Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.9970
Peer-review started: March 23, 2022
First decision: June 16, 2022
Revised: July 27, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 188 Days and 2.3 Hours
An outbreak of coronavirus disease 2019 (COVID-19) occurred in December 2019 due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a strain of SARS-CoV. Patients infected with the virus present a wide spectrum of manifestations ranging from mild flu-like symptoms, cough, fever and fatigue to severe lung injury, appearing as bilateral interstitial pneumonia or acute res
Core Tip: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) disease. Although SARS-CoV-2 infection predominantly offends the respiratory system, it leads to various cardiovascular complications as well. SARS-CoV-2 infected patients with a history of cardiovascular disease exhibit poor outcomes. The main COVID-19 related cardiovascular complications include acute coronary syndromes, myocarditis, heart failure and arrhythmias.
- Citation: Xanthopoulos A, Bourazana A, Giamouzis G, Skoularigki E, Dimos A, Zagouras A, Papamichalis M, Leventis I, Magouliotis DE, Triposkiadis F, Skoularigis J. COVID-19 and the heart. World J Clin Cases 2022; 10(28): 9970-9984
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/9970.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.9970
A global outbreak of coronavirus disease 2019 (COVID-19) occurred in December 2019 due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1,2]. Patients susceptible to the virus present a wide spectrum of manifestations ranging from mild flu-like symptoms, cough, fever and fatigue to severe lung injury, appearing as bilateral interstitial pneumonia or acute respiratory failure[3]. The majority of COVID-19 patients who progress to critically ill tend to have mild symptomatology in the early phase of the disease. The condition of these subjects abruptly worsens in the later phases of the disease or in the recovery process, usually due to “cytokine storm” and acute lung injury [acute respiratory distress syndrome (ARDS)][4]. In general, acute respiratory syndromes can be divided into those stemming from direct lung injury factors (i.e. bacterial, viral or fungal pneumonia) and those from indirect lung-injury risk factors (i.e. sepsis, hemorrhagic shock or pancreatitis)[5].
Until now, more than 50 million people worldwide have been infected with the virus, while it has been considered responsible for more than 2230000 deaths[6,7]. Recent literature indicates that SARS-CoV-2 infected patients with a history of cardiovascular disease display increased mortality. Patients with a history of hypertension, diabetes and obesity are also considered frail[8,9]. Additional risk factors are senility, chronic renal dysfunction, chronic respiratory diseases and concomitant malignancies[10]. Although SARS-CoV-2 infection predominantly offends the respiratory system, it is liable for various cardiovascular complications as well. The usual management of patients with COVID-19 includes self-isolation and supportive measures (antipyretics, rehydration) in mild cases (absence of signs of severe or critical disease), and hospitalization in severe (oxygen saturation < 90%on room air, signs of pneumonia, signs of respiratory distress) and critical cases (requires life sustaining treatment, ARDS, sepsis and septic shock)[11]. In this comprehensive state of the art review, we will attempt to summarize COVID-19 related cardiovascular complications, with a special concern in enlightening the subjacent mechanisms and pathophysiological traits below the main cardiovascular manifestations.
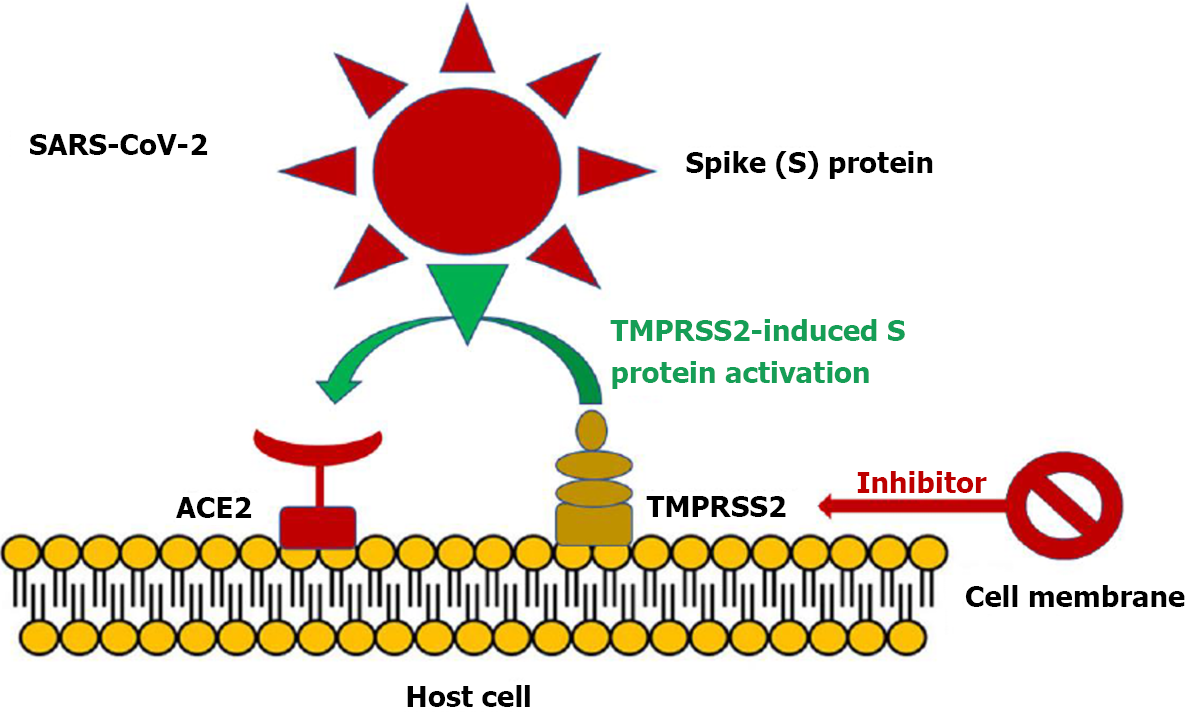
The affinity of SAR-CoV-2 infection to angiotensin converting enzyme 2 (ACE-2) receptor has been proposed to be the core of the disease’s pathophysiology, since it not only causes cardiovascular complications but also propagates disproportionately in cases with a pre-existing cardiovascular impairment[12]. ACE-2 is a membrane bound carboxypeptidase. Its normal function is to convert Ang II to Ang (1-7) and Ang I to Ang (1-9)[4]. After being enzymatically cleaved at sites S1/S2 by protease furin and at S2’ region by serine protease transmembrane protease serine 2 (TMPRSS2), SARS-CoV-2 anchors its surface glycoprotein spike (S) to ACE-2 to create its path to the host cells (Figure 1)[13].
Except for the transmembranic ACE-2 the receptor can also be found in a much smaller concentration in a soluble form[14]. Toll-like receptor 4 is a transmembranic receptor present on the surface and in the endosomes of host cells, such as macrophages and dendritic cells, that recognizes damage associated with molecular patterns and pathogen associated molecular patterns released by neighboring tissue after cell damage[15]. After its activation, it can trigger the release of proinflammatory cytokines as well as the release of anti-inflammatory cytokines and interferons. It has been proposed that its activation facilitates the expression of angiotensin converting enzyme 2 (ACE-2) receptors, participating in that way in a vicious cycle of cytokine storm establishment. Moreover, its activation on platelets induces a prothrombotic and procoagulant state, offering a conceivable explanation for the thrombogenic state in the COVID-19 set[16]. Except for the suggested contribution of ACE-2 to the disease’s commencement, additional receptors that could mediate the entry of SARS-CoV-2 in human cells is the recently recognized family of CD209. S viral glycoproteins present an elevated affinity to these sites and it is postulated that it could be effective for viral entry even in the absence of ACE-2. Ectopically expressed CD209 receptors could also intercede with viral entry[17]. Other receptors that interact with SARS-CoV-2, indicating additional possible routes for infection are MR/CD206, MGL/CLEC10A/CD301[18]. Variation in the above-mentioned receptors as well as in genes expressing priming proteases such as TMPRSS2, Cathepsin B and Cathepsin L could all provide a possible explanation for the diversity in infection susceptibility and range of symptoms in different individuals[19].
Despite the elevated incidence of myocardial infarction in COVID-19 patients, a substantial drop in the number of patients (up to 40%) addressing the emergency department due to acute coronary syndromes (ACS) has been observed in several countries, especially in the early stages of the pandemic[20-22]. In Greece, a country which implemented strict social measures, a reduction of approximately 30% in ACS hospitalizations during the COVID-19 outbreak was reported along with no excess in in-hospital mortality[23]. Several explanations for this trend have been proposed such as the poor participation in aerobic exercise, the reduction in air pollution as well as the changes in lifestyle and diet associated with the pandemic environment, or the fact that patients with less severe symptoms are reluctant to visit the hospital[24]. Several studies have reported the significant reduction in the total number of urgent and emergent coronary angiography performed in patients with ACS during the COVID-19 pandemic. COVID-19 patients with STEMI often did not receive guideline-recommended treatments, while the use of fibrinolysis over primary percutaneous coronary intervention (PCI) has been reported in a high number of cases[21,25-28]. However, an analysis from the Beijing Inpatient Database Study reported that the proportions of patients with ACS receiving PCI, the proportion of patients with STEMI receiving PCI within 24 h, and the proportion of patients with unstable angina receiving coronary artery bypass graft (CABG) were higher in the study period (December 1, 2019 to June 30, 2020), compared to those in the control period (December 1, 2018 to June 30, 2019)[29].
The cardiac involvement of COVID-19 as demonstrated by increased troponin levels at hospital admission has been associated with adverse prognosis in several cohorts[30,31]. A recent study by Nuzzi et al[32] demonstrated the prognostic significance of troponin trajectories in hospitalized patients with COVID-19[32]. The authors reported that the strongest independent predictor of increased mortality was the presence of normal troponin at admission and elevated troponin (defined as values above the 99th percentile of normal values) on day 2 (hazard ratio 3.78, 95% confidence interval: 1.10–13.09, P = 0.035). The aforementioned study suggests that COVID-19 patients deserve a serial troponin assessment, beyond baseline values, because it may have an additive prognostic role.
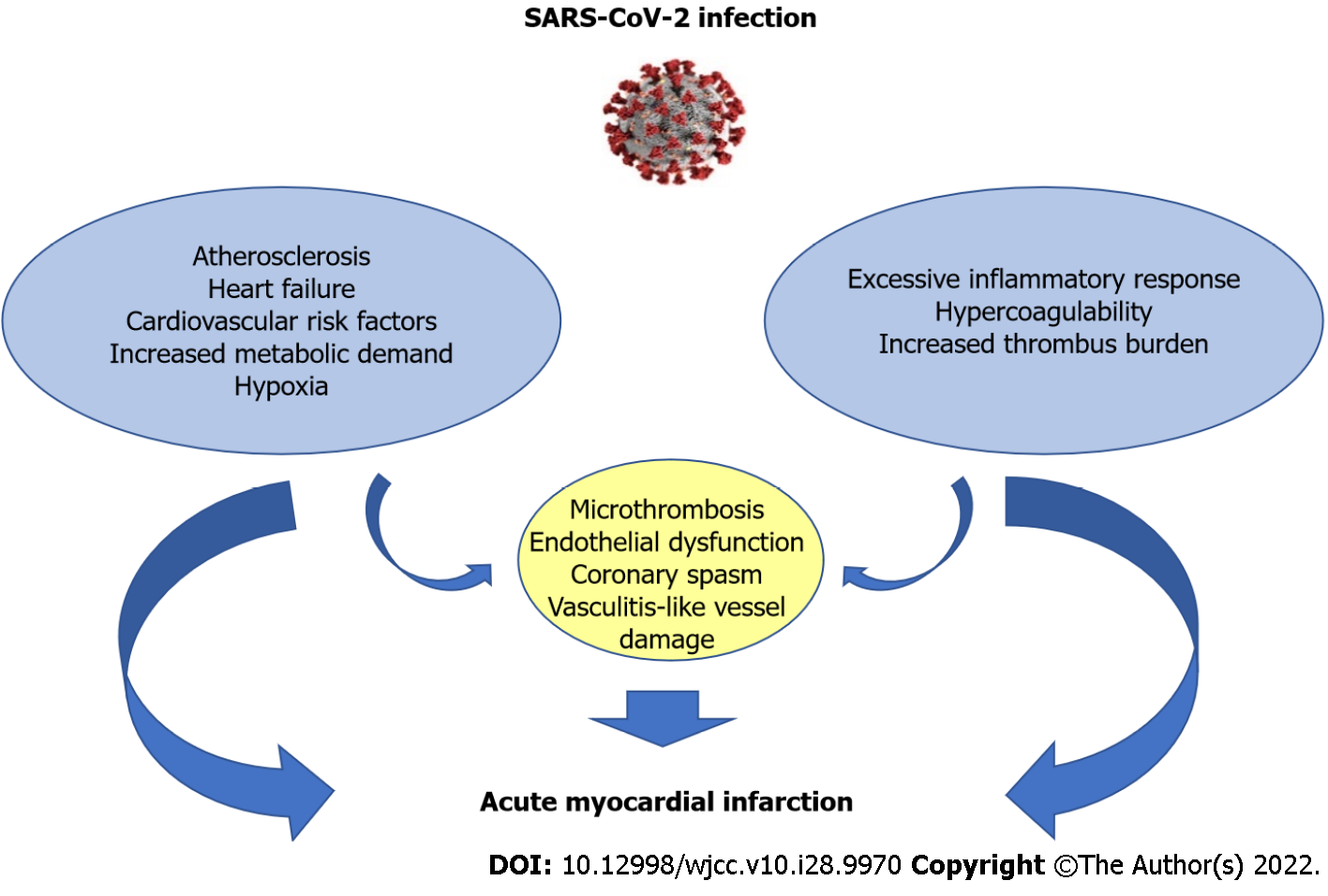
It is proposed that two different groups of COVID-19 patients with ACS exist. The first group includes patients with pre-existing atherosclerosis, a history of heart failure (HF) or multiple cardiovascular risk factors, who are prone to develop acute myocardial injury. Systemic hypoxia in patients with severe pneumonia or ARDS along with increased metabolic demands can precipitate imbalance between myocardial oxygen demand and supply, leading to type 2 myocardial infarction. Meanwhile, hypoxia-induced influx of calcium ions also leads to injury and apoptosis of cardiomyocytes[33]. Therefore, not surprisingly, hypoxemia (defined as pulse oximetry < 96%) has been reported to be an independent risk factor of ACS development in COVID-19 patients[34]. Moreover, it is well recognized that sepsis increases the risk for cardiovascular events and the risk of myocardial injury is linearly associated to the severity of respiratory infectious diseases[35].
In the second group, the excessive inflammatory response to the primary infection is the main mechanism. Patients may lack previous cardiovascular history or classic risk factors for coronary disease. Coronary occlusion occurs in the setting of procoagulant state and excessive autoimmune response[36,37]. Such patients are usually sick for at least 2 weeks with SARS-CoV-2, and ACS develops against the background of this infection[38]. A Swedish study which included 86742 patients with COVID-19 and 348481 matched control individuals reported an increased risk of myocardial infarction in COVID patients the first 2 weeks after the infection[39]. In an observational study of 119 SARS-CoV-2 positive patients presenting with COVID-19, a high thrombus burden evidenced with coronary thrombus, multivessel thrombosis and stent thrombosis during initial catheterization were observed[40]. In this case, patients are more often in need of thrombus aspiration, use of glycoprotein IIb/IIIa inhibitors and higher levels of heparin in order to achieve standard ACT levels[41]. Nonetheless, the two groups may share common pathophysiological mechanisms (Figure 2).
Multiple microthrombosis and endothelial dysfunction can complicate the clinical course in critically ill patients with sympathetic activation and increased metabolic demand and also in patients with high thrombus burden of epicardial coronary arteries as well. Coronary spasm and vasculitis-like vessel damage can occur in both groups[42].
Systemic inflammatory response due to cytokine storm can destabilize preexisting atherosclerotic plaques in patients with atherothrombosis, leading to rupture and subsequent excessive thrombus formation[43]. Below we discuss the main mechanisms associated with thrombosis and immune response presented in COVID-19 patients with ACS.
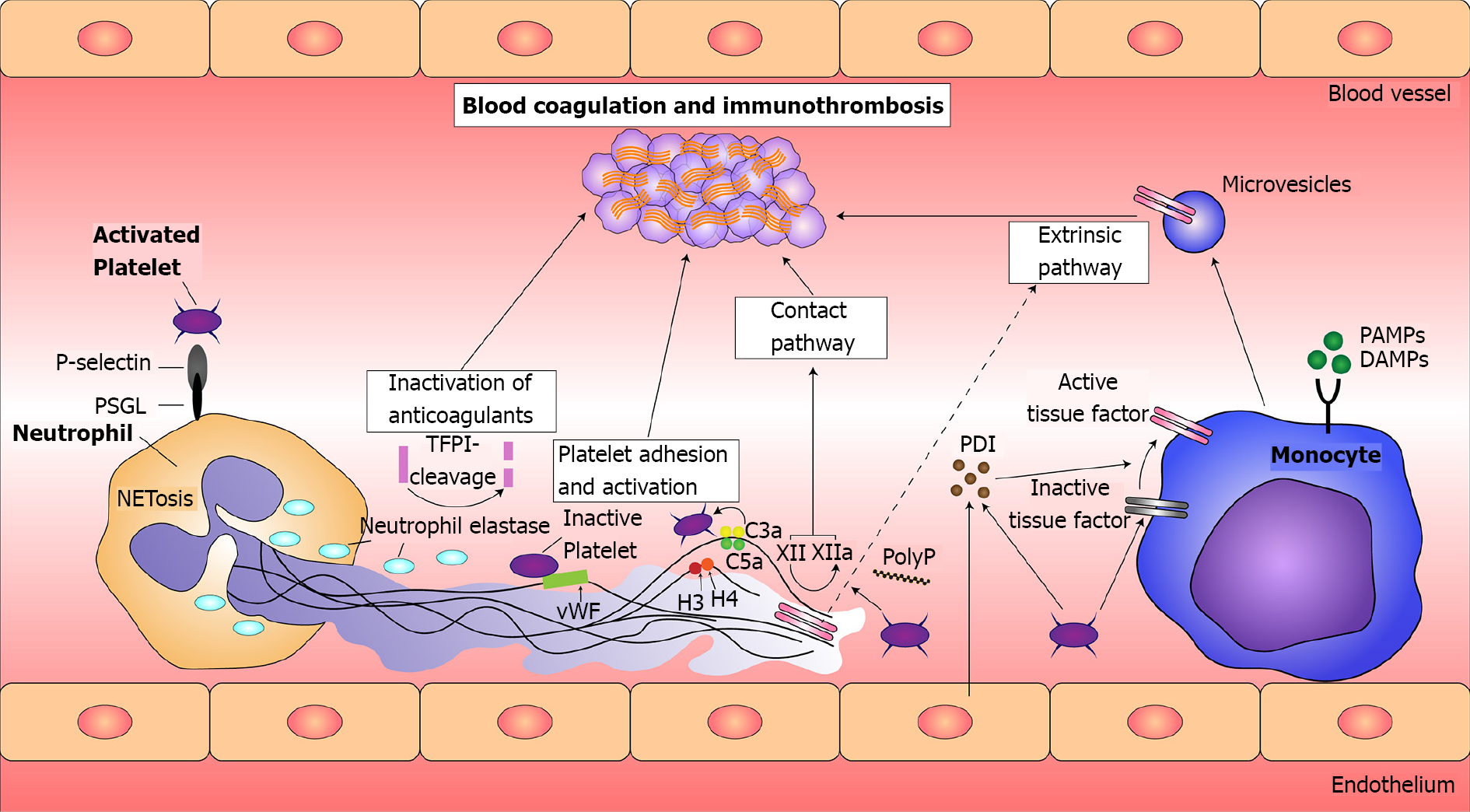
Viral mediated thrombosis: The occurrence of exacerbated thrombotic events in viral infection is not a novel concept. Immunopathological process in viral infections with influenza virus, SARS-CoV-1, Middle East respiratory syndrome (MERS) have been reported and attributed mainly to cytokine release syndrome. Secretion of proinflammatory cytokines interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, TNF-γ are postulated to aggravate myocardial function acutely and subacutelly through the secretion of neural sphingomyelinase and via nitric oxide-mediated blunting of beta-adrenergic signaling[44,45]. The core mechanisms responsible for myocardial injury is the disruption of endothelial integrity, the interaction of immune cells, mainly leukocytes, with coagulation factors and platelets, the constant secretion of cytokines and the release of prothrombotic mediators, such as tissue factor (TF) (Figure 3)[46].
Both enveloped and non-enveloped viruses are capable of mediating thrombosis mainly through up regulation of the extrinsic coagulation pathway, as demonstrated in former assays where respiratory viruses induced a 4-5 fold increase in TF and Xa factor and reduced the clotting time by 55%[47]. Similarly, herpes virus and measles virus induce procoagulant activity by increasing the affiliation of leukocytes and monocytes to endothelial monolayers[48,49]. Myocardial injury encountered at patients infected with SARS-CoV-2 resembles that caused by SARS-CoV-1, influenza virus and MERS-CoV. In a former study it was demonstrated that patients infected with influenza present a six- fold elevated risk of having a myocardial injury (both STEMI and NSTEMI) during the illness seasons. Nevertheless, COVID-19 infection has been associated with a significantly higher incidence of myocardial infarction compared to influenza. Moreover, patients infected with SAR-CoV-2 have elevated levels of pro
Immune response in acute myocardial infarction: Ischemic injury after acute myocardial infarction mobilizes a wide range of immune responses of innate and adaptive immunity. The progress commences with the release of preformed granules of resident mastic cells which induces the release of inflammatory cytokines and chemokines of the macrophages and endothelial cells. After that, neutrophils and macrophages mainly from the hematopoietic stem and progenitor cells in the bone marrow, infiltrate the cardiac muscle in a biphasic response, sequencing the inflammatory cascade[51]. Leukocytosis itself, although it starts initially as a compensatory mechanism for the preservation of cardiac muscle integrity, constitutes an independent cardiovascular risk factor. The absolute balance of the immune response is crucial for the outcome of the disease, since an over-accumulation of immune cells in the early stages leads to thinning of the infarct tissue while an absence of a vigorous immune response can lead to an excess of granulation tissue, making the myocardial wall less stable and prone to rupture. Acute deregulation of the immune system in SARS-CoV-2 infected patients can amplify immune response and precipitate multiple organ failure as well as acute myocardial infarction[52].
NETosis: Another recently studied pathway of immunothrombosis in COVID-19 is associated with the formation of neutrophil extracellular traps (NETs). In response to certain stimuli, neutrophils enhance their antimicrobial activities by releasing NETs, composed of extracellular chromatin[53]. Attached to these web-like DNA structures are histones and granule proteins such as myeloperoxidase (MPO), as well as cytosolic proteins. Activated neutrophils release nuclear DNA into the extracellular enviro
It has been suggested that NETs provide the scaffold for fibrin deposition and platelet entrapment and subsequent activation[54]. Subsequent platelet activation assisted by histones, also a key segment of NETs structure, leads to a vicious cycle with an end point of endothelial injury and the impairment of blood flow[54,55]. In addition to platelet entrapment and activation, NETs’ contribution to vascular thrombus formation is also mediated through erythrocytes entrapment and fibrin deposition[56]. The contribution of NETs to vascular thrombogenesis is further supported by the presence of TF on NETs. More specifically it was demonstrated that neutrophils in an inflammatory substrate released large amounts of TF and subsequently generated thrombin and concluded in platelet activation[57]. In a prospective cohort study of 33 COVID-19 patients and 17 sex-matched controls, NETs, Platelet Factor U and selected cytokines were measured and correlated directly with illness severity[58]. Lastly, in another study of 84 sera samples highly specific markers of NETs–MPO-DNA, citrullinated Histone 3 were reported in COVID-19 patients’ samples and directly correlated to acute phase reactants, C-reactive protein (CRP), D-dimmers and lactate dehydrogenase[59].
COVID-19 related myocarditis: Acute myocarditis has been reported as a possible complication in SARS-CoV-2 positive patients. Intracellular SARS-CoV-2 might impair stress granule formation via its accessory protein. Without the stress granules, the virus is allowed to replicate and damage the cell. Naïve T lymphocytes can be primed for viral antigens via antigen-presenting cells and the heart-produced hepatocyte growth factor. The primed CD8+ T lymphocytes migrate to the cardiomyocytes and may cause myocardial inflammation through cell-mediated cytotoxicity[60]. SARS-CoV-2 may also bring about myocardial damage via the infection of endothelial cells in the heart. This theory is reinforced by the histological discovery of SARS-CoV-2 in endothelial cells of several organs, including the heart[61].
The incidence of COVID-19 related myocarditis is estimated to be accountable for an average of 7% of all COVID-19 related deaths[60]. However COVID-19 related mortality displays a varying prevalence among different population groups. Increased troponin levels in COVID-19 are related to adverse outcomes, but the specific prognostic role of myocarditis is not known[62]. The clinical spectrum of SARS-CoV-2 myocarditis is relatively wide. Patients may present with symptoms of thoracic pain, fever and tachycardia. A retrospective cohort from 23 hospitals in the United States and Europe revealed chest pain and dyspnea as the most common symptoms at admission, whereas the majority of acute myocarditis occurred in the absence of pneumonia[63]. Patients with concomitant myocarditis and pneumonia exhibited the worse prognosis at 120 d. In more severe cases dyspnea and fatigue may be encountered. In an advanced clinical course patients may develop HF and cardiogenic shock. Signs and symptoms of right HF may also be encountered, with peripheral edema and jugular veins distention being the initial clinical manifestation[60]. Fulminant myocarditis, although uncommon, has also been reported as a complication secondary to COVID-19 infection[64]. In such occasions, cardiogenic shock may develop rapidly while the patient is at risk of fatal ventricular tachyarrhythmia or bradyarrhythmia[65]. Additionally, patients with COVID-19 myocarditis are at the risk of ventricular and supra
Skeletal muscle myopathy is an extra-pulmonary manifestation of COVID-19, observed in approximately one-third of symptomatic patients and varying from limited myalgia to myositis or rhabdomyolysis. A case series of patients with concurrent myopathy and inflammatory cardiac disease secondary to active COVID-19, revealed that the majority of patients were lacking of a major respiratory complication, whereas only two subjects experienced critical COVID-19 pneumonia[67].
The differential diagnosis includes COVID-19 related ACS, cardiomyopathy in the status of multiorgan dysfunction syndrome and Takotsubo cardiomyopathy. Elevated troponin levels accompanied by acute chest pain insinuate non-STEMI as the predominant situation. Predisposing factors of coronary heart disease such as dyslipidemia, arterial hypertension, family history and comorbidities should be scrutinized. With a high suspicion of ACS, epicardial disease has to be ruled out by coronary angiography. Sepsis-induced myocardial dysfunction in intensive care units ranges from 10% to 70% between studies[68]. Stress induced cardiomyopathy incidence appears to be elevated between human-coronavirus positive patients. A recent study demonstrated a substantial rise in the incidence of stress cardiomyopathy during the COVID-19 period (7.8%), compared to prepandemic timelines (1.5%-1.8%)[69,70]. Reverse Takotsubo cardiomyopathy has also been reported as the first direct evidence of myocardial inflammation in a woman with interstitial inflammatory lung disease due to COVID-19[71].
In the setting of high clinical suspicion of COVID-19 associated myocarditis, the diagnostic evaluation is based on the application of cardiac imaging methods–echocardiogram and cardiac magnetic resonance imaging (CMR)[65]. Echocardiographic signs of myocarditis depend on the disease’s severity and includes wall motion abnormalities, increased wall thickness, mildly or severely reduced myocardial contractility, cardiac chamber dilatation and pericardial effusion. In mild forms, echocardiographic evaluation may be otherwise normal, except for reduced myocardial strain. CMR imaging in myocarditis evaluation is usually applied early after the disease in order to denote the extent of myocardial injury. Myocardial edema and scarring have been reported in the majority of patients with COVID-19 myocarditis[72]. Cardiac computed tomography scan with contrast enhancement can be utilized when CMR is unavailable or contraindicated[73].
Currently, treatment for viral myocarditis is largely supportive. When left ventricular systolic dysfunction occurs, HF therapy is recommenced (i.e. angiotensin-converting enzyme-inhibitors and b-blockers) in a patient with sufficient cardiac output and hemodynamic stability. Several ongoing trials are investigating immunosuppressant therapy for the hyperinflammatory phase that may be useful to COVID-19-related myocarditis[62].
COVID-19 vaccine-associated myocarditis: Vaccination against SARS-CoV-2 has been anticipated worldwide as the redemption of the pandemic. Although overly infrequent, myocarditis has been discerned as a potential side effect of COVID-19 mRNA-vaccines[74]. COVID-19 mRNA-vaccine-associated myocarditis incidence is estimated to 0.3-0.5 per 100000 vaccinated people, which is significantly lower than the incidence of acute viral myocarditis (1–10 per 100000 people per year) or COVID-19-related myocarditis or myocardial injury (1%-4% of infected patients)[75]. In addition to its low incidence, mRNA-related myocarditis displays a favorable prognosis, with > 80% recovery and > 99% survival[76]. Among those who will present with vaccine-related myocarditis, an increased possibility of myocarditis during the 1st week after the second dose of mRNA vaccines have been observed. The population group mostly affected, seems to be young men[77]. In immunogenic susceptible patients, vaccine-associated myocarditis constitutes the manifestation of hyperimmunity of the preceded sensitization to the virus. SARS-CoV-2 mRNA vaccines contain nucleoside-modified mRNA encapsulated in lipid nanoparticles which, after the entrance in host cells drives the translation of the receptor binding spike glycoprotein of the virus[78]. Spike glycoprotein encoded by the vaccine mRNA stimulates the attraction of specific IgG antibodies and causes the activation of the acquired immune response, which has been adapted after the first vaccination dose. Hyperimmunity in genetically susceptible patients can activate proinflammatory cascades and stimulate an amplified immune response[79].
Molecular mimicry between cardiac muscle segments and the spike protein of SARS-CoV-2 is another potential mechanism[75]. Myocardial α-myosin heavy chain which is structurally similar to SARS-CoV-2 spike glycoprotein can be falsely identified as antigens and be objected by preformed antibodies[78]. However, new-onset myocardial injury following vaccination cannot be entirely attributed to cross reaction between self-antigens, letting this be the case only in immunogenetically predisposed patients.
The suggested pathophysiological mechanism to interpret the divergence in the incidence of vaccine-induced myocarditis between male and female patients lies in the differentiation in hormone signaling. Elevated testosterone levels are associated to inhibition of anti-inflammatory cells and predominance of Th-1 type response. Estrogen, on the other hand, seem to have a protective role, inhibiting the action of pro-inflammatory T cells and being associated with a prevalence of anti-inflammatory elements such as increased B cells, IL-10, domination of T regulatory cells and M2 macrophage activation pattern[76].
HF has been acknowledged as one of the most common critical complications during the COVID-19 pandemic along with acute cardiac injury, sepsis and ARDS[80]. COVID-19 patients may develop HF due to infiltration of the heart by inflammatory cells, destructive action of pro-inflammatory cytokines, micro-thrombosis and new onset or aggravated endothelial dysfunction and respiratory failure[81]. SARS-CoV-2 is accountable for viral inclusions in the myocardium and the consequent infiltration by monocytes/macrophages, neutrophils and lymphocytes[82]. COVID-19 related myocarditis may progress to dilated cardiomyopathy and advanced HF with reduced ejection fraction[83,84]. Other possible mechanisms include: (1) Cor-pulmonale secondary to ARDS or pulmonary embolism; (2) The use of steroids which can cause fluid overload; and (3) The administration of cardiotoxic drugs (i.e. hydroxychloroquine or azithromycin)[85].
Patients with HF hospitalized for COVID-19 exhibit a particularly increased risk of in-hospital complications and excess mortality. HF and SARS-CoV-2 infection share common risk factors such as senility, lung disease, chronic kidney disease and hypertension[86]. As reported in a retrospective study of 2184 patients hospitalized with COVID-19, the sub-group of HF patients experienced a longer hospitalization, and developed more frequently myocardial infarction or shock[87]. Likewise, in a retro
Infection is a usual trigger of HF hospitalization and it is related to increased mortality[89]. Patients with COVID-19 are not the exception[90]. The prevalence of coexisting HF ranged from 3.3% to 21% among SARS-CoV-2-infected patients, whereas during COVID-19 hospitalization, approximately one-third of patients with a history of HF had an acute decompensation of HF[85]. Cytokine storm in severe COVID-19 can increase metabolic demand and also provokes RAAS and sympathetic system activation which all entail high cardiac output and can lead to acute decompensation of chronic HF[91]. Critically ill COVID-19 patients are a specifically vulnerable group to developing acute renal injury. Except for the effect of nephrotoxic drugs on renal function, hemodynamic derangement and cytokine storm, kidneys are menaced by direct viral injury mediated by the abundant ACE2 receptors in their cells[92,93]. Microthrombosis in renal vasculature also participates in renal dysfunction and acute renal injury in COVID-19 patients[94]. Up to 11% of hospitalized patients and 25% of critically ill patients are estimated to be afflicted by acute kidney injury[95]. Loss of glomerular filtration rate accelerates the disease progression and predisposes to acute decompensations in patients with a history of HF[95].
Cardiac arrhythmias have been recognized as a frequent complication of COVID-19 infection. In a prospective observational study of 143 COVID-19 hospitalized patients followed with telemetry monitoring, sinus tachycardia afflicted 39.9% of patients, while premature ventricular contractions were observed in 28.7% and non-sustained ventricular tachycardia (VT) in 15.4% of patients (the mean follow-up was 23.7 d). The presence of sinus tachycardia was correlated with a less favorable outcome[96]. In a retrospective cohort of 241 patients hospitalized with COVID-19 the incidence of cardiac arrhythmias was 8.7%, whereas the presence of HF was associated with an increased hazard of new onset arrhythmia[97].
A prospective study including 113 SARS-CoV-2 positive patients admitted to intensive care unit (ICU), demonstrated a prevalence of sustained atrial arrhythmias in 44.2% of patients and a 33.6% of non-sustained atrial arrhythmias, with the most prevalent atrial arrhythmia being atrial fibrillation (AF)[98]. Ventricular arrhythmias were recorded in a total of 30.9% of patients; significantly higher than the prevalence of ventricular arrhythmias in non-COVID ICU patients reported in previous studies[99,100]. Approximately, 1 out of 4 of the patients had at least one bradycardic episode. In 8.9% of this subgroup, AV conduction disorders was the observed episode with third-degree AV block being the predominant subtype of AV conduction disorders[98].
During COVID-19 infection, diverse mechanisms participated in the genesis of arrhythmias. SARS-CoV-2 can engender arrhythmias through direct myocardial damage causing acute myocarditis or through HF decompensation or secondary, through respiratory failure or severe respiratory distress syndrome[101]. Direct viral myocardial injury to the conduction system can also predispose to arrhythmogenesis[42,102]. Other possible mechanisms include drug side effects, electrolyte derangements and autonomic or fluid imbalance.
Supraventricular arrhythmias: AF/atrial flutter occurred in approximately 15%-20% of patients hospitalized with COVID-19[103]. Factors like older age (senility) and obesity that make patients more susceptible to infection, also predispose to AF[104]. Hypoxemia and impaired gas exchange in patients with pneumonia increases the anaerobic fermentation and triggers imbalances in myocardial cells action potential promoting the development of AF[104,105]. Stimulation of the sympathetic nervous system may also contribute to AF development[105]. Macrophage infiltration, monocyte chemoattractant protein-1, IL-6, vascular endothelial growth factor, matrix metalloproteinase-2, TNF-α and matrix metalloproteinase-9 are encountered in the inflammatory state and have been positively related to AF and associated with atrial myocardial fibrosis[106]. Common drugs such as corticosteroids and aminophylline given in COVID-19 may have an effect on AF incidence[107]. QTc interval should be measured to avoid a marked prolongation in cases of co-administration of other QT-prolonging drugs[103,108]. Intravenous (IV) amiodarone is the recommended antiarrhythmic drug for rate and rhythm control. However, its combination with hydroxychloroquine and/or azithromycin should be avoided in COVID-19 patients[103]. COVID-19 is associated with a significant risk of venous, arterial, and microvascular thrombotic and thromboembolic disease. Therefore, special attention should be given to anticoagulation therapy administration, taking in mind that CHA2DS2-VASc score may underestimate risk of stroke[103,109].
Ventricular arrhythmias: Patients with myocardial injury and myocarditis present a high burden of ventricular ectopy. In the setting of COVID-19 infection accompanied by myocardial damage, various ventricular arrhythmias can complicate the clinical course, increasing the disease’s mortality. Electrolyte disturbances due to volume depletion or acute kidney injury, affect an average of 7.2% of COVID-19 patients and also predispose to the emergence of ventricular arrhythmias[110]. Non-sustained VT, multiple ventricular premature conductions, sustained VT and VF have all been encountered in COVID-19 patients[111]. In a single-center retrospective analysis the incidence of sustained VT or VF in hospitalized COVID-19 patients was reported to be 5.9%[31]. Polypharmacy in fragile patients or in patients with comorbidities may be culpable of QT prolongation leading to the development of polymorphic VT. Special attention should be paid to COVID-19 therapies that present an increased risk for TdP due to QT prolongation[108]. Hydroxychloroquine/chloroquine and azithromycin prolong action potential and trigger early after depolarization which can stimulate TdP. In a meta-analysis of forty-seven studies including 13087 COVID-19 patients the abovementioned treatment (hydroxychloroquine alone or in combination with azithromycin) lead to a significantly elevated prevalence of QTc prolongation[108]. COVID-19-associated cytokine surge may trigger ventricular ectopy in previously clinically silent cardiomyopathies or patients with chronic coronary syndromes. A VT storm occurring in an afebrile and otherwise asymptomatic SARS-CoV-2 positive patient has been reported, unmasking a previously unknown substrate of arrhythmogenic right ventricular cardiomyopathy[112].
Bradycardia and conduction abnormalities: In a small case series, transient sinus bradycardia has been reported as a manifestation of the COVID-19 disease. Sinus bradycardia episodes were unrelated to previous cardiovascular history[113]. Bradycardia episodes are attributed to hypoxia and inflammatory damage of the cardiac pacemaker cells and indicate a cross-talk between the autonomic nervous system and immune system[114]. Interestingly, bradycardia slightly precedes cytokine storm, denoting a timepoint of clinical deterioration[115]. Intermittent 3rd-degree AV block during hospitalization with COVID-19 has also been described.
Sinus bradycardia or AV block can be caused by drugs such as hydroxychloroquine, lopinavir/ rotinavir and azithromycin. Myocarditis affecting the sinus node can also be a cause of bradycardia[111]. Given the fact that bradycardia and atrioventricular conduction disorders in COVID-19 patients are mostly transient, the use of isoprenaline or implantation of a temporary pacemaker and subsequently evaluating the need of a permanent pacemaker, is a reasonable option[103,109,116].
The link between statin therapy and COVID-19 severity and outcomes is of intense interest at present. Several studies have demonstrated the association between statin therapy and the reduced risk of pneumonia, milder disease, as well as reduced mortality[117-119] However, an Italian multicenter observational study, which enrolled 842 hospitalized patients with COVID-19, revealed no association between the use of statin and in-hospital mortality (P = 0.185). Interestingly, statin use was associated with a more severe disease (National Early Warning Score ≥ 5, P = 0.025), reflecting the presence of cardiovascular risk factors as dyslipidemia or coronary artery disease in those patients[120].
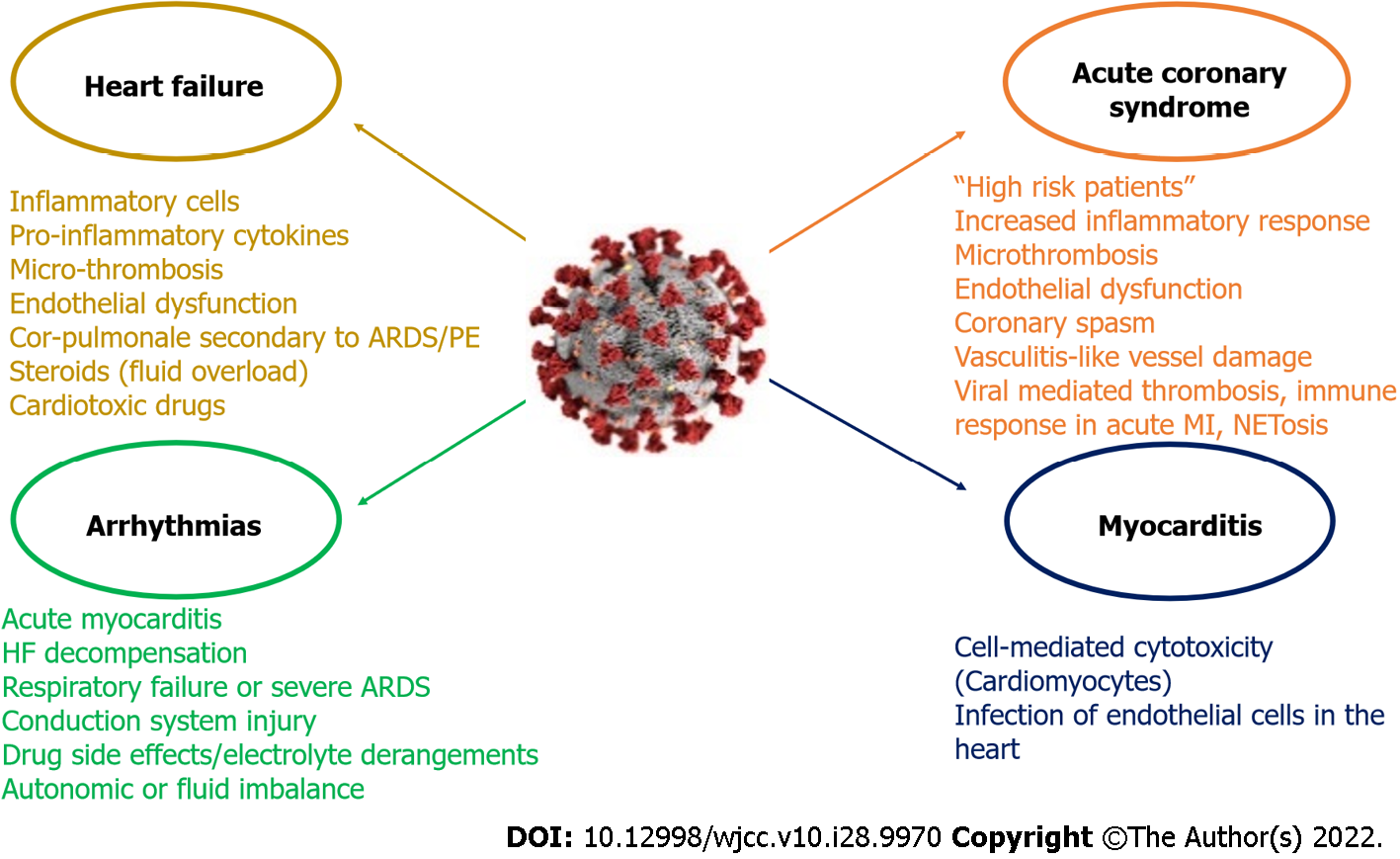
SARS-CoV-2, a single stranded RNA beta coronavirus, causes COVID-19 disease. The affinity of SAR-CoV-2 infection to ACE-2 receptor has been proposed to be the core of the disease’s pathophysiology. Although SARS-CoV-2 infection predominantly offends the respiratory system, it is liable for various cardiovascular complications such as ACS, myocarditis, HF and arrhythmias. Several mechanisms have been implicated such as excessive inflammatory response to the primary infection, immunothrombosis and myocardial injury (Figure 4). SARS-CoV-2 infected patients with a history of cardiovascular disease have increased mortality.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed S, Pakistan; Batalik L, Czech Republic; Freund O, Israel; Moshref RH, Saudi Arabia; Velikova TV, Bulgaria; Wang HD, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18844] [Article Influence: 3768.8] [Reference Citation Analysis (7)] |
| 2. | Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agrò FE. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288:192-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 732] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 3. | Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1531] [Article Influence: 306.2] [Reference Citation Analysis (0)] |
| 4. | Triposkiadis F, Starling RC, Xanthopoulos A, Butler J, Boudoulas H. The Counter Regulatory Axis of the Lung Renin-Angiotensin System in Severe COVID-19: Pathophysiology and Clinical Implications. Heart Lung Circ. 2021;30:786-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 1173] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 6. | Kurz DJ, Eberli FR. Cardiovascular aspects of COVID-19. Swiss Med Wkly. 2020;150:w20417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Colling ME, Kanthi Y. COVID-19-associated coagulopathy: An exploration of mechanisms. Vasc Med. 2020;25:471-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 8. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1950] [Article Influence: 390.0] [Reference Citation Analysis (0)] |
| 9. | Pepera G, Tribali MS, Batalik L, Petrov I, Papathanasiou J. Epidemiology, risk factors and prognosis of cardiovascular disease in the Coronavirus Disease 2019 (COVID-19) pandemic era: a systematic review. Rev Cardiovasc Med. 2022;23:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Magadum A, Kishore R. Cardiovascular Manifestations of COVID-19 Infection. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | WHO. Clinical management of COVID-19: Living guideline, 23 June 2022. [cited 19 July 2022]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2022-1. |
| 12. | Turshudzhyan A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Induced Cardiovascular Syndrome: Etiology, Outcomes, and Management. Cureus. 2020;12:e8543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Triposkiadis F, Xanthopoulos A, Giamouzis G, Boudoulas KD, Starling RC, Skoularigis J, Boudoulas H, Iliodromitis E. ACE2, the Counter-Regulatory Renin-Angiotensin System Axis and COVID-19 Severity. J Clin Med. 2021;10:3885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, Stein DA, Hardes K, Eickmann M, Dolnik O, Rohde C, Klenk HD, Garten W, Steinmetzer T, Böttcher-Friebertshäuser E. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 587] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 15. | Aboudounya MM, Heads RJ. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediators Inflamm. 2021;2021:8874339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 16. | Schattner M. Platelet TLR4 at the crossroads of thrombosis and the innate immune response. J Leukoc Biol. 2019;105:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Amraei R, Yin W, Napoleon MA, Suder EL, Berrigan J, Zhao Q, Olejnik J, Chandler KB, Xia C, Feldman J, Hauser BM, Caradonna TM, Schmidt AG, Gummuluru S, Muhlberger E, Chitalia V, Costello CE, Rahimi N. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. bioRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Gao C, Zeng J, Jia N, Stavenhagen K, Matsumoto Y, Zhang H, Li J, Hume AJ, Mühlberger E, van Die I, Kwan J, Tantisira K, Emili A, Cummings RD. SARS-CoV-2 Spike Protein Interacts with Multiple Innate Immune Receptors. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Chen L, Zheng S. Understand variability of COVID-19 through population and tissue variations in expression of SARS-CoV-2 host genes. Inform Med Unlocked. 2020;21:100443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Braiteh N, Rehman WU, Alom M, Skovira V, Breiteh N, Rehman I, Yarkoni A, Kahsou H, Rehman A. Decrease in acute coronary syndrome presentations during the COVID-19 pandemic in upstate New York. Am Heart J. 2020;226:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 21. | Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Roebuck C, Gale CP, Mamas MA, Deanfield JE, de Belder MA, Luescher TF, Denwood T, Landray MJ, Emberson JR, Collins R, Morris EJA, Casadei B, Baigent C. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 492] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 22. | Vecchio S, Fileti L, Reggi A, Moschini C, Lorenzetti S, Rubboli A. [Impact of the COVID-19 pandemic on admissions for acute coronary syndrome: review of the literature and single-center experience]. G Ital Cardiol (Rome). 2020;21:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 23. | Papafaklis MI, Katsouras CS, Tsigkas G, Toutouzas K, Davlouros P, Hahalis GN, Kousta MS, Styliadis IG, Triantafyllou K, Pappas L, Tsiourantani F, Varytimiadi E, Anyfantakis ZA, Iakovis N, Grammata P, Karvounis H, Ziakas A, Sianos G, Tziakas D, Pappa E, Dagre A, Patsilinakos S, Trikas A, Lamprou T, Mamarelis I, Katsimagklis G, Karmpaliotis D, Naka K, Michalis LK. "Missing" acute coronary syndrome hospitalizations during the COVID-19 era in Greece: Medical care avoidance combined with a true reduction in incidence? Clin Cardiol. 2020;43:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Sutherland N, Dayawansa NH, Filipopoulos B, Vasanthakumar S, Narayan O, Ponnuthurai FA, van Gaal W. Acute Coronary Syndrome in the COVID-19 Pandemic: Reduced Cases and Increased Ischaemic Time. Heart Lung Circ. 2022;31:69-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Esposito L, Cancro FP, Silverio A, Di Maio M, Iannece P, Damato A, Alfano C, De Luca G, Vecchione C, Galasso G. COVID-19 and Acute Coronary Syndromes: From Pathophysiology to Clinical Perspectives. Oxid Med Cell Longev. 2021;2021:4936571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Kwok CS, Gale CP, Kinnaird T, Curzen N, Ludman P, Kontopantelis E, Wu J, Denwood T, Fazal N, Deanfield J, de Belder MA, Mamas M. Impact of COVID-19 on percutaneous coronary intervention for ST-elevation myocardial infarction. Heart. 2020;106:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States During COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2871-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 754] [Cited by in RCA: 908] [Article Influence: 181.6] [Reference Citation Analysis (0)] |
| 28. | Gluckman TJ, Wilson MA, Chiu ST, Penny BW, Chepuri VB, Waggoner JW, Spinelli KJ. Case Rates, Treatment Approaches, and Outcomes in Acute Myocardial Infarction During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2020;5:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 29. | He L, Lu F, Du X, Long D, Sang C, Tang R, Dong J, Guo M, Ma C. Impact of COVID-19 Pandemic on Hospital Admissions of Acute Coronary Syndrome: A Beijing Inpatient Database Study. Lancet Reg Health West Pac. 2022;19:100335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Merlo M, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Specchia C, Metra M, Senni M. Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019: Results of a Multicenter Study. JAMA Cardiol. 2020;5:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 31. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2837] [Article Influence: 567.4] [Reference Citation Analysis (0)] |
| 32. | Nuzzi V, Merlo M, Specchia C, Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Volterrani M, Senni M, Metra M, Sinagra G. The prognostic value of serial troponin measurements in patients admitted for COVID-19. ESC Heart Fail. 2021;8:3504-3511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1235] [Article Influence: 247.0] [Reference Citation Analysis (0)] |
| 34. | Alquézar-Arbé A, Miró Ò, Castillo JGD, Jiménez S, Llorens P, Martín A, Martín-Sánchez FJ, García-Lamberechts EJ, Piñera P, Jacob J, Porrino JMM, Jiménez B, Río RD, García CP, Aznar JVB, Ponce MC, Fernández ED, Tost J, Mojarro EM, García AH, Quirós AM, Noceda J, Cano MJC, Almela AFS, Bayarri MJF, Tejera MG, Rodriguez AD, Burillo-Putze G; Spanish Investigators on Emergency Situations TeAm (SIESTA) Network. Incidence, Clinical Characteristics, Risk Factors and Outcomes of Acute Coronary Syndrome in Patients With COVID-19: Results of the UMC-19-S1010. J Emerg Med. 2022;62:443-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Musher DM, Abers MS, Corrales-Medina VF. Acute Infection and Myocardial Infarction. N Engl J Med. 2019;380:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 36. | Meizinger C, Klugherz B. Focal ST-segment elevation without coronary occlusion: myocardial infarction with no obstructive coronary atherosclerosis associated with COVID-19-a case report. Eur Heart J Case Rep. 2021;5:ytaa532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Tedeschi D, Rizzi A, Biscaglia S, Tumscitz C. Acute myocardial infarction and large coronary thrombosis in a patient with COVID-19. Catheter Cardiovasc Interv. 2021;97:272-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Shorikova DV, Shorikov EI. COVID-19 and acute coronary syndrome: emphasis on ACS without atherothrombosis. e-Journal of Cardiology Practice 2021;21(5). [cited 19 July 2022]. Available from: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-21/covid-19-and-acute-coronary-syndrome-emphasis-on-acs-without-atherothrombosis. |
| 39. | Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398:599-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 40. | Choudry FA, Hamshere SM, Rathod KS, Akhtar MM, Archbold RA, Guttmann OP, Woldman S, Jain AK, Knight CJ, Baumbach A, Mathur A, Jones DA. High Thrombus Burden in Patients With COVID-19 Presenting With ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2020;76:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 41. | Madkour M, Savoj J, Iftikhar S, Waked A. Extensive Coronary Thrombosis as a Sequelae of COVID-19 Presenting as a STEMI. Cureus. 2021;13:e15258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 492] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 43. | Hamadeh A, Aldujeli A, Briedis K, Tecson KM, Sanz-Sánchez J, Al Dujeili M, Al-Obeidi A, Diez JL, Žaliūnas R, Stoler RC, McCullough PA. Characteristics and Outcomes in Patients Presenting With COVID-19 and ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2020;131:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 44. | Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 45. | Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 526] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 46. | Jayarangaiah A, Kariyanna PT, Chen X, Jayarangaiah A, Kumar A. COVID-19-Associated Coagulopathy: An Exacerbated Immunothrombosis Response. Clin Appl Thromb Hemost. 2020;26:1076029620943293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 47. | Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost. 2000;84:319-324. [PubMed] |
| 48. | Span AH, Van Boven CP, Bruggeman CA. The effect of cytomegalovirus infection on the adherence of polymorphonuclear leucocytes to endothelial cells. Eur J Clin Invest. 1989;19:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Etingin OR, Silverstein RL, Hajjar DP. Identification of a monocyte receptor on herpesvirus-infected endothelial cells. Proc Natl Acad Sci U S A. 1991;88:7200-7203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Gopal R, Marinelli MA, Alcorn JF. Immune Mechanisms in Cardiovascular Diseases Associated With Viral Infection. Front Immunol. 2020;11:570681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Dutta P, Nahrendorf M. Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Rios-Navarro C, Dios E, Forteza MJ, Bodi V. Unraveling the thread of uncontrolled immune response in COVID-19 and STEMI: an emerging need for knowledge sharing. Am J Physiol Heart Circ Physiol. 2021;320:H2240-H2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular Mechanisms of NETosis. Annu Rev Cell Dev Biol. 2020;36:191-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 54. | Kambas K, Mitroulis I, Ritis K. The emerging role of neutrophils in thrombosis-the journey of TF through NETs. Front Immunol. 2012;3:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1798] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 56. | Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880-15885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1847] [Article Influence: 123.1] [Reference Citation Analysis (0)] |
| 57. | Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, Skendros P, Kourtzelis I, Koffa M, Kotsianidis I, Ritis K. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS One. 2012;7:e45427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 58. | Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD, Yost CC. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 1083] [Article Influence: 216.6] [Reference Citation Analysis (0)] |
| 59. | Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 60. | Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT Jr, Chahal CAA. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 61. | Ali M, Shiwani HA, Elfaki MY, Hamid M, Pharithi R, Kamgang R, Egom CB, Oyono JLE, Egom EE. COVID-19 and myocarditis: a review of literature. Egypt Heart J. 2022;74:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, Aimo A, Prasad S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022;27:251-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 63. | Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T, Hayek A, Piriou N, Leonardi S, Guida S, Turco A, Sala S, Uribarri A, Van de Heyning CM, Mapelli M, Campodonico J, Pedrotti P, Barrionuevo Sánchez MI, Ariza Sole A, Marini M, Matassini MV, Vourc'h M, Cannatà A, Bromage DI, Briguglia D, Salamanca J, Diez-Villanueva P, Lehtonen J, Huang F, Russel S, Soriano F, Turrini F, Cipriani M, Bramerio M, Di Pasquale M, Grosu A, Senni M, Farina D, Agostoni P, Rizzo S, De Gaspari M, Marzo F, Duran JM, Adler ED, Giannattasio C, Basso C, McDonagh T, Kerneis M, Combes A, Camici PG, de Lemos JA, Metra M. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation. 2022;145:1123-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 64. | Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ, Xie XJ, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 384] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 65. | Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny O; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e69-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 381] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 66. | Peretto G, Sala S, Rizzo S, De Luca G, Campochiaro C, Sartorelli S, Benedetti G, Palmisano A, Esposito A, Tresoldi M, Thiene G, Basso C, Della Bella P. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019;16:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 67. | Freund O, Eviatar T, Bornstein G. Concurrent myopathy and inflammatory cardiac disease in COVID-19 patients: a case series and literature review. Rheumatol Int. 2022;42:905-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, Brown SM. Septic Cardiomyopathy. Crit Care Med. 2018;46:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 69. | Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa'N A, Hedrick DP, Sleik KM, Mehta N, Chung MK, Khot UN, Kapadia SR, Puri R, Reed GW. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw Open. 2020;3:e2014780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 70. | Shah RM, Shah M, Shah S, Li A, Jauhar S. Takotsubo Syndrome and COVID-19: Associations and Implications. Curr Probl Cardiol. 2021;46:100763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 71. | Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861-1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 406] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 72. | Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1240] [Cited by in RCA: 1461] [Article Influence: 292.2] [Reference Citation Analysis (0)] |
| 73. | Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A, Sibley CT, Lima JA, Liu S, Bluemke DA. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012;264:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 74. | Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 75. | Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 180] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 76. | Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 619] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 77. | Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140-2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 425] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 78. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 79. | Maiese A, Frati P, Del Duca F, Santoro P, Manetti AC, La Russa R, Di Paolo M, Turillazzi E, Fineschi V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 80. | Sanderson JE, Fang F, Lu M, Ma CY, Wei YX. Obstructive sleep apnoea, intermittent hypoxia and heart failure with a preserved ejection fraction. Heart. 2021;107:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Adeghate EA, Eid N, Singh J. Mechanisms of COVID-19-induced heart failure: a short review. Heart Fail Rev. 2021;26:363-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 82. | Unudurthi SD, Luthra P, Bose RJC, McCarthy JR, Kontaridis MI. Cardiac inflammation in COVID-19: Lessons from heart failure. Life Sci. 2020;260:118482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 83. | Zhao W, Li H, Li J, Xu B, Xu J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J Med Virol. 2022;94:1886-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 84. | Komiyama M, Hasegawa K, Matsumori A. Dilated Cardiomyopathy Risk in Patients with Coronavirus Disease 2019: How to Identify and Characterise it Early? Eur Cardiol. 2020;15:e49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Italia L, Tomasoni D, Bisegna S, Pancaldi E, Stretti L, Adamo M, Metra M. COVID-19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front Cardiovasc Med. 2021;8:713560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 86. | Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, Ceirano A, Espinosa F, Saavedra E, Sanguine V, Tassara A, Cid C, Catalano HN, Agarwal A, Foroutan F, Rada G. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020;15:e0241955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 414] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 87. | Sokolski M, Reszka K, Suchocki T, Adamik B, Doroszko A, Drobnik J, Gorka-Dynysiewicz J, Jedrzejczyk M, Kaliszewski K, Kilis-Pstrusinska K, Konopska B, Kopec A, Larysz A, Lis W, Matera-Witkiewicz A, Pawlik-Sobecka L, Rosiek-Biegus M, Sokolska JM, Sokolowski J, Zapolska-Tomasiewicz A, Protasiewicz M, Madziarska K, Jankowska EA. History of Heart Failure in Patients Hospitalized Due to COVID-19: Relevant Factor of In-Hospital Complications and All-Cause Mortality up to Six Months. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Salinas-Botrán A, Sanz-Cánovas J, Pérez-Somarriba J, Pérez-Belmonte LM, Cobos-Palacios L, Rubio-Rivas M, de-Cossío-Tejido S, Ramos-Rincón JM, Méndez-Bailón M, Gómez-Huelgas R; en nombre del grupo SEMI-COVID-19. [Clinical characteristics and risk factors for mortality upon admission in patients with heart failure hospitalized due to COVID-19 in Spain]. Rev Clin Esp. 2022;222:255-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Drozd M, Garland E, Walker AMN, Slater TA, Koshy A, Straw S, Gierula J, Paton M, Lowry J, Sapsford R, Witte KK, Kearney MT, Cubbon RM. Infection-Related Hospitalization in Heart Failure With Reduced Ejection Fraction: A Prospective Observational Cohort Study. Circ Heart Fail. 2020;13:e006746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Rey JR, Caro-Codón J, Rosillo SO, Iniesta ÁM, Castrejón-Castrejón S, Marco-Clement I, Martín-Polo L, Merino-Argos C, Rodríguez-Sotelo L, García-Veas JM, Martínez-Marín LA, Martínez-Cossiani M, Buño A, Gonzalez-Valle L, Herrero A, López-Sendón JL, Merino JL; CARD-COVID Investigators. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22:2205-2215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 91. | Bader F, Manla Y, Atallah B, Starling RC. Heart failure and COVID-19. Heart Fail Rev. 2021;26:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 92. | Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 93. | Shahid Z, Kalayanamitra R, McClafferty B, Kepko D, Ramgobin D, Patel R, Aggarwal CS, Vunnam R, Sahu N, Bhatt D, Jones K, Golamari R, Jain R. COVID-19 and Older Adults: What We Know. J Am Geriatr Soc. 2020;68:926-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 589] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 94. | Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1267] [Article Influence: 253.4] [Reference Citation Analysis (0)] |
| 95. | Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12:610-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 96. | Cho JH, Namazi A, Shelton R, Ramireddy A, Ehdaie A, Shehata M, Wang X, Marbán E, Chugh SS, Cingolani E. Cardiac arrhythmias in hospitalized patients with COVID-19: A prospective observational study in the western United States. PLoS One. 2020;15:e0244533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 97. | Pimentel M, Magalhães APA, Novak CV, May BM, Rosa LGBD, Zimerman LI. Cardiac Arrhythmias in Patients with COVID-19. Arq Bras Cardiol. 2021;117:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 98. | Parwani AS, Haug M, Keller T, Guthof T, Blaschke F, Tscholl V, Biewener S, Kamieniarz P, Zieckler D, Kruse J, Angermair S, Treskatsch S, Müller-Redetzky H, Pieske B, Stangl K, Landmesser U, Boldt LH, Huemer M, Attanasio P. Cardiac arrhythmias in patients with COVID-19: Lessons from 2300 telemetric monitoring days on the intensive care unit. J Electrocardiol. 2021;66:102-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Boriani G, Fauchier L, Aguinaga L, Beattie JM, Blomstrom Lundqvist C, Cohen A, Dan GA, Genovesi S, Israel C, Joung B, Kalarus Z, Lampert R, Malavasi VL, Mansourati J, Mont L, Potpara T, Thornton A, Lip GYH; ESC Scientific Document Group. European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin American Heart Rhythm Society (LAHRS). Europace. 2019;21:7-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 100. | Annane D, Sébille V, Duboc D, Le Heuzey JY, Sadoul N, Bouvier E, Bellissant E. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, Chahal AA, Anesi G, Denduluri S, Domenico CM, Arkles J, Abella BS, Bullinga JR, Callans DJ, Dixit S, Epstein AE, Frankel DS, Garcia FC, Kumareswaram R, Nazarian S, Riley MP, Santangeli P, Schaller RD, Supple GE, Lin D, Marchlinski F, Deo R. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 102. | Tabatabai S, Abushabana M, Alhashmi J, M, Zaky H. High-Grade Atrioventricular Nodal Block: An Unusual Presentation of COVID-19 Infection. Dubai Med J. 2022;5:54-57. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Task Force for the management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2-care pathways, treatment, and follow-up. Eur Heart J. 2022;43:1059-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 104. | Blanchard M, Gervès-Pinquié C, Feuilloy M, Le Vaillant M, Trzepizur W, Meslier N, Paris A, Pigeanne T, Racineux JL, Balusson F, Oger E, Girault JM, Gagnadoux F. Association of Nocturnal Hypoxemia and Pulse Rate Variability with Incident Atrial Fibrillation in Patients Investigated for Obstructive Sleep Apnea. Ann Am Thorac Soc. 2021;18:1043-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 105. | Stone E, Kiat H, McLachlan CS. Atrial fibrillation in COVID-19: A review of possible mechanisms. FASEB J. 2020;34:11347-11354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 106. | Abe I, Teshima Y, Kondo H, Kaku H, Kira S, Ikebe Y, Saito S, Fukui A, Shinohara T, Yufu K, Nakagawa M, Hijiya N, Moriyama M, Shimada T, Miyamoto S, Takahashi N. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018;15:1717-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 107. | Kaakeh Y, Overholser BR, Lopshire JC, Tisdale JE. Drug-induced atrial fibrillation. Drugs. 2012;72:1617-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Diaz-Arocutipa C, Brañez-Condorena A, Hernandez AV. QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2021;30:694-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 109. | Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 1-epidemiology, pathophysiology, and diagnosis. Eur Heart J. 2022;43:1033-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 110. | Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30:451-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 111. | Dherange P, Lang J, Qian P, Oberfeld B, Sauer WH, Koplan B, Tedrow U. Arrhythmias and COVID-19: A Review. JACC Clin Electrophysiol. 2020;6:1193-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 112. | Mukhopadhyay S, Uppal A, Yusuf J, Muheeb G, Agarwal R. COVID-19 induced ventricular tachycardia storm unmasking a clinically silent cardiomyopathy: a case report. Eur Heart J Case Rep. 2021;5:ytab220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 113. | Amaratunga EA, Corwin DS, Moran L, Snyder R. Bradycardia in Patients With COVID-19: A Calm Before the Storm? Cureus. 2020;12:e8599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 114. | Ye F, Winchester D, Stalvey C, Jansen M, Lee A, Khuddus M, Mazza J, Yale S. Proposed mechanisms of relative bradycardia. Med Hypotheses. 2018;119:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Ye F, Hatahet M, Youniss MA, Toklu HZ, Mazza JJ, Yale S. The Clinical Significance of Relative Bradycardia. WMJ. 2018;117:73-78. [PubMed] |
| 116. | Saenz LC, Miranda A, Speranza R, Texeira RA, Rojel U, Enriquez A, Figuereido M. Recommendations for the organization of electrophysiology and cardiac pacing services during the COVID-19 pandemic : Latin American Heart Rhythm Society (LAHRS) in collaboration with: Colombian College Of Electrophysiology, Argentinian Society of Cardiac Electrophysiology (SADEC), Brazilian Society Of Cardiac Arrhythmias (SOBRAC), Mexican Society Of Cardiac Electrophysiology (SOMEEC). J Interv Card Electrophysiol. 2020;59:307-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Kouhpeikar H, Khosaravizade Tabasi H, Khazir Z, Naghipour A, Mohammadi Moghadam H, Forouzanfar H, Abbasifard M, Kirichenko TV, Reiner Ž, Banach M, Sahebkar A. Statin Use in COVID-19 Hospitalized Patients and Outcomes: A Retrospective Study. Front Cardiovasc Med. 2022;9:820260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 118. | Ganjali S, Bianconi V, Penson PE, Pirro M, Banach M, Watts GF, Sahebkar A. Commentary: Statins, COVID-19, and coronary artery disease: killing two birds with one stone. Metabolism. 2020;113:154375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 119. | Vahedian-Azimi A, Mohammadi SM, Banach M, Beni FH, Guest PC, Al-Rasadi K, Jamialahmadi T, Sahebkar A. Improved COVID-19 Outcomes following Statin Therapy: An Updated Systematic Review and Meta-analysis. Biomed Res Int. 2021;2021:1901772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 120. | Mitacchione G, Schiavone M, Curnis A, Arca M, Antinori S, Gasperetti A, Mascioli G, Severino P, Sabato F, Caracciolo MM, Arabia G, D'Erasmo L, Viecca M, Mancone M, Galli M, Forleo GB. Impact of prior statin use on clinical outcomes in COVID-19 patients: data from tertiary referral hospitals during COVID-19 pandemic in Italy. J Clin Lipidol. 2021;15:68-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |