Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10387
Peer-review started: June 6, 2022
First decision: August 4, 2022
Revised: August 13, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: October 6, 2022
Processing time: 113 Days and 12.3 Hours
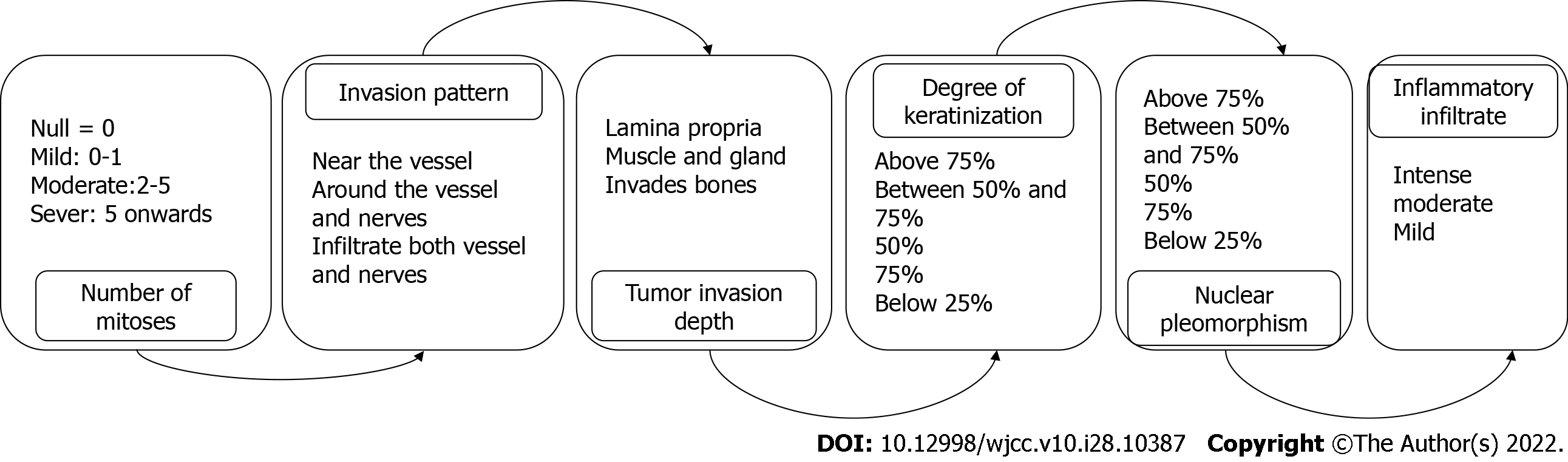
Oral squamous cell carcinoma is a neoplasm that originates from the epithelial mucosa. It is usually more frequent between the fifth and sixth decades of life, and more than 90% of carcinomas of the oral cavity are squamous cell carcinoma. It is an invasive neoplasia with a significant recurrence rate; 40% of patients present with metastases in the cervical lymph nodes at the time of diagnosis. The tumor invasion front is a characteristic of tumor growth, which can be infiltrative or noninvasive. The histopathological parameters examined include the number of mitoses, depth of the tumor, invasion pattern, degree of keratinization, and nuclear pleomorphism. For the pathologist, these parameters are routinely evaluated but are not reported to the treating physician in all cases, which we consider to be useful information when determining the therapeutic route.
Core Tip: The histopathological parameters of the tumor invasion front are evaluated by the pathologist at the diagnosis of squamous cell carcinoma. This information is not part of the microscopic description that the treating physician receives in all cases. Thus, we propose the evaluation and reporting of the tumor invasion front, thus providing the medical doctor with more objective criteria when establishing the therapeutic route for each patient.
- Citation: Cuevas-González JC, Cuevas-González MV, Espinosa-Cristobal LF, Donohue Cornejo A. Tumor invasion front in oral squamous cell carcinoma. World J Clin Cases 2022; 10(28): 10387-10390
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10387.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10387
Oral squamous cell carcinoma (SCC) is a neoplasm that originates from the epithelial mucosa. It is usually more frequent between the fifth and sixth decades of life, and more than 90% of carcinomas of the oral cavity are SCC[1]. Its reported incidence worldwide is 300400 cases, and it is responsible for 145,400 deaths per year with a 5-year survival rate of 50% to 60%. When the origin of the primary tumor is unknown in head and neck SCC, the 5-year survival rate is only 5% to 15%[2].
With regard to localization, approximately 32% of SCCs affect the oral mucosa, compared with 22% for the tongue, 11% for the lower lip, 11% for the palate, 8% for the vestibule, 5% for the alveolus, 5% for the floor of the mouth, and 3% for the gingiva[3].
SCC is an invasive neoplasia with a significant recurrence rate; 40% of patients present with metastases in the cervical lymph nodes at the time of diagnosis[4]. Thus, early detection and histopathological analysis are essential for the patient to be treated in an appropriate and timely manner.
The tumor invasion front (TIF) is a characteristic of tumor growth, which can be infiltrative or noninvasive. The histopathological parameters examined include the number of mitoses, depth of the tumor, invasion pattern, degree of keratinization, and nuclear pleomorphism (Figure 1)[5,6]. For the pathologist, these parameters are routinely evaluated but are not reported to the treating physician in all cases, which we consider to be useful information when determining the therapeutic route. Thus, these parameters were evaluated by our working group. We analyzed 10 cases diagnosed in the Oral Pathology Laboratory of the Stomatology Department of the Universidad Autonoma de Ciudad Juarez in Mexico; 9 corresponded to well-differentiated SCC and 1 was poorly differentiated. The mean age was 57 years, and the most frequent location was the lip (6/10). Regarding the TIF, mitosis was moderate (2-5) in 50% of cases; 3 cases had one mitosis and 2 cases had more than five mitoses in the 400 x field.
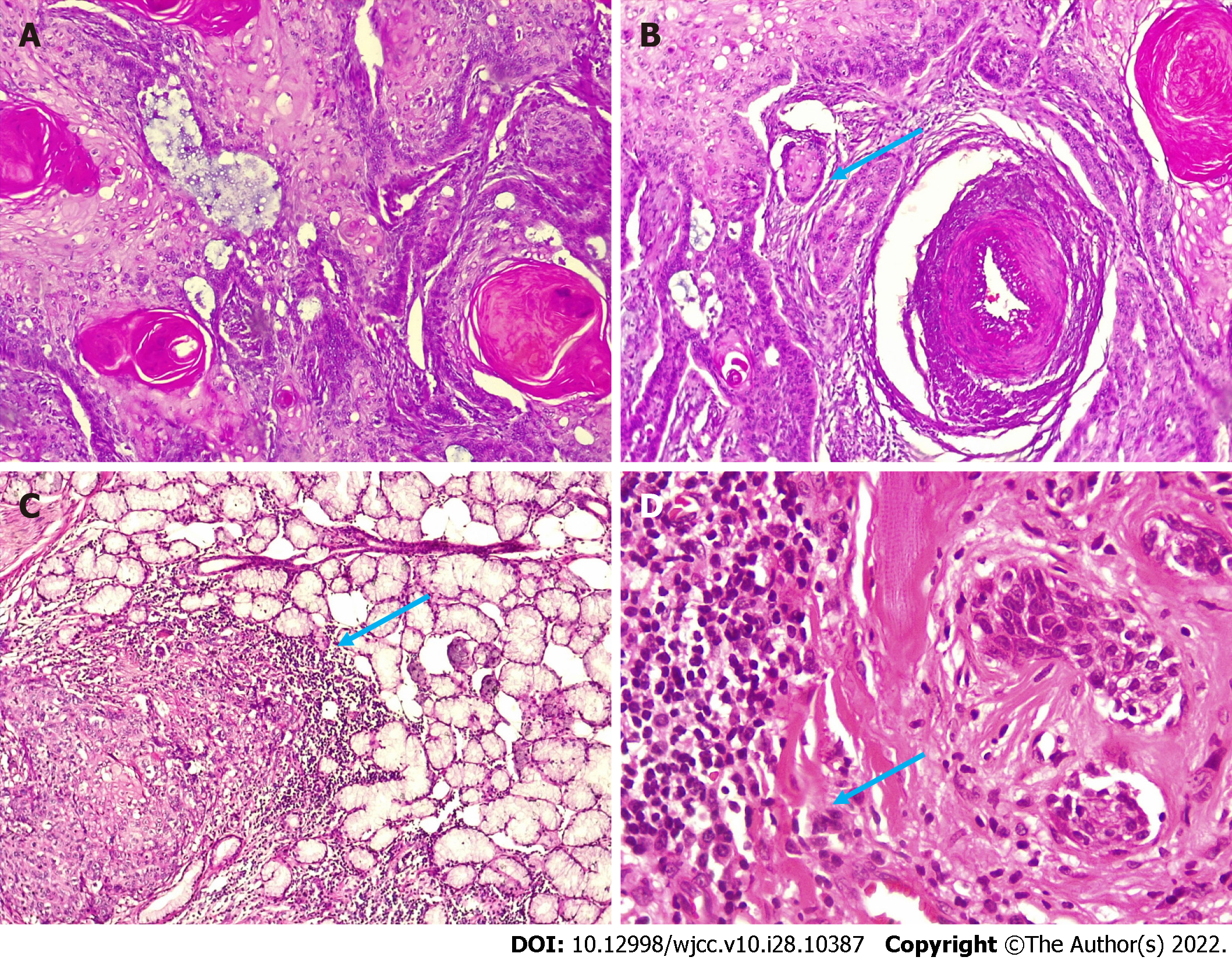
We noted a relationship among neoplastic cells, vascularity, and depth. In 4 cases, there was infiltration into the blood vessels; in 3 cases, the neoplasm surrounded the blood vessels; and in 3 cases, it was close to the blood vessels. The tumor depth reached the muscular and glandular levels in 6/10 neoplastic cells vs 4 in the lamina propria. These two characteristics are important to consider and should be reported to the clinician as support when establishing treatment and determining the prognosis of the carcinoma (Figure 2). Seven carcinomas presented with a keratin pearl and individual keratinization of at least 50%. Notably, although 90% of carcinomas were well differentiated, the tumor depth in most cases was infiltrating, which is a relevant histopathological finding. We consider that in cases of poorly differentiated carcinomas, complementary immunohistochemical studies (cytokeratins) would be very useful to confirm the epithelial origin of the neoplasm.
Currently, there are other efforts focused on elucidating the prognosis in oral SCC. Some of these have been reported by Nocini et al[7] and Girolami et al[8], who studied the expression of programmed death-ligand 1 (PD-L1) both in precancerous lesions of the head and neck, and in oral SCC. There are contrasting results for PD-L1 in the literature; the authors suggest that if an adequate standardization is carried out both in the performance and the evaluation of the marker, more reliable results can be obtained[7,8]. As shown here, this work is aimed at standardizing both histopathological characteristics and molecular biology techniques, with the sole purpose of facilitating the clinical management of oral SCC patients in a correct, precise, and timely manner.
In conclusion, although the histopathological parameters of TIF are evaluated by the pathologist at the diagnosis of SCC, this information is not part of the microscopic description that the treating physician receives in all cases. Thus, we propose the evaluation and reporting of the TIF, thus providing the medical doctor with more objective criteria when establishing the therapeutic route for each patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoun G, Lebanon; Eccher A, Italy S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweng PJ. Malignant surface epithelial tumours. WHO Classification of Head and Neck Tumours. 2016;109-111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (2)] |
| 2. | Große-Thie C, Maletzki C, Junghanss C, Schmidt K. Long-term survivor of metastatic squamous-cell head and neck carcinoma with occult primary after cetuximab-based chemotherapy: A case report. World J Clin Cases. 2021;9:7092-7098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. |
Korean Society of Thyroid-Head and Neck Surgery Guideline Task Force, Joo YH, Cho JK.
Guidelines for the Surgical Management of Oral Cancer: Korean Society of |
| 4. | Pavithra V, Kumari K, Haragannavar VC, Rao RS, Nambiar S, Augustine D, Sowmya SV. Possible Role of Bcl-2 Expression in Metastatic and Non Metastatic Oral Squamous Cell Carcinoma. J Clin Diagn Res. 2017;11:ZC51-ZC54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Cuevas-González JC, Maya-García IA, Villanueva-Sánchez FG, Gaitán-Cepeda LA, Leyva-Huerta ER. Estandarización en la Observación y Clasificación de Lesiones Epiteliales Premalignas y Malignas. Int J Morphol. 2011;29:706-710. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Slaton JW, Morgenstern N, Levy DA, Santos MW Jr, Tamboli P, Ro JY, Ayala AG, Pettaway CA. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol. 2001;165:1138-1142. [PubMed] |
| 7. | Nocini R, Vianini M, Girolami I, Calabrese L, Scarpa A, Martini M. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin Exp Dent Res. 2022;8:690-698. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Girolami I, Pantanowitz L, Munari E, Martini M, Nocini R, Bisi N, Molteni G, Marchioni D, Ghimenton C, Brunelli M, Eccher A. Prevalence of PD-L1 expression in head and neck squamous precancerous lesions: a systematic review and meta-analysis. Head Neck. 2020;42:3018-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |