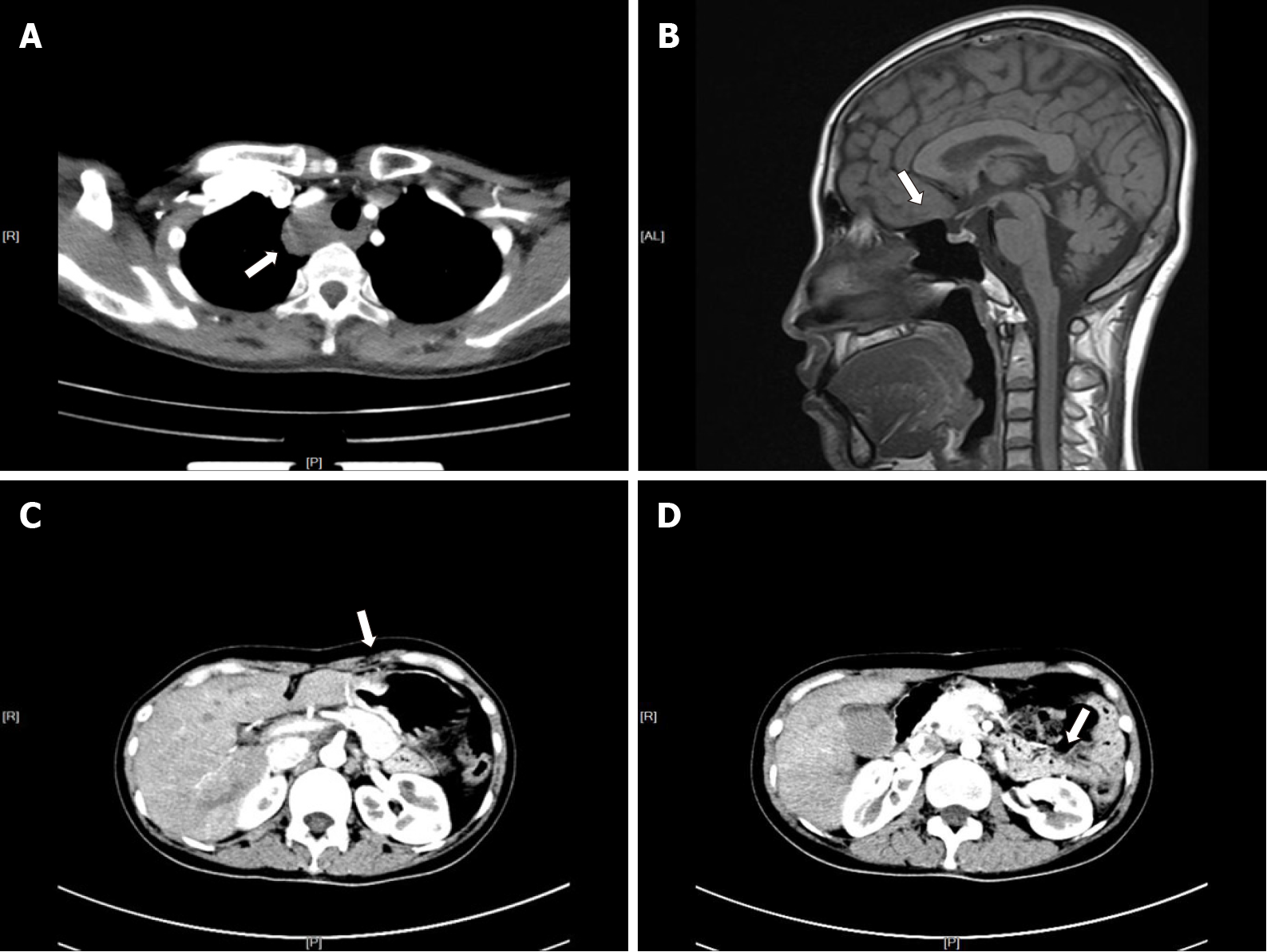
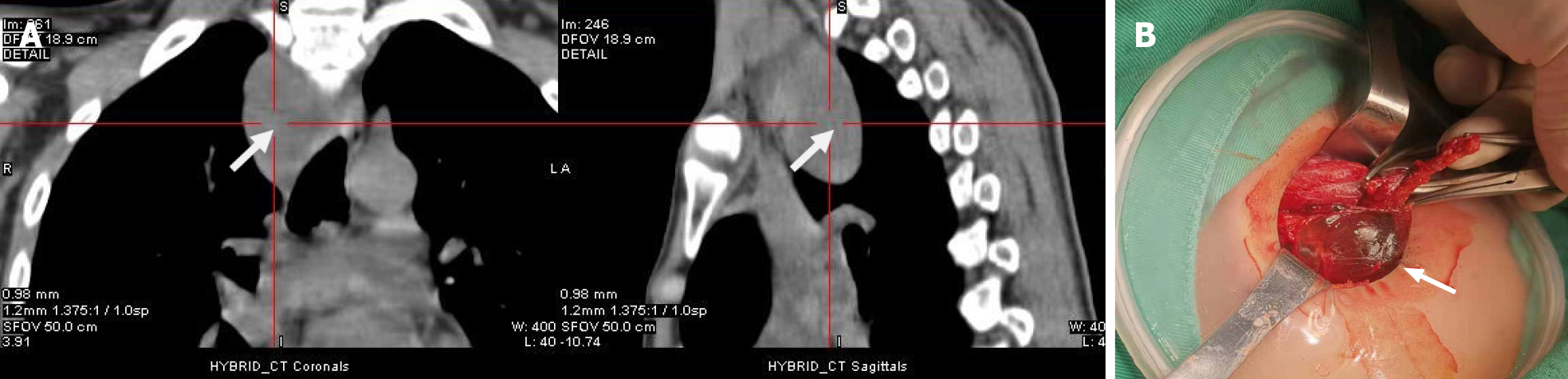
Neck ultrasound revealed a 64 mm × 28 mm × 45 mm cystic mass located below the right lobe of the thyroid gland with a well-defined smooth border, and several solid nodules were detected in the right thyroid lobe, with the largest (18 mm × 10 mm × 8 mm) in the right lower thyroid lobe. The largest nodule in the thyroid had an unclear boundary, dotted calcification and abundant internal blood flow (Figure 1A and B). Computed tomography (CT) or magnetic resonance imaging examination showed changes in the pituitary region; lesions in the right thyroid lobe and superior mediastinum; and changes after partial gastrectomy and in the tail of the pancreas (Figure 2A–D). In 99mTc-methoxyisobutyl isonitrile scintigraphy, tracer uptake was increased in the right lower region of the thyroid gland and mediastinum, and no abnormal retention of the tracer in the late phase was observed. No uptake was detected in other regions. Preoperative sestamibi single-photon emission computed tomography (SPECT)/CT found a lesion in the right lower thyroid lobe and part of which extended to the superior mediastinum (Figure 3A). Bone scanning showed T-scores -2.6 and Z-scores -2.0.
The final diagnosis of the presented case was thyroid neoplasm (right lobe).
Because the patient had a large functional parathyroid cyst, her serum calcium and iPTH levels were significantly abnormal and several solid nodules were detected in the right thyroid lobe (the largest nodule was suspected to be malignant by ultrasound). Fine needle aspiration (FNA) could not be performed for parathyroid cysts. And the patient refused FNA of the thyroid nodule before operation for fear of additional injury and requested to perform rapid intraoperative pathological diagnosis. Parathyroidectomy and unilateral thyroid lobectomy were recommended and performed with the patient’s consent.
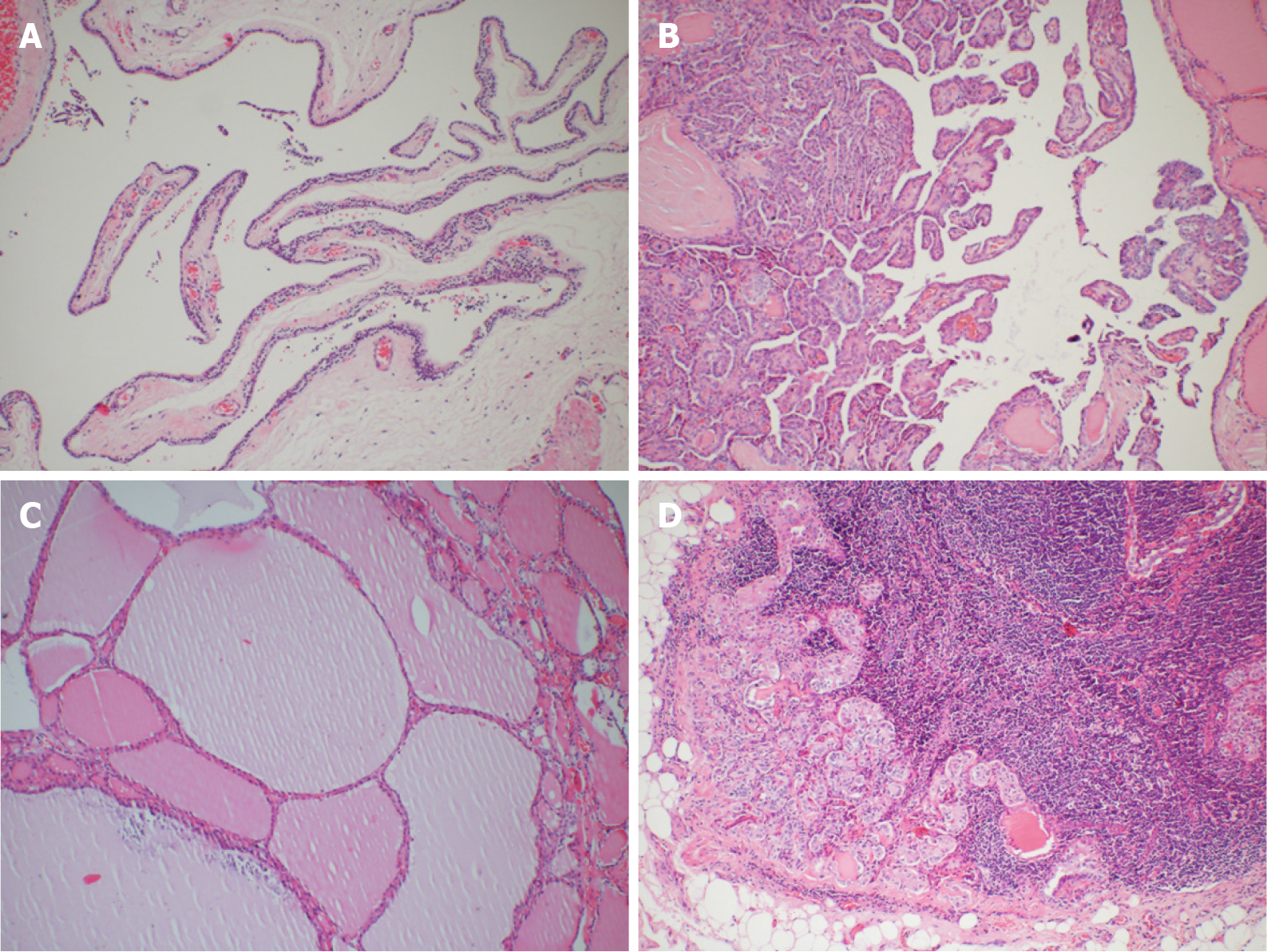
During the operation, gross examination of the largest cyst showed that it was partially surrounded and contiguous with the right lower thyroid lobe and extended to the superior mediastinum, and it was peeled off easily from the right lower thyroid lobe and mediastinum. Furthermore, it was filled with clear watery fluid, suggesting that it was a parathyroid cyst (Figure 3B). The largest cyst and right lobe of the thyroid were removed. The central lymph nodes were cleared. Intraoperative frozen section pathology showed that the largest cyst was the source of parathyroid, and parathyroid carcinoma was excluded. A PTC (maximum diameter 1 cm, invading the capsule) was found in the thyroid right lobe. In the central lymph nodes, 4/5 had cancer metastasis. iPTH at 20 min after resection was decreased to 253.4 pg/mL. Other parathyroid glands were explored. Two upper parathyroid glands were normal. The left lower parathyroid was enlarged and removed. Rapid intraoperative pathological examination revealed that the left lower parathyroid had adenomatoid hyperplasia. After another 20 min, serum iPTH decreased to 63.9 pg/mL. The postoperative pathological results were PTC (maximum diameter 1cm, invading the capsule) in the right thyroid lobe and nodular goiter. In the central lymph nodes, 4/5 had PTC metastasis. In the right cervical cysts, parathyroid cysts (monolocular) showed adenomatous hyperplasia. In the left lower parathyroid, adenomatous hyperplasia was observed (Figure 4). The patient had indications for a total thyroidectomy because of central compartment lymph node metastasis. But the patient had a strong desire to preserve the thyroid gland and refused to remove the left lobe.
After surgery, the patient was closely monitored on serum calcium and was pumped calcium gluconate 2.0 g/day through a central venous catheter for 5 d. After taking 1.5 g calcium carbonate daily for 3 mo, her serum calcium levels returned to normal. Meanwhile the patient received endocrine suppression therapy after the operation.
After the operation, genetic analysis was performed, and a germline MEN1 gene mutation was detected. There was a heterozygous mutation in the second exon of MEN1 gene which was 357-360delCTGT. During follow-up, there was no hypoparathyroidism or other complications. The laboratory data on postoperative day 2 showed that serum calcium was 1.98 mmol/L and iPTH was 24.1 pg/mL. After taking 1.5 g calcium carbonate daily for 3 mo, the patient’s laboratory data improved: calcium 1.98 mmol/L and iPTH 43.7 pg/mL. Calcium was 2.47 mmol/L and iPTH was 61 pg/mL after 18 mo. One month after the operation, the dose of levothyroxine was reduced from 75 mg to 50 mg.
Currently, the role of inactivating mutations of MEN1 gene in tumorigenesis of PTC and/or nodular goiter is still controversial. It remains to be determined by more case reports and further research.
MEN1 is a rare hereditary tumor syndrome inherited in an autosomal dominant manner and presents mostly as the parathyroid[15], endocrine pancreas (such as gastrinoma)[16] and anterior pituitary tumors[17]. Other endocrine and nonendocrine lesions of MEN1, such as adrenal cortical tumors, carcinoids of the bronchi, gastrointestinal tract and thymus lipomas, angiofibromas, and collagenomas, have also been described[1,18]. MEN1 with a large functioning parathyroid cyst is rare. Cavalli et al[15] found that approximately 300 cases of sporadic parathyroid cysts had been reported up to 2017, and only two cases have been described in MEN1. Parathyroid cysts can be divided into functioning and nonfunctioning, and most parathyroid cysts are nonfunctioning. The functioning parathyroid cysts are more likely to be caused by degenerative changes in parathyroid adenoma than hyperplasia in our case[19]. In many cases, it is difficult to diagnose the nature of the cyst merely by ultrasound before surgery. Parathyroid cysts need to be differentiated from lymphatic cysts, cystic thyroid nodules and hemangioma[20,21]. The diagnostic rate can be improved by laboratory examination and other imaging examinations. Preoperative SPECT/CT is useful in localizing parathyroid cysts in most patients, with an accuracy rate up to 79% if it is interpreted in combination with cervical ultrasound images[22]. Postoperative pathological diagnosis is the gold standard. The clinical features of hyperparathyroidism (HPT) with MEN1 are similar to those with sporadic HPT, but the former is often more aggressive. For patients who have HPT with MEN1, early surgical treatment is preferred. Surgical treatment should be considered in asymptomatic patients when (1) serum calcium is higher than the reference range 2.52mmol/L; (2) glomerular filtration < 60 mL/min; (3) bone mineral density at any point is 2.5 or lower, or patient has fragility fractures; and (4) age < 50 years. Whether early surgery can reduce the incidence rate and mortality is not clear. For patients who have HPT with MEN1, especially in asymptomatic or mild and young patients, early parathyroidectomy can reduce the long-term effects of HPT on the patients, especially reducing the bone loss. Although our case was a young woman with normal upper parathyroid glands on both sides, and bilateral lower parathyroid glands showed adenoma-like changes and hyperplasia, parathyroid hormone and serum calcium levels had increased significantly. To avoid a permanent hypoparathyroidism, we did not perform subtotal parathyroidectomy or a total parathyroidectomy with parathyroid tissue autotransplantation. And based on our past experience, unless it is a parathyroid cancer, surgery can achieve a better treatment effect by removing the problematic parathyroid glands. In the present case, the parathyroid hormone had decreased from 676.3 pg/mL to 63.9 pg/mL after the removal of the bilateral inferior parathyroid glands, and the patient’s serum parathyroid hormone was 47.6 pg/mL at 27 mo postoperatively. FNA is the most useful means for diagnosis of thyroid nodules, however, it is not widely accepted for diagnosis of parathyroid tumors due to the risk of dissemination of tumor cells.
Two different forms of MEN1, sporadic and familial, have been described[23]. The sporadic form presents with two of the three principal MEN1-related endocrine tumors (parathyroid adenoma, enteropancreatic tumor and pituitary tumor) within a single patient, while the familial form consists of MEN1 with at least one first-degree relative showing one of the endocrine characteristic tumors[24-27]. In our case, the patient did not provide a clear family history. It is still unclear what form of MEN1 our patient had. There is no evidence to exclude the accidental occurrence of MEN1 with PTC and nodular goiter in this patient.
So far, few cases of thyroid carcinoma and/or nodular goiter combined with MEN1 have been reported. Whether there is a correlation among them is still controversial[12,28,29]. Hill et al[30] investigated the probability of concomitant thyroid cancer in patients with MEN1. They found that in patients with MEN1, a 28% substantial incidence of thyroid cancer was observed and all cancers in MEN1 patients were common PTCs histologically (100%). We noticed that only PTCs that measured >1 cm in diameter were considered in the report by Hill et al[30]. But at present, papillary thyroid microcarcinoma (PTMC) accounts for more than half of all PTCs in clinical practice. If we take these PTMC cases into account, the actual incidence of papillary thyroid carcinoma would be higher in MEN1 patients. Our case was PTMC. MEN1 is caused by inactivating mutations of the MEN1 gene that encodes the protein menin[2]. Menin is a nuclear protein whose interaction with several other nuclear proteins indicates a role in transcriptional regulation. Previous studies have supported a role for MEN1 in controlling cell growth and differentiation, and in sensing or repairing DNA damage as well. The loss of menin function in tumor precursor cells is involved in the mechanism underlying tumor formation in MEN1[8-10,31-33]. Research showed that the inactivation of menin in the thyroid gland of young mice affected the proliferation of follicular cells[13]. Capraru et al[11] showed that the expression of menin was positive, identical to normal thyroid tissue, but it could be decreased or absent in some thyroid tumors including PTC. As is well known, mutations of the MEN1 gene cause deficiency in the menin protein in MEN1 patients. There was a heterozygous 357-360delCTGT mutation in the second exon of MEN1 gene in our case. Further molecular studies are needed to evaluate the role of menin protein deficiency in tumorigenesis of PTC and nodular goiter. Kazubskaia et al[34] investigated follicular cell (papillary and follicular) thyroid carcinoma, genetic inheritance and molecular diagnostic markers. They believed that familial PTC and PTC may be a component of multitumor syndromes, such as MEN1, Cowden syndrome, and familial adenomatous polyposis. We speculate upon possible reasons for the association between MEN1 and PTC/nodular goiter. Loss of heterozygosity (LOH) studies have been used to identify sites harboring tumor suppressor genes involved in tumor initiation or progression. MEN1 is a tumor suppressor gene located on human chromosome 11q13. Specific genomic areas, such as 3p22, 7q31, 11q13 and 11q23, have been reported to be involved in some epithelial or endocrine tumor types[35]. PTC is also one of the endocrine tumors. Whether inactivating mutations of 11q13 can induce PTC remains to be further studied. Chu et al[36,37] found MEN1 deletion in neurotrophic tyrosine kinase receptor (NTRK)-rearranged PTC patients. They also proved that nucleotide variants and indels in pTERT, MEN1 and CDH1 were observed in several kinase fusion-related PTCs. The relationship among MEN1, PTC and NTRK needs to be further studied. In the analysis of MEN1 gene, about 20% may have false negative results due to the diversity of the causative mutation and scattered position in the entire open reading frame. Moreover, approximately 10% new germline mutations are being detected in the overall MEN1 patients, which may be the reason why the genotype- phenotype correlations could not be identical in 10%-30% of patients[38]. In 2008, Kim et al[29] reported the first case of PTC combined with MEN1 in Korea. Their patient’s genetic analysis of DNA had revealed no germline mutation in MEN1 gene locus. But there was a genetic mutation in our case. Menin, the protein encoded by MEN1 gene is ubiquitously expressed in endocrine tissues, is less in many endocrine tumors including PTC. Deletion of the MEN1 tumor suppression still might be etiologically related to the oncogenesis of PTC. DNA analysis of more samples with PTC combined with MEN1 may be helpful. Now it is very difficult to confirm the LOH of MEN1 gene completely and accurately, which has dozens of polymorphic markers[29]. The clinical aspects and molecular genetics of MEN1 were reviewed together with the reported 1336 mutations[39]. It has been proved that many of the diseases that have been widely believed to be associated with MEN1 mutations, such as pituitary tumors, lung carcinoids, etc., sometimes failed to exhibit meaningful LOH at 11q13[40]. For PTC, some people have used three of polymorphic markers to test one patient’s sample, with limited results[12]. The exact significance remains to be determined by more case reports and further research. It is another possibility that MEN1 patients who develop PTC may have specific MEN1 mutations of the affected allele that act like dominant oncogenes with regard to thyroid cancer oncogenesis. If any of these scenarios was the case, the MEN1 gene could play a role in the development of the papillary cancer without obvious LOH of the gene locus.
In summary, we presented a rare case of MEN1 combined with PTC and nodular goiter, in which a germline mutation of the MEN1 gene was detected. It is possible there is a potential correlation between MEN1 syndrome and PTC/nodular goiter. However, further studies and additional case reports are required to clarify it.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casella C S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Marini F, Falchetti A, Del Monte F, Carbonell Sala S, Gozzini A, Luzi E, Brandi ML. Multiple endocrine neoplasia type 1. Orphanet J Rare Dis. 2006;1:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Kamilaris CDC, Stratakis CA. Multiple Endocrine Neoplasia Type 1 (MEN1): An Update and the Significance of Early Genetic and Clinical Diagnosis. Front Endocrinol (Lausanne). 2019;10:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 3. | Giudici F, Cavalli T, Giusti F, Gronchi G, Batignani G, Tonelli F, Brandi ML. Natural History of MEN1 GEP-NET: Single-Center Experience After a Long Follow-Up. World J Surg. 2017;41:2312-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Shi J, Gao Z, Gao J, Li G, Chen Y. Predictors and outcome surgery for posterior cortex epilepsies. Clin Neurol Neurosurg. 2018;171:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Tsukada T, Nagamura Y, Ohkura N. MEN1 gene and its mutations: basic and clinical implications. Cancer Sci. 2009;100:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Marx SJ. Recent Topics Around Multiple Endocrine Neoplasia Type 1. J Clin Endocrinol Metab. 2018;103:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Suzuki J, Yamada T, Inoue K, Nabe S, Kuwahara M, Takemori N, Takemori A, Matsuda S, Kanoh M, Imai Y, Yasukawa M, Yamashita M. The tumor suppressor menin prevents effector CD8 T-cell dysfunction by targeting mTORC1-dependent metabolic activation. Nat Commun. 2018;9:3296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Li JWY, Hua X, Reidy-Lagunes D, Untch BR. MENIN loss as a tissue-specific driver of tumorigenesis. Mol Cell Endocrinol. 2018;469:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Cheng P, Li G, Yang SS, Liu R, Jin G, Zhou XY, Hu XG. Tumor suppressor Menin acts as a corepressor of LXRα to inhibit hepatic lipogenesis. FEBS Lett. 2015;589:3079-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Dreijerink KMA, Groner AC, Vos ESM, Font-Tello A, Gu L, Chi D, Reyes J, Cook J, Lim E, Lin CY, de Laat W, Rao PK, Long HW, Brown M. Enhancer-Mediated Oncogenic Function of the Menin Tumor Suppressor in Breast Cancer. Cell Rep. 2017;18:2359-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Capraru OM, Decaussin-Petrucci M, Joly MO, Borda A, Fanfaret IS, Borson-Chazot F, Selmi-Ruby S. EXPRESSION OF MENIN IN THE HUMAN THYROID GLAND. Acta Endocrinol (Buchar). 2017;13:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Desai D, McPherson LA, Higgins JP, Weigel RJ. Genetic analysis of a papillary thyroid carcinoma in a patient with MEN1. Ann Surg Oncol. 2001;8:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Căpraru OM, Berger N, Gadot N, Decaussin-Petrucci M, Zhang C, Borda A, Szilágyi T, Borson-Chazot F, Selmi-Ruby S. Histopathological changes induced by selective inactivation of menin on the thyroid gland in RET÷PTC3 and E7 transgenic mice. A study of 77 cases. Rom J Morphol Embryol. 2016;57:91-98. [PubMed] |
| 14. | Lodewijk L, Bongers PJ, Kist JW, Conemans EB, de Laat JM, Pieterman CR, van der Horst-Schrivers AN, Jorna C, Hermus AR, Dekkers OM, de Herder WW, Drent ML, Bisschop PH, Havekes B, Rinkes IH, Vriens MR, Valk GD. Thyroid incidentalomas in patients with multiple endocrine neoplasia type 1. Eur J Endocrinol. 2015;172:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Cavalli T, Giudici F, Nesi G, Amorosi A, Santi R, Brandi ML, Tonelli F. Cystic parathyroid glands in MEN1: A rare entity? Fam Cancer. 2017;16:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore). 2013;92:135-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Delemer B. MEN1 and pituitary adenomas. Ann Endocrinol (Paris). 2012;73:59-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Carroll RW. Multiple endocrine neoplasia type 1 (MEN1). Asia Pac J Clin Oncol. 2013;9:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Tamiya H, Miyakawa M, Suzuki H, Takeshita A, Ohashi K, Usui T, Miura D, Takeuchi Y. A large functioning parathyroid cyst in a patient with multiple endocrine neoplasia type 1. Endocr J. 2013;60:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Papavramidis TS, Chorti A, Pliakos I, Panidis S, Michalopoulos A. Parathyroid cysts: A review of 359 patients reported in the international literature. Medicine (Baltimore). 2018;97:e11399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Arduc A, Tutuncu YA, Dogan BA, Arikan Ileri AB, Tuna MM, Ozcan HN, Isik S, Berker D, Guler S. Parathyroid cysts. Am Surg. 2015;81:E163-E165. [PubMed] |
| 22. | Johnson NA, Yip L, Tublin ME. Cystic parathyroid adenoma: sonographic features and correlation with 99mTc-sestamibi SPECT findings. AJR Am J Roentgenol. 2010;195:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Makri A, Bonella MB, Keil MF, Hernandez-Ramirez L, Paluch G, Tirosh A, Saldarriaga C, Chittiboina P, Marx SJ, Stratakis CA, Lodish M. Children with MEN1 gene mutations may present first (and at a young age) with Cushing disease. Clin Endocrinol (Oxf). 2018;89:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Hendy GN, Cole DE. Genetic defects associated with familial and sporadic hyperparathyroidism. Front Horm Res. 2013;41:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Beijers HJBH, Stikkelbroeck NML, Mensenkamp AR, Pfundt R, van der Luijt RB, Timmers HJLM, Hermus ARMM, Kempers MJE. Germline and somatic mosaicism in a family with multiple endocrine neoplasia type 1 (MEN1) syndrome. Eur J Endocrinol. 2019;180:K15-K19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Horvath A, Stratakis CA. Clinical and molecular genetics of acromegaly: MEN1, Carney complex, McCune-Albright syndrome, familial acromegaly and genetic defects in sporadic tumors. Rev Endocr Metab Disord. 2008;9:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Karges W, Jostarndt K, Maier S, Flemming A, Weitz M, Wissmann A, Feldmann B, Dralle H, Wagner P, Boehm BO. Multiple endocrine neoplasia type 1 (MEN1) gene mutations in a subset of patients with sporadic and familial primary hyperparathyroidism target the coding sequence but spare the promoter region. J Endocrinol. 2000;166:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kasaian K, Chindris AM, Wiseman SM, Mungall KL, Zeng T, Tse K, Schein JE, Rivera M, Necela BM, Kachergus JM, Casler JD, Mungall AJ, Moore RA, Marra MA, Copland JA, Thompson EA, Smallridge RC, Jones SJ. MEN1 mutations in Hürthle cell (oncocytic) thyroid carcinoma. J Clin Endocrinol Metab. 2015;100:E611-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Kim HJ, Park JS, Kim CS, Kang ES, Cha BS, Lim SK, Kim KR, Lee HC, Ahn CW. A case of multiple endocrine neoplasia type 1 combined with papillary thyroid carcinoma. Yonsei Med J. 2008;49:503-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Hill KA, Yip L, Carty SE, McCoy KL. Concomitant Thyroid Cancer in Patients with Multiple Endocrine Neoplasia Type 1 Undergoing Surgery for Primary Hyperparathyroidism. Thyroid. 2019;29:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Razmara M, Monazzam A, Skogseid B. Reduced menin expression impairs rapamycin effects as evidenced by an increase in mTORC2 signaling and cell migration. Cell Commun Signal. 2018;16:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Dreijerink KMA, Timmers HTM, Brown M. Twenty years of menin: emerging opportunities for restoration of transcriptional regulation in MEN1. Endocr Relat Cancer. 2017;24:T135-T145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Wu G, Yuan M, Shen S, Ma X, Fang J, Zhu L, Sun L, Liu Z, He X, Huang, Li T, Li C, Wu J, Hu X, Li Z, Song L, Qu K, Zhang H, Gao P. Menin enhances c-Myc-mediated transcription to promote cancer progression. Nat Commun. 2017;8:15278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Kazubskaia TP, Kozlova VM, Kondrat'eva TT, Pavlovskaia AI, Marakhonov AV, Baranova AV, Ivanova NI, Stepanova AA, Poliakov AV, Belev NF, Brzhezovskiĭ VZh. [Follicular cell (papillary and follicular) thyroid carcinoma, genetic inheritance, and molecular diagnostic markers]. Arkh Patol. 2014;76:3-12. [PubMed] |
| 35. | Oriola J, Halperin I, Mallofré C, Muntané J, Angel M, Rivera-Fillat F. Screening of selected genomic areas potentially involved in thyroid neoplasms. Eur J Cancer. 2001;37:2470-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Chu YH, Dias-Santagata D, Farahani AA, Boyraz B, Faquin WC, Nosé V, Sadow PM. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod Pathol. 2020;33:2186-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 37. | Chu YH, Wirth LJ, Farahani AA, Nosé V, Faquin WC, Dias-Santagata D, Sadow PM. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol. 2020;33:2458-2472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Guru SC, Manickam P, Crabtree JS, Olufemi SE, Agarwal SK, Debelenko LV. Identification and characterization of the multiple endocrine neoplasia type 1 (MEN1) gene. J Intern Med. 1998;243:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 40. | Dong Q, Debelenko LV, Chandrasekharappa SC, Emmert-Buck MR, Zhuang Z, Guru SC, Manickam P, Skarulis M, Lubensky IA, Liotta LA, Collins FS, Marx SJ, Spiegel AM. Loss of heterozygosity at 11q13: analysis of pituitary tumors, lung carcinoids, lipomas, and other uncommon tumors in subjects with familial multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 1997;82:1416-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |