Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10375
Peer-review started: July 5, 2022
First decision: July 14, 2022
Revised: July 21, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 84 Days and 1.6 Hours
Tethered cord syndrome (TCS) secondary to split cord malformation (SCM) is rare in adulthood. There is as yet no consensus about the optimal treatment method for adult patients with SCMs and degenerative spine diseases such as lumbar stenosis, spondylolisthesis and ossification of the ligamentum flavum (OLF). The tethered cord poses a great challenge to the decompression and fusion procedures for the intraoperative stretching of the spinal cord, which might lead to deteriorated neural deficits. Here, we report on a case to add our treatment experience to the medical literature.
We treated a 67-year-old female patient with type II SCM suffering from lumbar disc herniation, degenerative lumbar spondylolisthesis and thoracic OLF. The patient underwent thoracolumbar spinal fusion and decompression surgery for severe lower back pain, extensive left lower limb muscle weakness and inter
For adult patients with underlying TCS secondary to SCM coupled with thoracic OLF and lumbar spondylolisthesis, a thoracolumbar fusion surgery could be safe and effective with the tethered cord untreated. It is critical to design individualized surgical protocols to reduce the stretch of the low-lying spinal cord.
Core Tip: Tethered cord syndrome (TCS) secondary to split cord malformation (SCM) is rare in adulthood. We present a patient who underwent thoracolumbar surgery for thoracic ossification of the ligamentum flavum and lumbar spondylolisthesis complicated with TCS. A thoracolumbar fusion surgery could be safe and effective with the tethered cord untreated. It is critical to design individualized surgical protocols to reduce the stretch of the low-lying spinal cord. A literature review of SCM in adults was also performed.
- Citation: Wang YT, Mu GZ, Sun HL. Thoracolumbar surgery for degenerative spine diseases complicated with tethered cord syndrome: A case report. World J Clin Cases 2022; 10(28): 10375-10383
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10375.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10375
Split cord malformation (SCM), one of the most common intraspinal malformations, is a rare disease of the spinal cord and cauda equina caused by embryonic dysplasia[1,2]. SCM refers to a spinal cord divided longitudinally into two distinct hemicords that later rejoin[1,3], which has been categorized into two types: Type I SCM refers to two hemicords, with their own dural tubes and separated by a dural-sheathed rigid osteocartilaginous median septum, and type II SCM also refers to two hemicords but housed in a single dural tube separated by a nonrigid, fibrous median septum[4]. It has been demonstrated that the presence of a bony fibrous septum anchoring the cord interferes with its normal upward ascension during growth, resulting in tethered cord syndrome (TCS)[1,3]. The typical symptoms might result from stretch-induced ischemia by traction on the cord.
SCM is commonly presented during childhood but rarely diagnosed in adults[2]. Moreover, SCM is more likely to be diagnosed in females[1,5]. Neurologic symptoms of type I SCM can progressively deteriorate if left untreated[6]. Unidentified adult SCM might also result in various degenerative spinal diseases with age, and the tethered spinal cord could pose a great challenge to the spinal fusion surgery[7]. However, there is not enough experience on spine diseases complicated by TCS secondary to SCM.
In the present study we report an adult-onset SCM patient of type II suffering from degenerative lumbar spondylolisthesis and thoracic ossification of the ligamentum flavum (OLF) who needed a spinal fusion surgery for extensive left lower limb muscle weakness.
A 67-year-old female was hospitalized with severe low back pain with radiating pain in the left lower limb for 5 years. Symptoms worsened in the last 1 wk. She had exhausted conservative treatments and intended to proceed with surgery.
Five years before hospitalization, the patient began to experience aching pain in the waist, accompanied by pain and numbness in the left lower limb ranging from the posterior thigh and the posterolateral crus to the dorsolateral foot, especially obvious at the 4th and 5th toes. There was no weakness of lower limbs and no urination difficulty. The patient was not able to walk more than 100 m without rest. The uncomfortable symptoms often occurred after overwalking and catching a cold, but could be relieved via physical therapy and NSAIDs. One week before hospitalization, the above symptoms were significantly aggravated, accompanied by progressively extensive weakness of the left lower extremity and with conservative treatment giving unsatisfactory results. Lumbar computed tomography (CT) scan demonstrated L4/5 intervertebral disc prolapse and lumbar X-ray imaging showed grade I L4 anterolisthesis from another hospital.
The patient was diagnosed with atrial septal defect five years before hospitalization and treated with repair surgery. She underwent operation because of lipoma located on the left trunk during the same year. She denied history of hypertension or diabetes. No history of trauma or malignant tumors were identified.
There was no special history or personal history. The patient was unaware of SCM before and denied family history of SCM.
Examination showed normal curvature of the lumbar spine without scoliosis deformity and no foot abnormality. There was a round skin sag of 1.5 cm in diameter located in the sacrococcygeal region with chromatosis but no hair. Slight tenderness and percussion pain in the paraspinal muscles were found. The left lower extremity had slight hypoalgesia and hypopselaphesia. Waist activities in different directions were somewhat limited because of pain, especially in extension. There was extensive weakness in the left lower limb, with hip flexors strength graded IV, knee flexion muscle strength graded III+, knee extension strength graded III and foot dorsiflexion strength graded III. The Lasegue sign was positive in the left lower limb. Bilateral tendon reflexes showed suspicious hyperactivity with lower limbs’ muscle tension slightly increased.
Her baseline severity of low back pain and left lower limb pain was 90 mm and 90 mm, respectively, on a 100-mm visual analog scale (VAS) when she was admitted into our hospital. We used the Oswestry Disability Index (ODI) to evaluate lumbar function and the score was 64.
The routine blood and blood biochemical parameters of the patient were within normal limits.
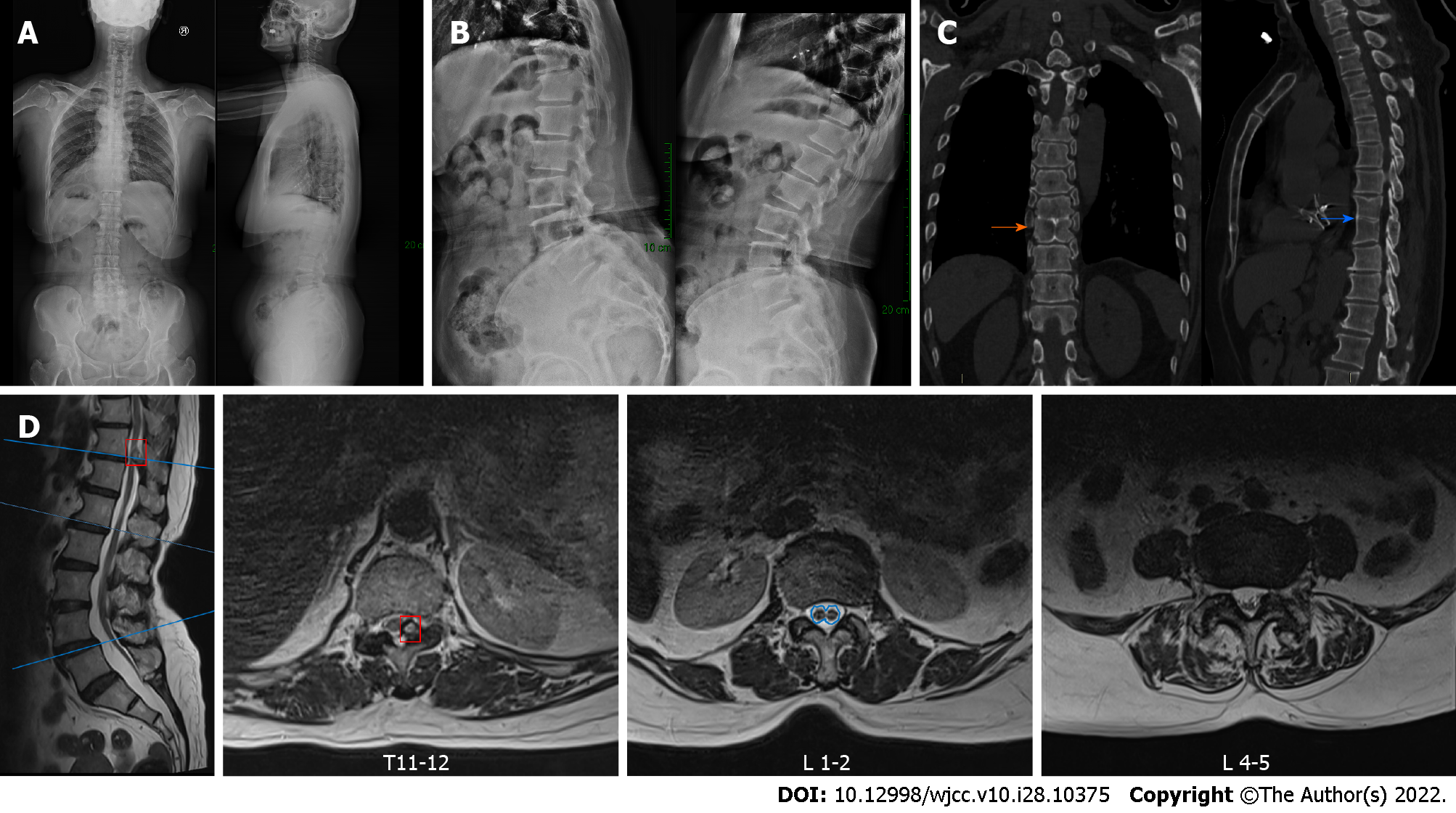
Anteroposterior and lateral X-ray imaging showed a grade I L4 spondylolisthesis and the flexion-extension X-ray imaging demonstrated instability at that level. Lumbar CT scan showed the L2-S1 intervertebral disc was swollen with the dura sac compressed to varying degrees, in addition to the L4 spondylolisthesis. Thoracic CT scan showed that the left part of the ligamentum flavum was thickened and ossified at the T11-12 Level with the posterior dura sac obviously compressed and that there were block vertebrae (T8/T9) and a butterfly vertebra (T9). Lumbar magnetic resonance imaging (MRI) showed L4 spondylolisthesis and the L4-5 intervertebral disc bulge compressing bilateral nerve roots, which was more serious on the left side. Additionally, SCM was observed; the spinal was split into two hemicords from the lower aspect of T12 to the upper aspect of L2, accompanied by a low-lying conus terminating at S1 with a thickened terminal filament deposited by fatty tissue (Figure 1).
The clinical diagnosis was T11-12 thoracic OLF, L4 Lumbar spondylolisthesis (grade I) and TCS.
Considering there was a tethered cord due to the silent SCM, neurosurgery was requested to evaluate the feasibility of concurrent transection of the filum terminale during the spine surgery. However, this was not recommended because simultaneous operation for the tethered cord required opening of the dura sac, which could lead to cerebrospinal fluid extravasation and interfere with decompression and fusion manipulations. After identifying the lesions responsible for neural symptoms, we decided to perform a thoracolumbar combined surgery to treat the thoracic OLF and lumbar spondylolisthesis but not the TCS over the same period. Posterior thoracic canal decompression through laminectomy followed by ossification removal with pedicle screw fixation (T11-12) was conducted for the thoracic spinal stenosis resulting from the thoracic OLF. For the lumbar spinal stenosis due to the L4-5 intervertebral disc bulge and segmental instability caused by L4 spondylolisthesis, an L4-5 midline lumbar fusion (MIDLF) procedure was performed simultaneously to pursue bilateral L5 nerve root and spinal canal decompression, cortical bone trajectory screw fixation and intervertebral and posterior-lateral fusion. Given there was a preoperatively existing extensive decrease in left lower limb muscle strength, the intraoperative interference of the tethered cord would carry a great risk of paralysis, posing a substantial challenge for the surgeon to conduct the thoracolumbar combined operation. As a result, we adopted neural electrophysiological monitoring during the whole operation, which showed good sensory and motor conduction before and after decompression of the spinal cord (Table 1). The operation duration was 315 min and the estimated blood loss was 500 mL.
| SEP (left) | SEP (right) | MEP (left) | MEP (right) | |||||||||
| P40 in ms | N50 in ms | Amplitude in μV | P40 in ms | N50 in ms | Amplitude in μV | P40 in ms | N50 in ms | Amplitude in μV | P40 in ms | N50 in ms | Amplitude in μV | |
| Baseline | 41.0 | 50.3 | 1.3 | 40.0 | 46.5 | 1.0 | 50.2 | 47.7 | 85.8 | 45.0 | 52.2 | 61.3 |
| After lamina exposure | 41.5 | 49.8 | 1.3 | 39.5 | 47.7 | 1.0 | 51.5 | 49.0 | 83.7 | 44.0 | 47.0 | 41.9 |
| After thoracic decompression | 42.5 | 51.2 | 0.7 | 40.5 | 46.5 | 0.8 | 48.3 | 46.0 | 186.6 | 52.5 | 50.5 | 284.2 |
| After lumbar decompression | 38.8 | 49.5 | 0.6 | 38.5 | 46.5 | 0.7 | 48.0 | 44.0 | 149.4 | 50.0 | 46.0 | 336.1 |
The principle of the combined operation was that the thoracic spinal decompression and pedicle screw fixation was taken as the prior task and then the bilateral compression to the T11-12 fixation was carried out to relieve and shorten the strained spinal cord for providing compensatory space. The L4-5 MIDLF was performed to avoid excessive opening of the intervertebral space, so as to reduce the stretch of the dura sac and the spinal cord. The patient's lower limbs moved well after awakening from anesthesia.
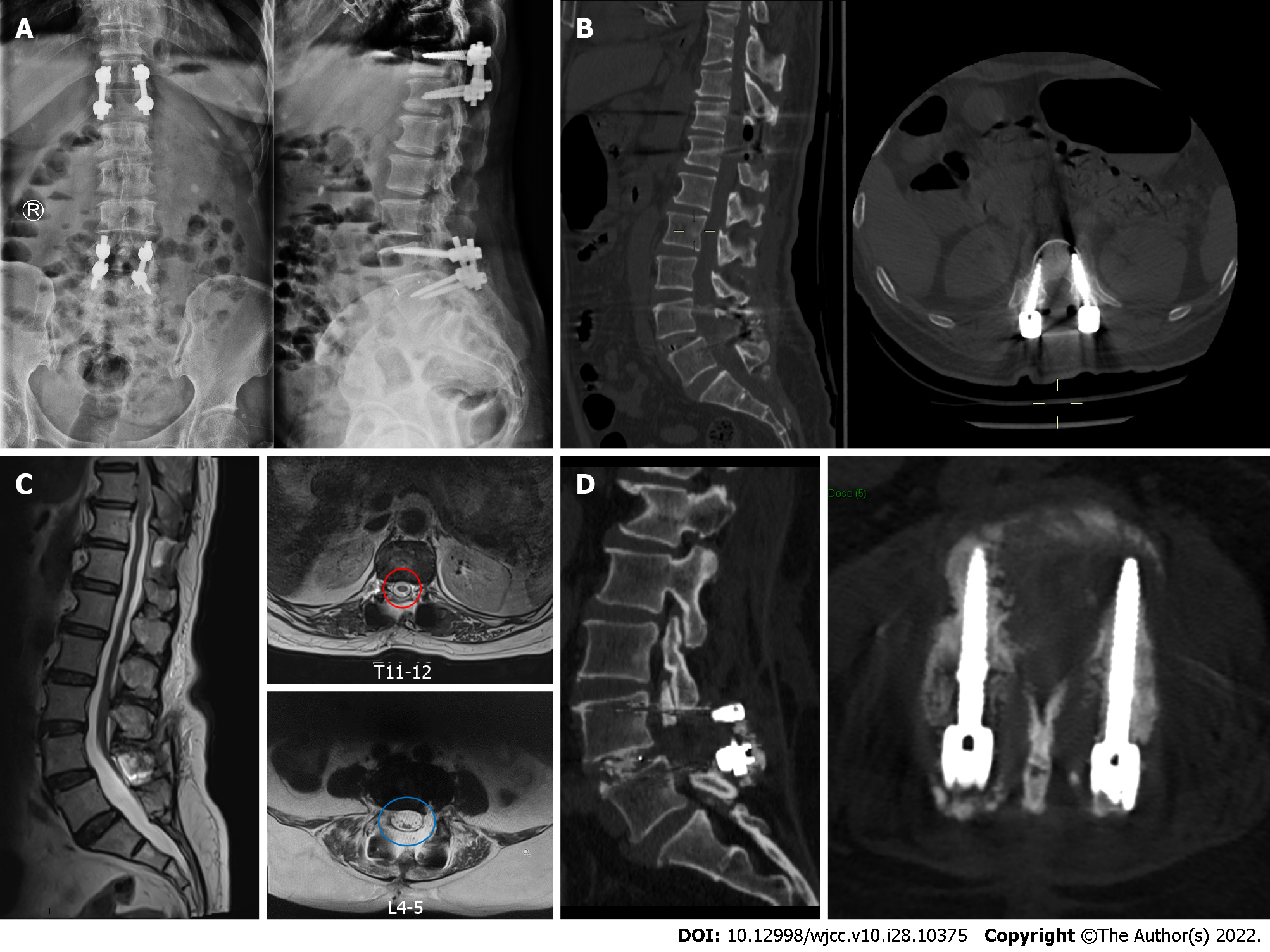
The patient's symptoms were significantly alleviated postoperatively. There were no operation-related complications after surgery. By the time she was discharged, her low back pain VAS score had dropped to 3 and her leg pain VAS score to 2. She could ambulate well after leaving the bed with left lower limb muscle strength enhancement. The MRI at 1 mo after surgery showed fine decompression of the spinal canal (Figure 2). Up to the final follow-up (3 mo after surgery), her low back pain VAS score had dropped to 2 and her leg pain VAS score to 1; the ODI had dropped to 26; and the lumbar CT scan showed the intervertebral fusion status and implant position were fine (Figure 2).
SCM is described as a congenital spinal dysraphism with often a bone spur and a membranous or fibrous septum resulting in two split hemicords, with a single or separated dural layer surrounding it[4,5,8]. SCM is often noted due to the appearance of scoliosis, skin stigmas, progressive foot deformities, calf and foot atrophy and bowel or bladder disturbances in childhood. As a result, this pathologic phenomenon is most frequently seen in childhood but rarely presented in the adult population[1,3,9]. D'Agostino et al[3] reviewed SCM in adults and from 1936 to 2018, only 25 cases of concurrent split and radiographic tethered cords were identified. Patients averaged 37 years of age at the time of diagnosis and 56% were female. A recent review summarized 146 adult SCM patients diagnosed at a mean age of 26.8 years, of which 74.6% were female[8]. SCM is often accompanied by vertebral anomalies, spina bifida occulta being the most common[9]. The female patient we presented was 67-years-old and she was not diagnosed with SCM until she was admitted to our hospital. The CT scan revealed that there were T8 and T9 block vertebrae with a T9 butterfly vertebra.
Adult SCM can occur at any level along the spine but is more common in the lumbar region, followed by lumbosacral segments and thoracic regions[2,9-11]. According to the number of dural tubes and the characteristics of the median septum, Pang et al[4] classified SCM into two types, with type I referring to two hemicords, each in a separate dura sac separated by a rigid osseocartilaginous median septum, and type II involving two hemicords in a single dural tube with a nonrigid septum. A low-lying cord is usually associated with this dysraphism because the septum could prevent the spinal cord from moving upward, leading to excess strain on the spinal cord, which results in TCS. It appears that type II SCM lesions are more likely to tether than type I lesions though more data are needed to confirm this[3,5]. TCS is rarely secondary to SCM in adulthood. SCM was reported to account for 10%-38% of adult TCS diagnoses[3]. Studies revealed that adult-onset TCS is usually associated with precipitating events such as stretching of the conus medullaris, narrowing of the spinal canal or trauma[12].
The clinical presentation of SCM is variable. The most common symptom is back pain associated with the level of pathology, and radiculopathy and lower extremity weakness were also reported as common manifestations with sometimes bowel or bladder disturbance[1,13-16]. Moreover, some cases of SCM remained asymptomatic or only caused subtle symptoms until there was a factor that stretched the tethered spinal cord. The factors might be various degenerative spine diseases such as lumbar/thoracic disc herniation, spondylolisthesis, lumbar/thoracic spinal stenosis or scoliosis[17,18]. Physicians should be cautious when recommending or giving prophylactic surgery for asymptomatic SCM. Goldberg et al[19] reported 28 patients who underwent prophylactic operations of split cords. Of these patients, 10 patients had reduced lower extremity strength after treatment. For symptomatic SCM, such as pain and lower extremity weakness, if no other responsible lesions such as lumbar disc herniation or spondylolisthesis were identified on imaging, surgery for SCM or TCS such as removal of the bony diaphragm or cutting the filum terminale tended to result in good clinical outcomes[20-22].
Refractory pain and neurologic deficits in adult patients with TCS usually implicate that there might be stenosis or compression factors and that surgery is required[5]. We reviewed the reported cases of adult TCS coupled with degenerative spine abnormalities that remained asymptomatic until the compression or instability required treatment. The characteristics of these cases are summarized in the Table 2. It seems that operation on these patients with TCS was challenging because it could interfere with the existing balancing of the tethered spinal cord, causing paralysis or neurological deficits. The main principle of this kind of operation was to minimize the stretching of the spinal cord. Some surgeons argued to untether the spinal cord by filamentectomy or resection of the bony spur before treating the compression factors[5,13]. The simultaneous removal of bony spur while treating the degenerative spine lesions could be challenging and may be more suitable for type I SCM, because there is a high risk of complication of the opening of the dura sac, causing cerebrospinal fluid leakage or infection[9,13,23]. In addition, some surgeons resorted to minimally invasive procedures, such as endoscopic surgery, to remove the lesions or decompress the spinal canal in order to decrease the disturbance of the unreleasing low-lying cord[15,24]. A few cautious surgeons performed operations avoiding stretching of the spinal cord through indirect compression operation without removing the compressive lesions, adopting an anterior approach to fusion, or pursuing posterolateral fusion without intervertebral fusion[17,23,25]. Another important method to protect the tethered cord is to shorten the spine, such as vertebral column resection or pedicle subtraction osteotomy[26-28]. As for the instability and compression from spondylolisthesis, fixation to acquire stability is fairly necessary. In order to minimize manipulation of neural structures, in situ fusion instead of reduction is a good choice[25].
| Ref. | Country | Study type | Number of patients | Gender/age in yr | SCM type | Symptoms | Main diagnosis | Operation | Principles | Problems |
| Oh et al[13], 2021 | United States | Case report | 1 | Female/50 | Type II | Back and radiating leg pain; leg numbness; bowel/bladder incontinence | Spondylolisthesis | L5/S1 ALIF with posterior fixation | First to perform untethering procedure before spinal fusion surgery | High-volume drain output; pseudomeningocele; surgical exploration with wound debridement and repair |
| Kobets et al[5], 2021 | United States | Case Series | 6 | 4 Females/35-62 | Type II | Radiating leg pain; lower extremity paresthesia; bladder and bowel dysfunction | / | / | Filamentectomy | Symptom recurrence |
| Chang et al[24], 2020 | United States | Case report | 1 | Male/40 | NM | leg pain; sensory changes; hyperreflexia, and gait disturbance | Lumbar disc herniation | Full endoscopic lumbar diskectomy | Treating the responsible lesions in a minimally invasive method without spinal cord detethering surgery | NM |
| Kaminker et al[9], 2000 | United Kingdom | Case report | 1 | Male/38 | Type I | Bilateral leg pain and neurogenic claudication | Lumbar spinal stenosis | Posterior decompression | Decompression with subtotal resection of the bony bar | Cerebrospinal fluid leak |
| Breton et al[15], 2020 | United States | Case report | 1 | Female/79 | NM | Leg pain; progressive gait deterioration and bilateral leg weakness | Lumbar spinal stenosis; spondylolisthesis | Sublaminoplasty for spinal cord decompression with onlay arthrodesis | Conduct a minimally invasive surgery with tethered cord untreated | NM |
| Hui et al[26], 2014 | China | Case report | 1 | Male/23 | Type I | Unstable walking and progressed numbness in the lower limbs | Kyphoscoliosis | Posterior segmental pedicle screw instrumented fusion with vertebral column resection | Vertebral column resection above bony spur to shorten the spine and decrease the stretched power on the spinal cord. | No complications |
| Endo et al[17], 2014 | Japan | Case report | 1 | Male/43 | NM | Progressive spastic gait disturbance; numbness; muscle weakness and pyramidal tract signs in the lower limbs | Lumbar disc herniation | Herniotomy via a posterolateral approach and instrumented posterolateral fusion | Decompression and posterolateral fusion without intervertebral fusion | No complications |
| Srinivas et al[23], 2012 | United Kingdom | Case report | 1 | Female/77 | NM | Severe low back pain and progressive paraparesis | Lumbar disc herniation | Posterior decompression | Indirectly decompression by the falling back spinal cord | Deep wound infection |
| König et al[25], 2012 | United Kingdom | Case report | 1 | Female/26 | Type II | Severe low back pain, and bilateral L5/S1 sciatica | Spondylolisthesis | Anterior in situ fusion coupled with pedicle screw fixation | Anterior fusion to minimize manipulation of neural structures | No complications |
| Kawamura et al[27], 2010 | Japan | Case report | 2 | Male/69; Male/36 | NM | 1 Legs and low back pain with intermittent claudication; 2 Numbness and severe muscle weakness in the lower limbs | Lumbar spinal stenosis | Pedicle subtraction osteotomy and yellow ligament resection | Pedicle subtraction osteotomy to shorten the spine | NM |
| Kramer[28], 2009 | Canada | Case report | 1 | Female/54 | NM | Progressive pain and sensorimotor symptoms in the lower back and limbs | Thoracic disc herniation | Posterolateral partial vertebral body resection and decompression | Osteotomy to shorten the spine | NM |
To the best of our knowledge, this is the first case report of an adult patient with TCS due to SCM coupled with both thoracic OLF and lumbar spondylolisthesis who needs thoracolumbar combined surgery. Given the complexity of this case, we treated the lesions according to the following principles: (1) First, we treated the thoracic OLF by removing the ossification to decompress the thoracic spinal canal; (2) After the thoracic pedicle screw fixation was completed, bilateral fixation compression was conducted to reduce the spinal cord strain to provide compensatory space for the following L4-5 MIDLF; (3) There was no reduction operation for the lumbar spondylolisthesis when performing the in situ MIDLF, so the split and tethered cord was not stretched during the whole operation period; and (4) Finally, a safe and uneventful intraoperative neural electrophysiological monitoring enhanced the confidence of the surgeon and improved the safety of this combined surgery. The follow-up outcomes demonstrated our treatment was successful.
This is only a case report and it remains to be confirmed that our treatment strategy is optimal through studies with larger sample sizes. Moreover, the follow-up was relatively short; longer follow-up is needed to provide information on the long-term decompression effects.
For adult patients with underlying TCS secondary to SCM coupled with thoracic OLF and lumbar spondylolisthesis, combined thoracolumbar fusion surgery could be safe and effective with the tethered cord untreated. It is critical to design individual surgical protocols to reduce the stretch of the low-lying spinal cord.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Shariati MBH, Iran S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Alnefaie N, Alharbi A, Alamer OB, Khairy I, Khairy S, Saeed MA, Azzubi M. Split Cord Malformation: Presentation, Management, and Surgical Outcome. World Neurosurg. 2020;136:e601-e607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Akay KM, Izci Y, Baysefer A, Timurkaynak E. Split cord malformation in adults. Neurosurg Rev. 2004;27:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | D'Agostino EN, Calnan DR, Makler VI, Khan I, Kanter JH, Bauer DF. Type I split cord malformation and tethered cord syndrome in an adult patient: A case report and literature review. Surg Neurol Int. 2019;10:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: Part I: A unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 362] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Kobets AJ, Oliver J, Cohen A, Jallo GI, Groves ML. Split cord malformation and tethered cord syndrome: case series with long-term follow-up and literature review. Childs Nerv Syst. 2021;37:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Cheng B, Li FT, Lin L. Diastematomyelia: a retrospective review of 138 patients. J Bone Joint Surg Br. 2012;94:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Lewandrowski KU, Rachlin JR, Glazer PA. Diastematomyelia presenting as progressive weakness in an adult after spinal fusion for adolescent idiopathic scoliosis. Spine J. 2004;4:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Karim Ahmed A, Howell EP, Harward S, Sankey EW, Ehresman J, Schilling A, Wang T, Pennington Z, Gray L, Sciubba DM, Goodwin CR. Split cord malformation in adults: Literature review and classification. Clin Neurol Neurosurg. 2020;193:105733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Kaminker R, Fabry J, Midha R, Finkelstein JA. Split cord malformation with diastematomyelia presenting as neurogenic claudication in an adult: a case report. Spine (Phila Pa 1976). 2000;25:2269-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Huang SL, He XJ, Xiang L, Yuan GL, Ning N, Lan BS. CT and MRI features of patients with diastematomyelia. Spinal Cord. 2014;52:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Jiblawi A, Chanbour H, Tayba A, Khayat H, Jiblawi K. MRI Characteristics of Split Cord Malformation. Cureus. 2021;13:e18328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Klekamp J. Tethered cord syndrome in adults. J Neurosurg Spine. 2011;15:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Oh T, Avalos LN, Burke JF, Mummaneni N, Safaee M, Gupta N, Clark AJ. A Type II Split Cord Malformation in an Adult Patient: An Operative Case Report. Oper Neurosurg (Hagerstown). 2021;20:E148-E151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Menezes AH, Seaman SC, Iii MAH, Hitchon PW, Takacs EB. Tethered spinal cord syndrome in adults in the MRI era: recognition, pathology, and long-term objective outcomes. J Neurosurg Spine. 2021;1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Breton JM, Yang MJ, Riesenburger RI. The use of decompressive segmental sublaminoplasty to treat myelopathy caused by lumbar stenosis in tethered cord syndrome. J Surg Case Rep. 2020;2020:rjaa041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Assaker R, El Hasbani G, Vargas J, Parashar K, Thomas GA, Rodrigue P, Yagan N. Incidentally discovered type 1 split cord malformation in an adult patient. Radiol Case Rep. 2020;15:1756-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Endo F, Iizuka H, Iizuka Y, Kobayashi R, Mieda T, Takagishi K. Myelopathy due to lumbar disc herniation in the presence of a tethered cord. Spinal Cord. 2014;52 Suppl 1:S11-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Martinez-Lage JF, Piqueras C, Poza M. Lumbar canal stenosis: a cause of late neurological deterioration in patients with spina bifida. Surg Neurol. 2001;55:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Goldberg C, Fenelon G, Blake NS, Dowling F, Regan BF. Diastematomyelia: a critical review of the natural history and treatment. Spine (Phila Pa 1976). 1984;9:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Viswanathan VK, Minnema AJ, Farhadi HF. Surgical management of adult type 1 split cord malformation. Report of two cases with literature review. J Clin Neurosci. 2018;52:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Sack AM, Khan TW. Diastematomyelia: Split Cord Malformation. Anesthesiology. 2016;125:397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Barutcuoglu M, Selcuki M, Selcuki D, Umur S, Mete M, Gurgen SG, Umur. Cutting filum terminale is very important in split cord malformation cases to achieve total release. Childs Nerv Syst. 2015;31:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Srinivas S, Shetty R, Collins I. Symptomatic lumbar disc protrusion causing progressive myelopathy in a low-lying cord. Global Spine J. 2012;2:115-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Chang HK, Wegner AM, Lu ML, Hsu CC, Wu RW, Chen SH, Yin TC. Full Endoscopic Lumbar Diskectomy for Lumbar Disk Herniation in the Presence of a Low-Lying Cord. World Neurosurg. 2020;137:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | König MA, Boszczyk BM. Limited access surgery for 360 degrees in-situ fusion in a dysraphic patient with high-grade spondylolisthesis. Eur Spine J. 2012;21:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Hui H, Zhang ZX, Yang TM, He BR, Hao DJ. Vertebral column resection for complex congenital kyphoscoliosis and type I split spinal cord malformation. Eur Spine J. 2014;23:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Kawamura I, Ishido Y, Zenmyo M, Yamamoto T, Kagawa Y, Komiya S, Ijiri K. Pedicle subtraction osteotomy for adult tethered cord syndrome with lumbar canal stenosis: report of two cases. Int J Neurosci. 2010;120:735-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kramer JL, Dvorak M, Curt A. Thoracic disc herniation in a patient with tethered cord and lumbar syringomyelia and diastematomyelia: magnetic resonance imaging and neurophysiological findings. Spine (Phila Pa 1976). 2009;34:E484-E487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |