Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10346
Peer-review started: June 17, 2022
First decision: July 12, 2022
Revised: August 2, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 6, 2022
Processing time: 102 Days and 4.9 Hours
Many genetic and metabolic diseases affect the liver, but diagnosis can be difficult because these diseases may have complex clinical manifestations and diverse clinical patterns. There is also incomplete clinical knowledge of these many different diseases and limitations of current testing methods.
We report a 53-year-old female from a rural area in China who was hospitalized for lower limb edema, abdominal distension, cirrhosis, and hypothyroidism. We excluded the common causes of liver disease (drinking alcohol, using traditional Chinese medicines, hepatitis virus infection, autoimmunity, and hepatolenticular degeneration). When she was 23-years-old, she developed night-blindness that worsened to complete blindness, with no obvious cause. Her parents were first cousins, and both were alive. Analysis of the patient’s family history indicated that all 5 siblings had night blindness and impaired vision; one sister was completely blind; and another sister had night-blindness complicated with cirrhosis and subclinical hypothyroidism. Entire exome sequencing showed that the patient, parents, and siblings all had mutations in the cytochrome P450 4V2 gene (CYP4V2). The CYP4V2 mutations of the parents and two sisters were heterozygous, and the others were homozygous. Two siblings also had heterozygous dual oxidase activator 2 (DUOXA2) mutations.
Mutations in the CYP4V2 gene may affect lipid metabolism and lead to chronic liver injury, fibrosis, and cirrhosis.
Core Tip: We describe two patients from consanguineous parents who had liver cirrhosis, were completely blind, and had familial mutations in the cytochrome P450 4V2 (CYP4V2) gene. The four other siblings of these patients were night-blind, and one also had cirrhosis. CYP4V2 functions in hepatic lipid metabolism and inflammatory responses. We suggest that this CYP4V2 gene mutation affects hepatic lipid metabolism and inflammatory responses, which leads to chronic liver injury and may subsequently progress to liver fibrosis and cirrhosis.
- Citation: Jiang JL, Qian JF, Xiao DH, Liu X, Zhu F, Wang J, Xing ZX, Xu DL, Xue Y, He YH. Relationship of familial cytochrome P450 4V2 gene mutation with liver cirrhosis: A case report and review of the literature. World J Clin Cases 2022; 10(28): 10346-10357
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10346.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10346
“Liver disease of unknown cause” includes a wide range of pathologies. The specific history of a patient, laboratory results, and liver biopsy results can help to identify common liver diseases, such as autoimmune liver disease, drug-induced liver damage, and non-alcoholic fatty liver disease. Genetic metabolic liver disease (GLD) accounts for about 1% of all cases of unexplained liver disease. GLD is characterized by metabolic abnormalities and incomplete liver function[1], which may occur due to consanguinity, and is characterized by early liver involvement, extensive liver damage, and is often accompanied by damage of other organs. Diagnosis of GLD is most common in children, but symptoms may manifest at any age.
GLD usually results from the combined effects of genetic and environmental factors. The liver typically has a good compensatory function, and some of these patients may be asymptomatic under normal physiological conditions, with no evidence of abnormal liver function or late onset of liver disease. Thus, the possibility of a GLD in an adult who presents with an unknown liver disease or cirrhosis should be considered[2,3]. Most GLDs are autosomal recessive diseases, although some are autosomal dominant, X-linked, or mitochondrial diseases[4]. The absence of a family history of liver disease cannot exclude the possibility of GLD.
Diagnosis of GLDs is difficult in clinical practice due to their rarity, complex and wide spectrum of clinical manifestations, significant individual differences, and insufficient clinical understanding and limitations of testing methods[5,6]. At present, there are more than 600 kinds of GLD, including primary hemochromatosis, hepatolenticular degeneration, hereditary hemochromatosis, glycogen storage disease, α1-antitrypsin deficiency, hereditary hyperbilirubinemia, Dubin-Johnson syndrome, Rotor syndrome, hereditary hyperbilirubinemias, and congenital liver fibrosis[7]. At onset, GLD most commonly manifests as a chronic disease, but acute disease may occur under certain circumstances. Some laboratory and imaging tests can identify genetic abnormalities and can be used to diagnose certain hereditary metabolic diseases[8]. In particular, some GLD can be confirmed by measurements of specific enzymes in the serum, skin fibroblasts, or liver tissue cells[9]. Pathological examination and gene testing are also very important for the diagnosis of GLD.
The patient was admitted for recurrent lower extremity edema for 9 mo, abdominal distension for 7 mo, and aggravation for half a month.
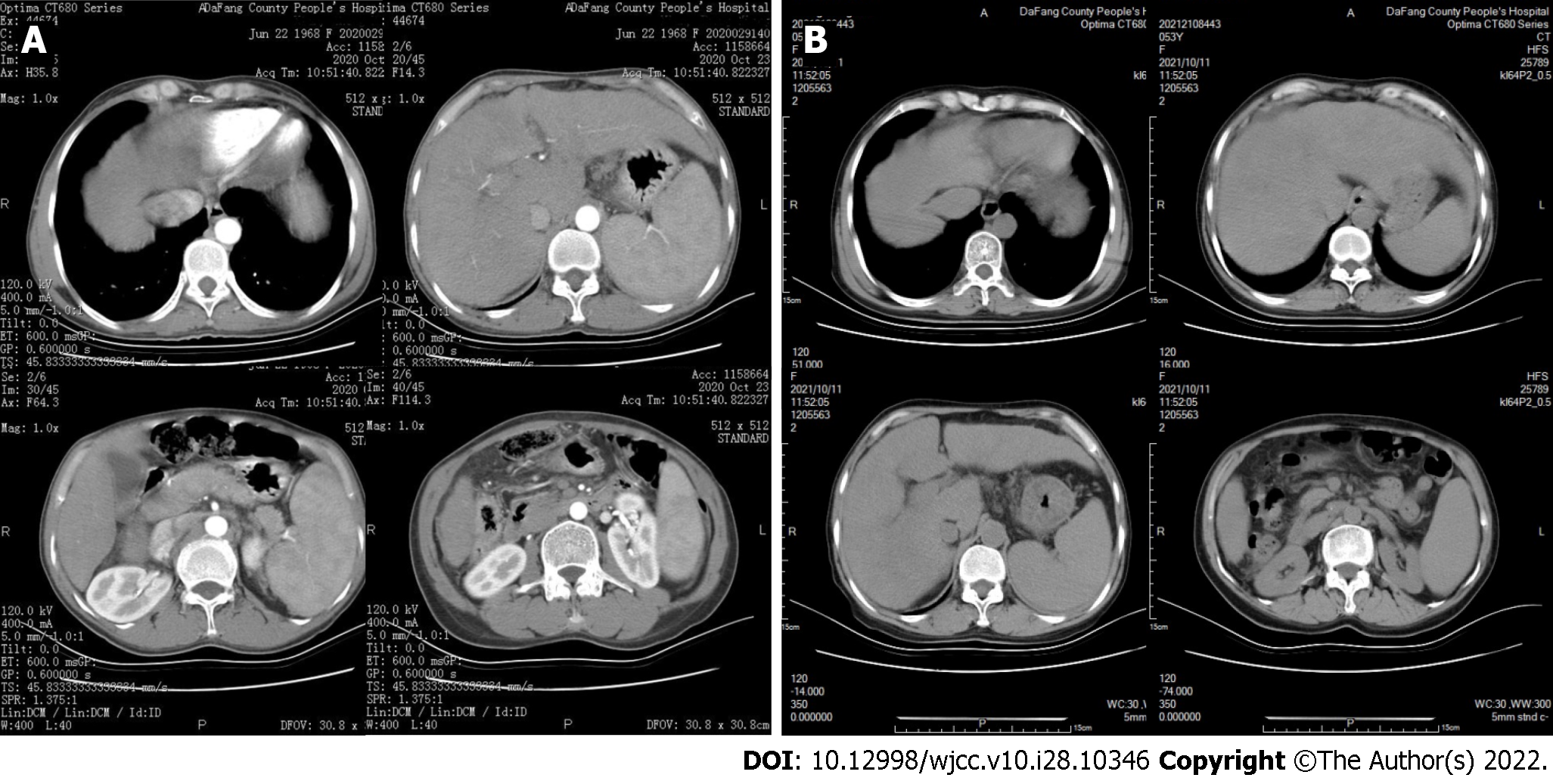
A 53-year-old rural Chinese woman visited Dafang County People’s Hospital. Symptoms began to appear nine months ago, during which edema and abdominal distension of both lower extremities recurred, and these gradually worsened during the previous two months. Our initial liver function examination showed elevated alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL); decreased albumin/globulin (ALB/GLO) ratio (Table 1). Examination of blood cells indicated decreased white blood cells (WBCs), red blood cells (RBCs), hemoglobin (HB), and platelets (PLTs) (Table 2). Examination of coagulation function indicated an increased international normalized ratio (INR), prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT), and decreased fibrinogen (FIB) (Table 3). An upper abdominal computed tomography (CT) showed liver cirrhosis (Figure 1). Examination of thyroid function showed an elevated level of thyroid stimulating hormone (TSH) and a decreased level of free triiodothyronine (FT3) (Table 4). We therefore initially considered a diagnosis of “decompensated stage of cirrhosis and subclinical hypothyroidism”.
| Date | ALT, U/L | AST, U/L | TBIL, μmol/L | DBIL, μmol/L | IBIL μmol/L | GGT, U/L | ALP, U/L | ALB, g/L | GLO, g/L |
| Ref: 0-40 | Ref: 0-34 | Ref: 5.1-19.0 | Ref: 1.7-6.8 | Ref: 1.7-13.2 | Ref: 0-50 | Ref: 40-150 | Ref: 38-51 | Ref: 20-30 | |
| 2020-10-14 | 89.9↑ | 259.6↑ | 94.13↑ | 44.45↑ | 49.68↑ | 40.70 | 178.7↑ | 25.30↓ | 51.7↑ |
| 2020-10-20 | 58.9↑ | 164.7↑ | 90.92↑ | 39.89↑ | 51.03↑ | 35.77 | 158.5↑ | 33.03↓ | 43.0↑ |
| 2021-01-14 | 86.9↑ | 182.5↑ | 109.91↑ | 60.62↑ | 49.28↑ | 37.60 | 69.9 | 21.20↓ | 55.3↑ |
| 2021-01-26 | 61.0↑ | 159.2↑ | 71.90↑ | 44.26↑ | 27.64↑ | 28.60 | 87.5 | 27.70↓ | 30.0 |
| 2021-04-18 | 63.7↑ | 204.4↑ | 102.32↑ | 68.26↑ | 34.06↑ | 31.40 | 117.4 | 24.00↓ | 35.6↑ |
| 2021-04-25 | 46.2↑ | 151.8↑ | 79.40↑ | 53.25↑ | 26.15↑ | 28.80 | 105.3 | 30.60↓ | 32.3↑ |
| 2021-07-14 | 53.3↑ | 164.7↑ | 95.24↑ | 65.73↑ | 29.51↑ | 34.00 | 116.7 | 25.30↓ | 34.7↑ |
| 2021-07-25 | 36.72 | 113.5↑ | 80.00↑ | 57.63↑ | 22.37↑ | - | - | 33.78↓ | 27.7 |
| Date | WBCs, × 109/L | RBCs, × 1012/L | HB, g/L | PLTs, × 109/L |
| Ref: 3.5-9.5 | Ref: 3.8-5.1 | Ref: 115-150 | Ref: 125-350 | |
| 2020-10-14 | 3.25↓ | 3.5↓ | 120 | 62↓ |
| 2021-01-13 | 8.94 | 3.3↓ | 122 | 46↓ |
| 2021-01-26 | 2.56↓ | 2.8↓ | 97↓ | 50↓ |
| 2021-04-18 | 3.21↓ | 2.9↓ | 102↓ | 58↓ |
| 2021-04-22 | 2.49↓ | 2.7↓ | 108↓ | 51↓ |
| 2021-07-14 | 3.13↓ | 3.0↓ | 105↓ | 56↓ |
| Date | INR | PT, s | APTT, s | FIB, g/L |
| Ref: 0.8-1.3 | Ref: 10.0-15.0 | Ref: 21.0-35.0 | Ref: 2.0-4.0 | |
| 2020-10-14 | 1.53↑ | 17.03↑ | 56.73↑ | 1.54↓ |
| 2021-01-13 | 1.25 | 15.60↑ | 31.63 | 2.06 |
| 2021-01-26 | 1.47↑ | 17.90↑ | 38.77↑ | 1.24↓ |
| 2021-04-18 | 1.54↑ | 18.79↑ | 42.95↑ | 1.30↓ |
| 2021-04-22 | 1.58↑ | 19.36↑ | 43.93↑ | 1.18↓ |
| 2021-07-15 | 1.43↑ | 17.02↑ | 39.93↑ | 1.37↓ |
| Date | T3, ng/mL | T4, µg/dL | FT3, pg/mL | FT4, ng/dL | TSH, µIU/mL |
| Ref: 1.3-3.1 | Ref: 5.0-14.5 | Ref: 2.3-4.0 | Ref: 0.6-1.2 | Ref: 0.35-5.10 | |
| 2020-10-14 | 0.66↓ | 9.27 | 2.14↓ | 0.94 | 5.18↑ |
| 2021-01-13 | 0.62↓ | 7.65 | 2.17↓ | 0.96 | 4.74 |
| 2021-07-26 | 0.43↓ | 5.62 | 1.57↓ | 0.91 | 6.56↑ |
She tested negative for hepatitis viruses and negative for autoimmune hepatitis, and the cause of cirrhosis was unknown. She was subsequently hospitalized twice for abdominal distension with yellow staining of the sclera, but these symptoms resolved after administration of a liver protectant and a diuretic to reduce swelling. After discharge, she still reported repeated abdominal distension and discomfort, which gradually became worse from 2020 Oct 1. Since the initial presentation, her liver function indexes have fluctuated. The thyroid function tests indicated a decreasing level of triiodothyronine (T3) and an increasing level of TSH, indicating progression from subclinical hypothyroidism to clinical hypothyroidism.
She reported loss of vision 30 years ago, with no obvious cause, and had no history of long-term heavy drinking, taking a Chinese medicine, exposure to toxic substances, surgery, or trauma.
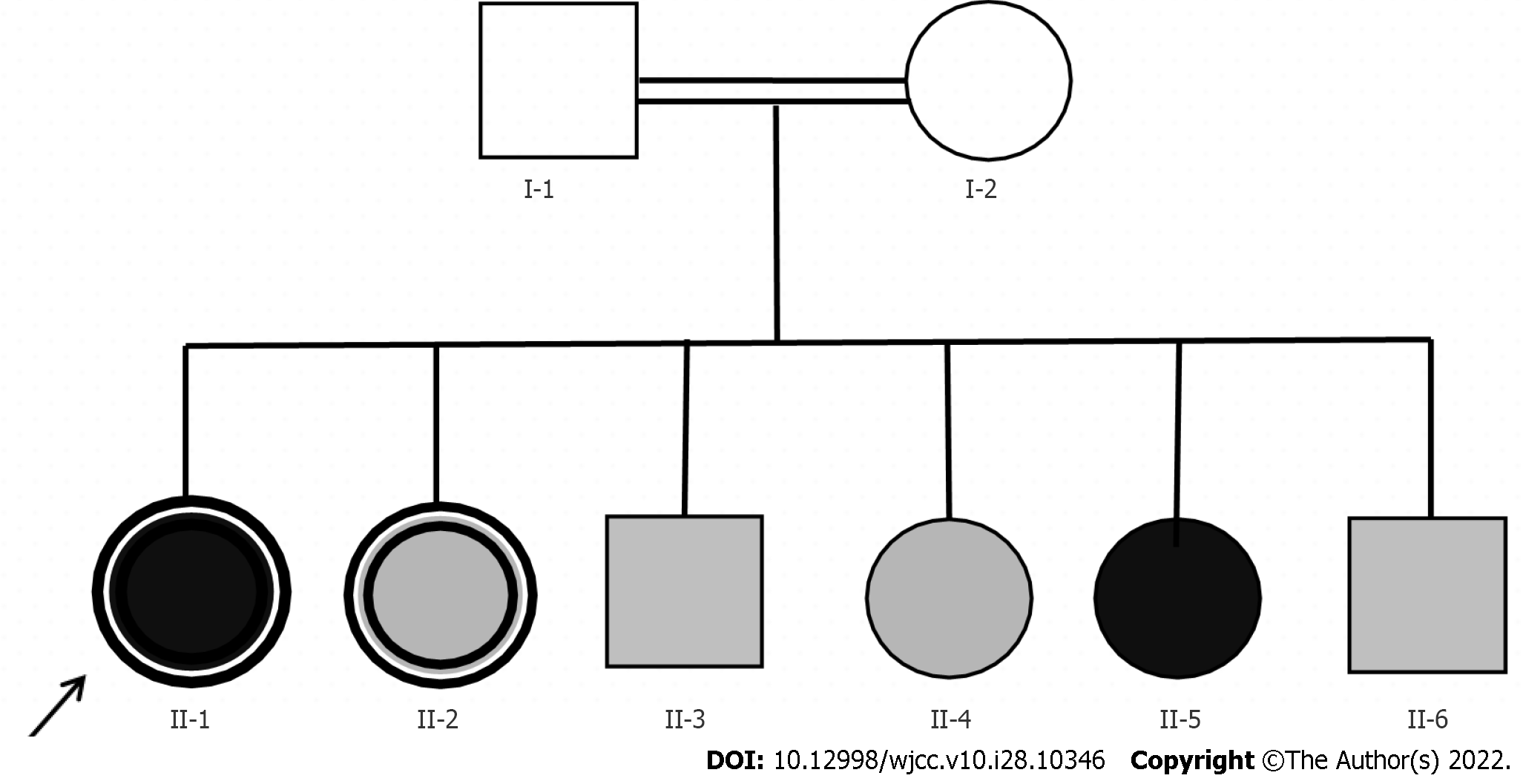
We designated the index patient as proband II-1, and analyzed her pedigree (Figure 2). She had five siblings (3 sisters, 2 brothers), all of whom had night blindness, and one sister (II-4) was completely blind. Her parents had no night blindness or impaired vision, but their marriage was consanguineous (first cousins).
We performed serum biochemical examinations of the father (I-1) and all siblings (Table 5). The mother (I-2) was too old to travel to our institution for testing. The AST level was elevated in all 7 individuals, and fasting blood glucose was decreased in the father and 4 siblings. Two siblings had elevated levels of high density lipoprotein cholesterol (HDL-C), and one had a low level. The T3 Level was low in two siblings, and the TSH level was high in three siblings. Some of the siblings also had elevated levels of TBIL and apolipoprotein A-I (APOA1).
| Patient | ALT, U/L | AST, U/L | TBIL, μmol/L | HDL-C, mmol/L | APOA1, g/L | T3, ng/mL | TSH, µIU/mL |
| Ref:0-40 | Ref: 0-34 | Ref: 5.1-19.0 | Ref: 1.29-1.55 | Ref: 1.0-1.6 | Ref: 1.3-3.1 | Ref: 0.35-5.1 | |
| I-1 | 23 | 41↑ | 10.1 | 1.51 | 1.57 | 1.74 | 2.99 |
| II-1 | 53.3↑ | 164.7↑ | 95.2↑ | 1.42 | 1.50 | 0.43↓ | 6.56↑ |
| II-2 | 19 | 54↑ | 18.2 | 1.88↑ | 1.79↑ | 1.96 | 6.54↑ |
| II-3 | 26 | 59↑ | 13.2 | 1.42 | 1.52 | 1.83 | 1.70 |
| II-4 | 20 | 50↑ | 29.8↑ | 1.17↓ | ND | 1.50 | 5.29↑ |
| II-5 | 10 | 28 | 22.8↑ | 1.94↑ | 1.83↑ | 1.29↓ | 1.55 |
| II-6 | 22 | 41↑ | 16.4 | 1.52 | 1.71↑ | 2.14 | 3.13 |
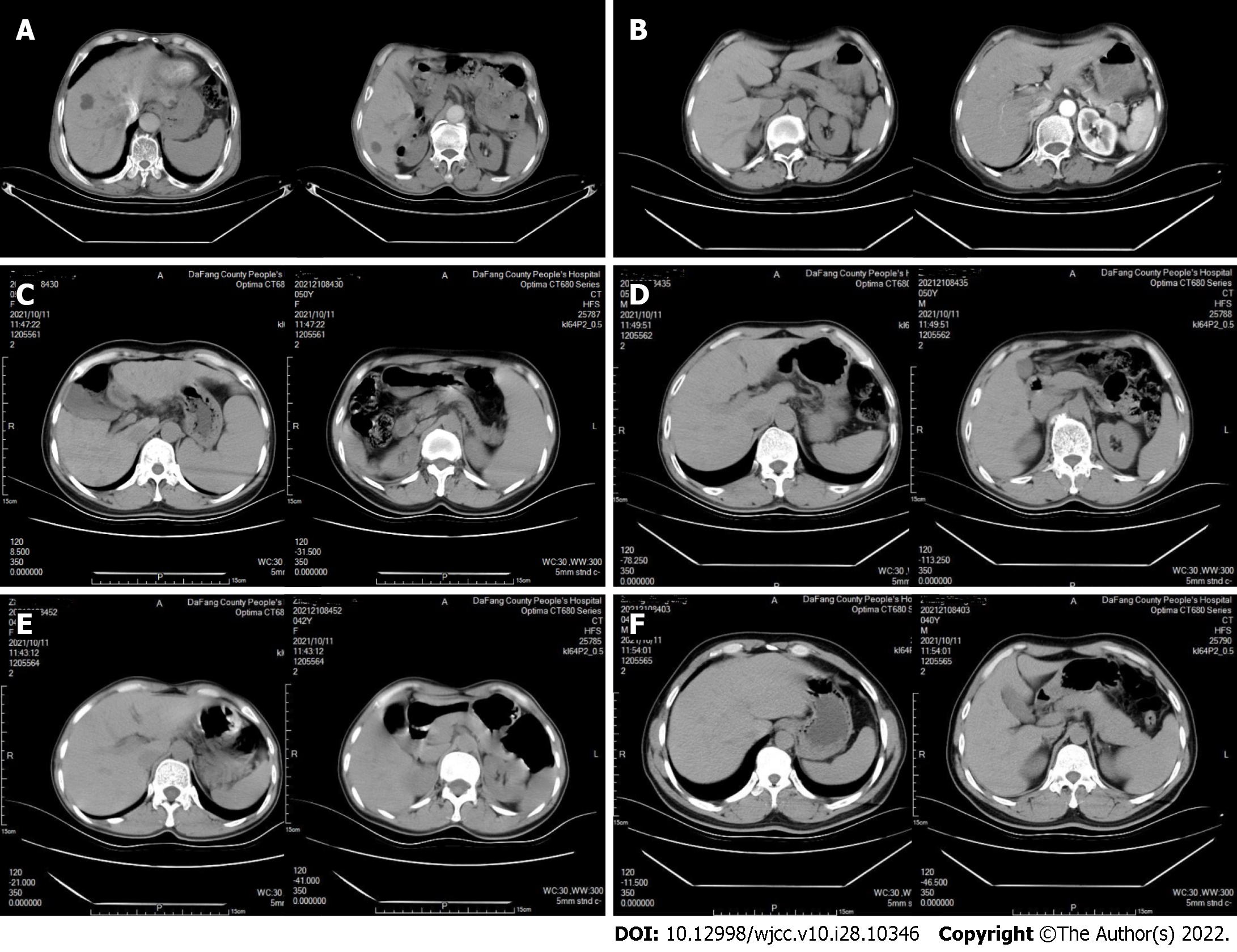
CT examinations showed that neither the father (I-1) nor the mother (I-2) had cirrhosis (Figure 3A and B). Sibling II-2 had cirrhosis (Figure 3C), but siblings II-3, II-5, and II-6 did not (Figure 3D-F). Abdominal color ultrasound indicated that sibling II-4 had a fatty liver.
A physical examination indicated she had a BMI of 22.22. There was also evidence of chronic liver disease, visible spider nevi, and palmar erythema. She had no vision in either eye, and no K-F rings in the corneas. A cardiopulmonary examination showed no obvious abnormalities. Her abdomen was slightly distensible and was positive for shifting dullness. There was moderate pitting edema in both lower limbs.
2020 Oct 14: Blood lipid analysis indicated an HDL of 1.42 mmol/L. Hepatitis A virus, hepatitis B virus, and hepatitis C virus related tests were all negative.
2021 Jan 17: All antibody tests for autoimmune liver diseases were negative.
2021 Jul 16: Examination of ascites indicated the Rivalta test was negative, the WBC count was 50 × 106/L, total protein (TP) was 5.50 g/L, adenosine deaminase (ADA) was 3.4 U/L, glucose (GLU) was 8.71 mmol/L, and lactate dehydrogenase (LDH) was 48.8 U/L.
2020 Oct 15: Color Doppler ultrasonography of the thyroid showed bilateral thyroid cysts.
2020 Oct 23: A plain CT scan of the upper abdomen with enhancement showed cirrhotic ascites with splenomegaly (Figure 1).
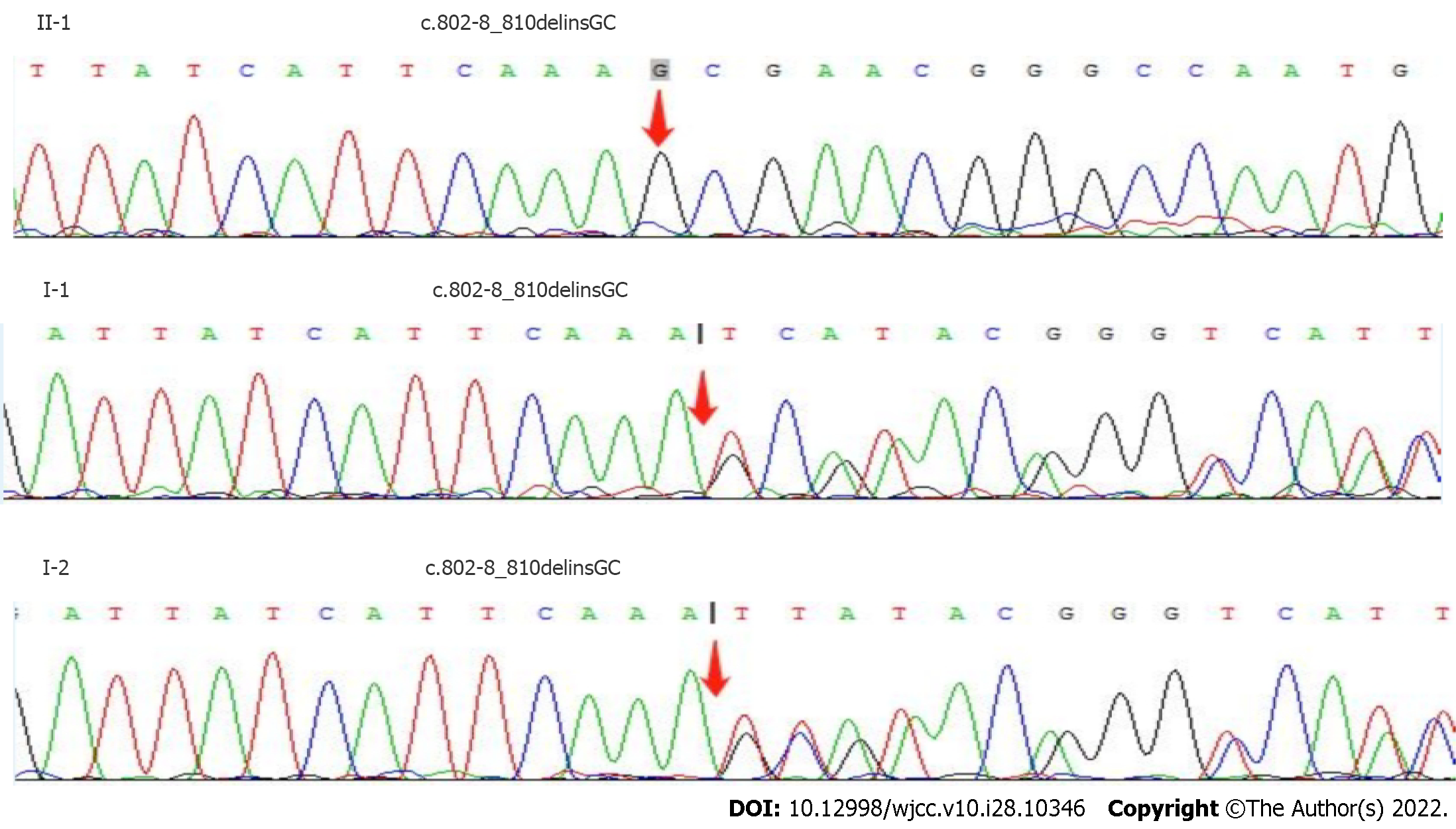
We suggested more extensive examination of a liver biopsy to clarify the cause of cirrhosis, but this was not possible because the patient did not consent. The index patient was one of 6 siblings; II-2 was similar (night blindness complicated with cirrhosis and hypothyroidism), II-5 was completely blind in both eyes, and the other three had obvious night blindness. We considered the possibility of GLD, and suggested molecular genetic analysis. After receiving informed consent, we collected peripheral blood samples for whole-exome sequencing (Figure 4, Table 6). The results indicated the presence of the deletion/insertion mutation C.802-8_810delinsGC (homozygous) in the cytochrome P450 4V2 (CYP4V2) gene and the transcoding mutation C.413dupA (heterozygous) in the dual oxidase activator 2
| Patient | Gene | Chromosome location | Basic variation | Zygosity | Inheritance pattern |
| I-1 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HTZ | AR |
| I-2 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HTZ | AR |
| II-1 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HMZ | AR |
| DUOXA2 | chr15: 45408785 | c.413dupA | HTZ | AR | |
| II-2 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HTZ | AR |
| II-3 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HMZ | AR |
| DUOXA2 | chr15: 45408785 | c.413dupA | HTZ | AR | |
| II-5 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HTZ | AR |
| DUOXA2 | chr15: 45408785 | c.413dupA | HTZ | AR | |
| II-6 | CYP4V2 | chr4: 187122319 | c.802-8_810delinsGC | HMZ | AR |
Combined with the patient’s medical history, the final diagnosis was decompensated active cirrhosis and hypothyroidism.
Drugs were given to protect the liver, reduce jaundice, and promote diuresis to eliminate ascites.
After discharge, the patient continued to experience abdominal distension and jaundice repeatedly, and the treatments had limited efficacy.
We used whole exome sequencing to identify a CYP4V2 gene mutation in the index patient and in her parents and siblings. Both parents were heterozygous for the mutation, and had no night blindness or vision loss. Among the five siblings of the index patient (II-1), II-2 and II-5 were heterozygous, II-3 and II-6 were homozygous, and all of them had night blindness or more severe visual impairment. Patients II-1, II-3, and II-5 also had a DUOXA2 heterozygous mutation, but II-2 and II-6 only had the CYP4V2 mutations. Notably, upper abdominal CT of II-1 and II-2 indicated liver cirrhosis. CYP4V2 is in the cytochrome P450 family, and functions in the hydroxylation of long-chain fatty acids and in anti-inflammatory reactions. This suggests that a defective CYP4V2 gene may cause chronic liver injury by altering fatty acid metabolism and increasing inflammation in the liver, which subsequently progresses to liver fibrosis and cirrhosis.
The CYP4V2 gene has 11 exons, is located at 4q35.1-q35.2, encodes a protein with 525 amino acids, and is expressed in almost all tissues and organs of the human body. It has high expression in the retinal pigment epithelium (RPE) and in cultured RPE cells (ARPE-19), but is only rarely detected in the corneal epithelium. The outer section of the retina contains a disc membrane rich in rhodopsin, and the lipids of these discs are mostly palmitic acid, stearic acid, oleic acid, and docosahexaenoic acid (DHA); eicosapentaenoic acid (EPA) is a minor retinal lipid. CYP4V2 dominates the metabolism of EPA and DHA in the retina[10].
A pathogenic variation of CYP4V2 can lead to Bietti crystalline corneal and retinal dystrophy (BCD), a disease with autosomal recessive inheritance. BCD is characterized by choroidal retinal degeneration with yellow-white crystals or complex lipid deposits in the retina and cornea. BCD is rare in Caucasians, but has a higher prevalence in Asians. It is a progressive disease, and most patients experience some vision loss at the age of 10 to 40 years-old, and legal blindness often occurs when the patient is 40 or 50 years-old[11]. The CYP4V2 gene functions in the decomposition and elimination of fatty acids from the retina, so a mutation in this gene presumably affects lipid metabolism in the RPE. At the age of 23, the index patient had gradually decreased vision, and she developed legal blindness when she was 30 years-old. The other five siblings all had different degrees of visual impairment (mainly night blindness), although one of them was completely blind. These findings are consistent with the clinical characteristics of BCD.
CYP4V2 has high expression in the RPE and in tissues and cells that function in the metabolism of fatty acids and steroids, such as hepatocytes, the thyroid, and islet cells[12]. However, the CYP4V2 mutation responsible for BCD has little effect on these other tissues and organs[13]. There is evidence that BCD patients have increased serum levels of triglycerides (TGs), total cholesterol (TC), and low density lipoprotein cholesterol (LDL-C)[14]. Previous research reported that CYP4V2 was a selective hydroxylase of saturated medium-chain fatty acids, and had high catalytic efficiency for creatine and lauric acid[15]. In addition, CYP4V2 expression is also associated with production of arachidonic acid ω-hydroxylase, suggesting it may contribute to arachidonic acid metabolism. There is also evidence that overexpression of functional CYP4V2 alters lipid homeostasis in cultured liver cancer cells (Hep G2). In particular, the levels of EPA and DHA were significantly increased in Hep G2 cells that expressed the mutant enzyme CYP4V2[10]. These many studies indicate that CYP4V2 functions in fat metabolism, is strongly expressed in the liver, and that mutations can affect liver fatty acid metabolism. In agreement, we found abnormalities in the blood lipids of the index patient and her family, and the level of HDL-C was high or borderline-high in patients II-1, II-2, II-5, and II-6, although the molecular mechanism of this effect remains unclear. However, elevated HDL-C promotes reverse transport of peripheral cholesterol into the liver, and thus increases hepatic cholesterol accumulation. Long-term disorders of fatty acid metabolism that lead to hepatic lipid accumulation can cause or exacerbate liver disease.
There is evidence that leukotriene B4 (LTB4) reduces the uptake of free fatty acids by adipocytes, and increases leukocyte tissue infiltration and inflammatory responses via the production of inflammatory cytokines and chemokines, thus contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD)[16]. CYP ω-hydroxylase converts LTB4 into its 20-hydroxyl metabolite (20-OH LTB4), which reduces chemotactic activity against leukocytes. Notably, research indicated the activity of CYP ω-hydroxylase was significantly decreased and the level of LTB4 was significantly increased in the colonic mucosa of patients with inflammatory bowel disease[17,18]. Other studies showed that reduced activity of CYP ω-hydroxylase was associated with sclerosis and atopic dermatitis[19-21] and with neuroinflammation[22]. All of these results are consistent with our interpretation that the CYP4V2 gene mutation identified here promotes liver inflammation and injury.
The DUOX2 gene is located at 15q15.3, contains 34 exons, encodes a protein with 1548 amino acids, and is mainly expressed in thyroid tissues. This gene functions in the iodination of thyroid hormone, and catalyzes the production of H2O2. A pathogenic variant of this gene leads to thyroid dyshormonogenesis 5 (TDH5), an autosomal recessive disorder. The clinical manifestations of this disease include partial or complete loss of thyroid function, and elevated serum levels of TSH. There is also ultrasonography evidence of goiter in these patients[23]. However, mutation of the DUOX2 gene prevents the iodination of thyroid hormone, reduces the synthesis of thyroxine, and thus leads to hypothyroidism[24]. We found elevations of TSH in patients II-1, II-2, and II-4, and decreased levels of T3 in patients II-1 and II-5, consistent with mutations of the DUOXA2 gene.
Thyroid hormone contributes to the regulation of liver triglycerides. Thyroid hormone binds to a specific thyroid hormone receptor (THR), which is a transcription factor that regulates the expression of many genes that function in adipogenesis, such as adiposynthase, ACC1, and THRSP[25]. In addition, thyroxine indirectly regulates the transcription of other transcription factors, such as steroid regulatory element binding protein 1C (SREBP1C), liver X receptor (LXR), and carbohydrate reaction element binding protein (ChREBP), and thus regulates hepatic adipogenesis[26]. Although thyroid hormones stimulate fat production, patients with hyperthyroidism experience a net decrease in total liver triglycerides because the rate of fatty acid metabolism exceeds that of fat synthesis.
The mobilization, degradation, and β-oxidation of fatty acids by thyroid hormones contribute to an increased overall rate of fatty acid metabolism. The release of FFA in hepatocytes is caused by cellular lysozyme activity[27]. Thyroxine promotes the oxidative catabolism of hepatic fat by increasing the expression and activity of hepatic lipase and other mitochondrial enzymes required for fatty acid beta oxidation, including medium-chain acetyl-CoA dehydrogenase. In hypothyroidism, fat synthesis is reduced, but fatty acid mobilization, degradation, and oxidation are reduced even more, and this leads to the accumulation of triglycerides in the liver.
Thyroid hormone functions in the regulation of cholesterol metabolism in the liver. On the one hand, thyroid hormone can strongly induce the expression of APOA1, scavanger receptor class B member 1 (SR-B1), and steroid regulatory element binding protein 2 (SR-EBP2), thus increasing the level of low density lipoprotein receptor (LDLR). This increases the transport of cholesterol from surrounding tissues into the liver[28]. On the other hand, thyroid hormone can also up-regulate hydroxymethyl glutarate monoacyl coenzyme A reductase (HMGR), thereby promoting cholesterol synthesis. The key enzyme in the transformation of cholesterol to bile acid is cholesterol 7α hydroxylase (CYP7A); thyroid hormone can increase the transcription of CYP7A, thus promoting the transformation of cholesterol to bile acid. Patients with hypothyroidism have reduced cholesterol synthesis, excretion, and transformation, but thyroid hormone has a greater effect on the LDL-C receptor than on HMGR. Thus, the overall effect of hypothyroidism is an increase in total cholesterol. In addition, patients with hypothyroidism have reduced oxidative stress and basal metabolic rate, leading to fat accumulation[29].
Thyroid hormone deficiency leads to increased TSH secretion, and TSH regulation of hepatic triglyceride metabolism is independent of thyroid hormone. TSH binds to the TSHR on liver cells, activates the cAMP/PKA pathway, thus increasing the expression and activity of SREBP-1C and triglyceride synthesis and promoting hepatocyte steatosis[30]. TSH also inhibits the synthesis of liver bile acids via the SREBP2/HNF4/CYP7A1 signaling pathway, resulting in reduced cholesterol conversion. As an upstream molecule, TSH increases hepatic gluconeogenesis and HMGC receptor (HMGCR) expression by regulating the cAMP/PKA/CREB signaling pathway, and it also promotes cholesterol and triglyceride synthesis[31-33]. Hypothyroidism can lead to chronic liver disorders in glycolipid metabolism, and the subsequent accumulation of triglycerides and cholesterol in the liver can lead liver disease. Promotes synthesis of triglycerides and cholesterol.
Our results indicated that a CYP4V2 gene mutation disrupted lipid metabolism in the liver, presumably due to an increase in inflammation. A DUOXA2 gene mutation causes insufficient thyroxine production and secondary TSH secretion, and these also affect glucose and lipid metabolism. Thus, mutations of both genes lead to even greater dysregulation of sugar and fat metabolism and contribute to chronic liver inflammation and injury. Two siblings in the family (II-1 and II-2) had cirrhosis. However, patient II-1 had mutations in both genes, but II-2 only had the CYP4V2 mutation. This suggests that the CYP4V2 mutation may have a stronger effect in causing chronic liver injury.
The onset of GLD usually occurs at an early age, but two patients in the family were more than 50 years-old. The mutations of the CYP4V2 or DUOXA2 genes described here may not have directly caused liver damage, but instead may have directly or indirectly altered the metabolism of glucose and lipids in the liver, thereby increasing liver inflammation. Notably, the index patient lived in a rural area with poor living conditions and had a low-fat diet, conditions that may be conducive to slowing the progression of liver disease. This may be one of the reasons for her late onset of liver disease. The mutations of the CYP4V2 or DUOXA2 genes indicated they have important functions in liver fat metabolism, and suggest there should also be hepatocyte steatosis. However, our color ultrasound and upper abdominal CT examinations did not indicate a fatty liver. We wanted to conduct a liver biopsy for confirmation, but the patient refused. The lack of a complete pathological diagnosis is thus a deficiency of our study. The apparent lack of a fatty liver in the index patient might be because the patient was close to end-stage cirrhosis, had a poor diet after disease onset, delayed seeking medical care, and did not show metabolic abnormalities. Although we examined the presence of GLD and CYP4V2 gene mutations in 8 individuals from a consanguineous family, it is generally considered necessary to analyze 3 separate families to establish a causal relationship. Unfortunately, this is often impossible for very rare conditions.
GLD is rare and can have complex clinical manifestations. This case report provides an improved understanding of one type of GLD. The onset of symptoms in patients with GLD can occur at any age, so the possibility of GLD should also be considered in adult patients who present with unexplained liver disease or cirrhosis. The diagnosis of GLD requires careful examination of clinical manifestations, a comprehensive analysis of family history, assessment of symptoms and signs in family members, and detailed pathological examinations and genetic testing. Clinicians who encounter patients with “liver disease of unknown cause” should therefore consider GLD, regardless of patient age.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kreisel W, Germany; Kumar R, India; Maslennikov R, Russia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Kim JS, Kim KM, Oh SH, Kim HJ, Cho JM, Yoo HW, Namgoong JM, Kim DY, Kim KH, Hwang S, Lee SG. Liver transplantation for metabolic liver disease: experience at a living donor dominant liver transplantation center. Pediatr Gastroenterol Hepatol Nutr. 2015;18:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Song P, Gao J, Inagaki Y, Kokudo N, Tang W. Intractable and rare diseases research in Asia. Biosci Trends. 2012;6:48-51. [PubMed] |
| 3. | Wong LJ, Scaglia F, Graham BH, Craigen WJ. Current molecular diagnostic algorithm for mitochondrial disorders. Mol Genet Metab. 2010;100:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Cox C, Bosley M, Southerland LB, Ahmadi S, Perkins J, Roman S, Sosa JA, Carneiro-Pla D. Lobectomy for treatment of differentiated thyroid cancer: can patients avoid postoperative thyroid hormone supplementation and be compliant with the American Thyroid Association guidelines? Surgery. 2018;163:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Alam S, Sood V. Metabolic Liver Disease: When to Suspect and How to Diagnose? Indian J Pediatr. 2016;83:1321-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 6. | Yamaguchi K, Wakimizu R, Kubota M. Difficulties in Daily Life and Associated Factors, and QoL of Children with Inherited Metabolic Disease and Their Parents in Japan: A Literature Review. JIMD Rep. 2017;33:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 812] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 8. | Xiong QF, Yang YF. [Histopathological features and diagnostic considerations for genetic metabolic liver disease]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Koenig MK. Presentation and diagnosis of mitochondrial disorders in children. Pediatr Neurol. 2008;38:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Nakano M, Kelly EJ, Wiek C, Hanenberg H, Rettie AE. CYP4V2 in Bietti’s crystalline dystrophy: ocular localization, metabolism of ω-3-polyunsaturated fatty acids, and functional deficit of the p.H331P variant. Mol Pharmacol. 2012;82:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Wang W, Chen W, Bai X, Chen L. Multimodal imaging features and genetic findings in Bietti crystalline dystrophy. BMC Ophthalmol. 2020;20:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Jarrar YB, Lee SJ. Molecular Functionality of Cytochrome P450 4 (CYP4) Genetic Polymorphisms and Their Clinical Implications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | García-García GP, Martínez-Rubio M, Moya-Moya MA, Pérez-Santonja JJ, Escribano J. Current perspectives in Bietti crystalline dystrophy. Clin Ophthalmol. 2019;13:1379-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Nakano M, Lockhart CM, Kelly EJ, Rettie AE. Ocular cytochrome P450s and transporters: roles in disease and endobiotic and xenobiotic disposition. Drug Metab Rev. 2014;46:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid omega-hydroxylase. Drug Metab Dispos. 2009;37:2119-2122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Horrillo R, González-Périz A, Martínez-Clemente M, López-Parra M, Ferré N, Titos E, Morán-Salvador E, Deulofeu R, Arroyo V, Clària J. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol. 2010;184:3978-3987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Costea I, Mack DR, Israel D, Morgan K, Krupoves A, Seidman E, Deslandres C, Lambrette P, Grimard G, Levy E, Amre DK. Genes involved in the metabolism of poly-unsaturated fatty-acids (PUFA) and risk for Crohn’s disease in children & young adults. PLoS One. 2010;5:e15672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Curley CR, Monsuur AJ, Wapenaar MC, Rioux JD, Wijmenga C. A functional candidate screen for coeliac disease genes. Eur J Hum Genet. 2006;14:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Du L, Yin H, Morrow JD, Strobel HW, Keeney DS. 20-Hydroxylation is the CYP-dependent and retinoid-inducible leukotriene B4 inactivation pathway in human and mouse skin cells. Arch Biochem Biophys. 2009;484:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Kalsotra A, Du L, Wang Y, Ladd PA, Kikuta Y, Duvic M, Boyd AS, Keeney DS, Strobel HW. Inflammation resolved by retinoid X receptor-mediated inactivation of leukotriene signaling pathways. FASEB J. 2008;22:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Zhang X, Hardwick JP. Regulation of CYP4F2 leukotriene B4 omega-hydroxylase by retinoic acids in HepG2 cells. Biochem Biophys Res Commun. 2000;279:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Sehgal N, Agarwal V, Valli RK, Joshi SD, Antonovic L, Strobel HW, Ravindranath V. Cytochrome P4504f, a potential therapeutic target limiting neuroinflammation. Biochem Pharmacol. 2011;82:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, Onigata K, Fugazzola L, Refetoff S, Persani L, Weber G. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Vaisman M, Rosenthal D, Carvalho DP. [Enzymes involved in thyroid iodide organification]. Arq Bras Endocrinol Metabol. 2004;48:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Radenne A, Akpa M, Martel C, Sawadogo S, Mauvoisin D, Mounier C. Hepatic regulation of fatty acid synthase by insulin and T3: evidence for T3 genomic and nongenomic actions. Am J Physiol Endocrinol Metab. 2008;295:E884-E894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 504] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 27. | Quiroga AD, Lehner R. Liver triacylglycerol lipases. Biochim Biophys Acta. 2012;1821:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Lopez D, Abisambra Socarrás JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771:1216-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Messarah M, Boumendjel A, Chouabia A, Klibet F, Abdennour C, Boulakoud MS, Feki AE. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp Toxicol Pathol. 2010;62:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Yan F, Wang Q, Lu M, Chen W, Song Y, Jing F, Guan Y, Wang L, Lin Y, Bo T, Zhang J, Wang T, Xin W, Yu C, Guan Q, Zhou X, Gao L, Xu C, Zhao J. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol. 2014;61:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Li Y, Wang L, Zhou L, Song Y, Ma S, Yu C, Zhao J, Xu C, Gao L. Thyroid stimulating hormone increases hepatic gluconeogenesis via CRTC2. Mol Cell Endocrinol. 2017;446:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Zhang X, Song Y, Feng M, Zhou X, Lu Y, Gao L, Yu C, Jiang X, Zhao J. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J Lipid Res. 2015;56:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Duntas LH, Brenta G. A Renewed Focus on the Association Between Thyroid Hormones and Lipid Metabolism. Front Endocrinol (Lausanne). 2018;9:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |