Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10339
Peer-review started: June 20, 2022
First decision: July 14, 2022
Revised: July 18, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 98 Days and 22.5 Hours
Chronic myeloid leukemia (CML) is a malignant hematologic malignancy that can progress to blast phase with a myeloid or lymphoid phenotype. Some patients with CML can also progress to blast crisis phase; however, the transformation of CML into Philadelphia-positive lymphoma is extremely rare.
We present a patient with CML who experienced a sudden transformation to anaplastic large-cell lymphoma (ALCL) after 7 mo of treatment with imatinib, during which she had achieved partial cytogenetic response as well as early molecular response. The patient noticed a mass in her left shoulder, the biopsy data of which were consistent with ALCL; moreover, her lymphoma cells exhi
Unexplained lymphadenopathy or an extramedullary mass in a patient with CML may warrant a biopsy and testing for BCR-ABL fusion.
Core Tip: We describe a patient with chronic myeloid leukemia (CML) whose disease underwent a sudden transformation to anaplastic large-cell lymphoma (ALCL) following partial cytogenetic remission and early molecular response with 7 months of imatinib treatment. The patient developed a mass and felt pain in her left shoulders; a biopsy of the mass revealed ALCL, and BCR/ABL fusion was detected via fluorescence in situ hybridization. The patient was then treated with nilotinib and achieved complete cytogenetic remission and undetectable molecular residual disease within 6 mo. To the best of our knowledge, our patient is the first reported to have Philadelphia-positive ALCL transformed from CML.
- Citation: Wu Q, Kang Y, Xu J, Ye WC, Li ZJ, He WF, Song Y, Wang QM, Tang AP, Zhou T. Sudden extramedullary and extranodal Philadelphia-positive anaplastic large-cell lymphoma transformation during imatinib treatment for CML: A case report. World J Clin Cases 2022; 10(28): 10339-10345
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10339.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10339
Chronic myeloid leukemia (CML) is a malignant disease of clonal hematopoietic stem cells that is characterized by the presence of the t(9;22)(q34;q11.2) translocation indicating BCR-ABL fusion. The natural course of CML is divided into 3 distinct phases: chronic phase, accelerated phase, and blast crisis. In the era of tyrosine kinase inhibitor (TKI) agents, the 10-year survival rate for patients with CML is 83.3%[1], and only 6.9% reportedly progress to accelerated phase or blast crisis[2]. According to the 2020 National Comprehensive Cancer Network guideline for CML, the criteria for blast crisis are the percentage of blast cells in the blood and/or marrow exceeding 30% or evidence of extramedullary infiltration of leukemic cells. During blast phase, CML cells commonly transform to acute leukemia; nearly 70% of patients progress to acute myeloblastic leukemia and 25% to B lymphoblastic leukemia. However, T cell blast transformation is exceedingly rare.
Specific types of blast crisis transformation have been described in patients with CML. One is sudden blast transformation, which is defined as unexpected transformation occurring in patients with complete hematologic remission[3,4]; the other is extramedullary blast crisis, in which leukemic blasts infiltrate areas other than bone marrow[5]. In some rare cases, patients with CML may further progress to a specific type of lymphoma, such as mantle cell leukemia, when extramedullary blast crisis occurs[6]. Here, we report a rare episode where a patient with CML experienced sudden transformation to extramedullary and extranodal Philadelphia-positive (Ph-positive) anaplastic large-cell lymphoma (ALCL) after initially achieving complete hematologic response, partial cytogenetic response, and early molecular response with imatinib therapy.
A Chinese woman with CML who had been undergoing imatinib treatment for the past 7 mo presented at our hospital with systemic muscular pain and a mass growing in her right shoulder.
In August 2015, the patient (then aged 50 years) was found to have elevated white blood cell (WBC) counts during a routine physical examination. This led to a comprehensive evaluation wherein physical examination revealed no enlarged lymph nodes or hepatosplenomegaly. Her complete blood count revealed WBC 23 × 109/L, hemoglobin 127 g/L, and platelets 362 × 109/L. A peripheral blood smear revealed 1% blasts, 10% myelocytes, 16% band neutrophils, 59% segmented neutrophils, 7% basophils, 6% lymphocytes and 1% monocytes. A bone marrow smear showed hypercellularity with severe granulopoiesis (95% granulocytes and 1% blast cells) with a neutrophil alkaline phosphatase score of 6. The karyotype of the bone marrow cells was 46, XX, t(9; 22)(q34; q11) in all the 20 metaphase cells. The BCR-ABL/ABL (p210) proportion was 188.82%. The patient was diagnosed with low-risk CML and treated with imatinib (400 mg daily). Three months later, the patient achieved complete hematologic response, partial cytogenetic response, and early molecular response. In April 2016 (7 mo after initiating imatinib therapy), the patient was hospitalized again due to systemic muscular pain and a mass growing in her right shoulder, whereupon imatinib was discontinued.
The patient had no previous medical history.
The patients personal and family histories were unremarkable.
Physical examination revealed that the patient had a slightly drooping upper eyelid and a smaller pupil in the right eye in addition to a swelling mass in her right shoulder. She had no enlarged lymph nodes or hepatosplenomegaly.
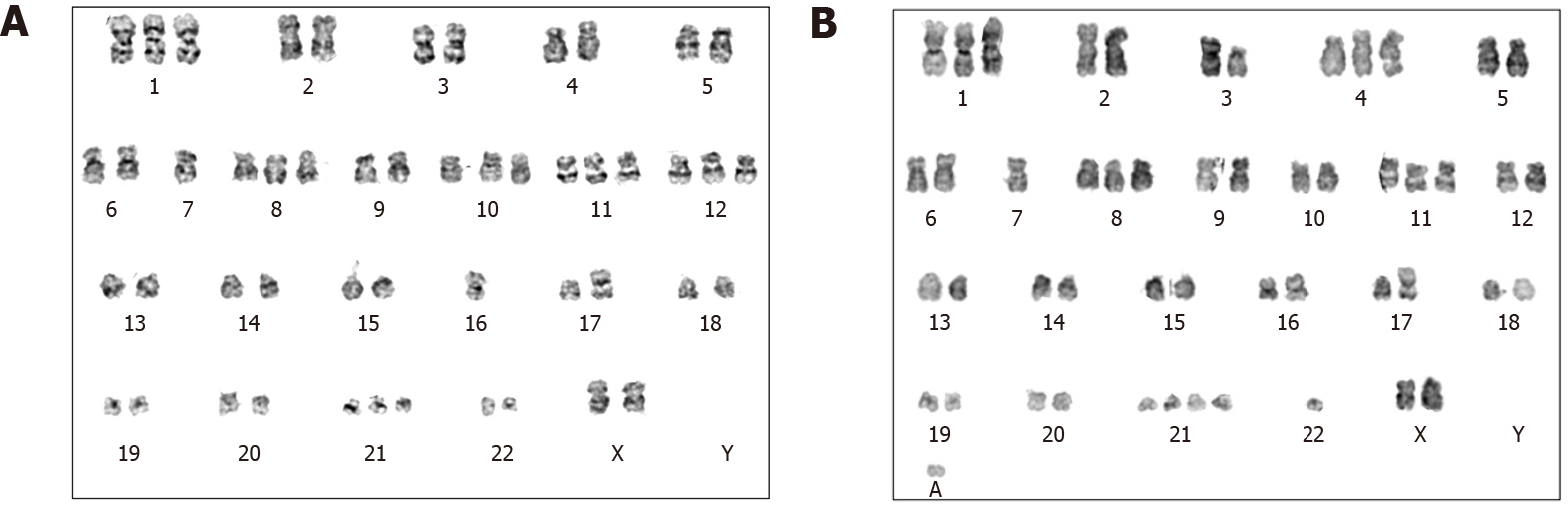
Her complete blood count showed a WBC count 7.89 × 109/L, hemoglobin 100 g/L, and platelet count 207 × 109/L. No blast cells were found in the peripheral blood smear. Bone marrow examination showed hypercellularity with 0.5% blasts. Chromosome karyotype analysis showed 49-51, XX, +1, -7, +8, t(9; 22)(q34; q11), +10, +11, add(16)(p13), ? add(17)(p11), +18, +21, -22, +mar, 1min, inc [cp10]/46, XX[10] (Figure 1). Her BCR-ABL/ABL (international scale) proportion was 1.6%, and no mutations were detected in the kinase domain of the ABL gene.
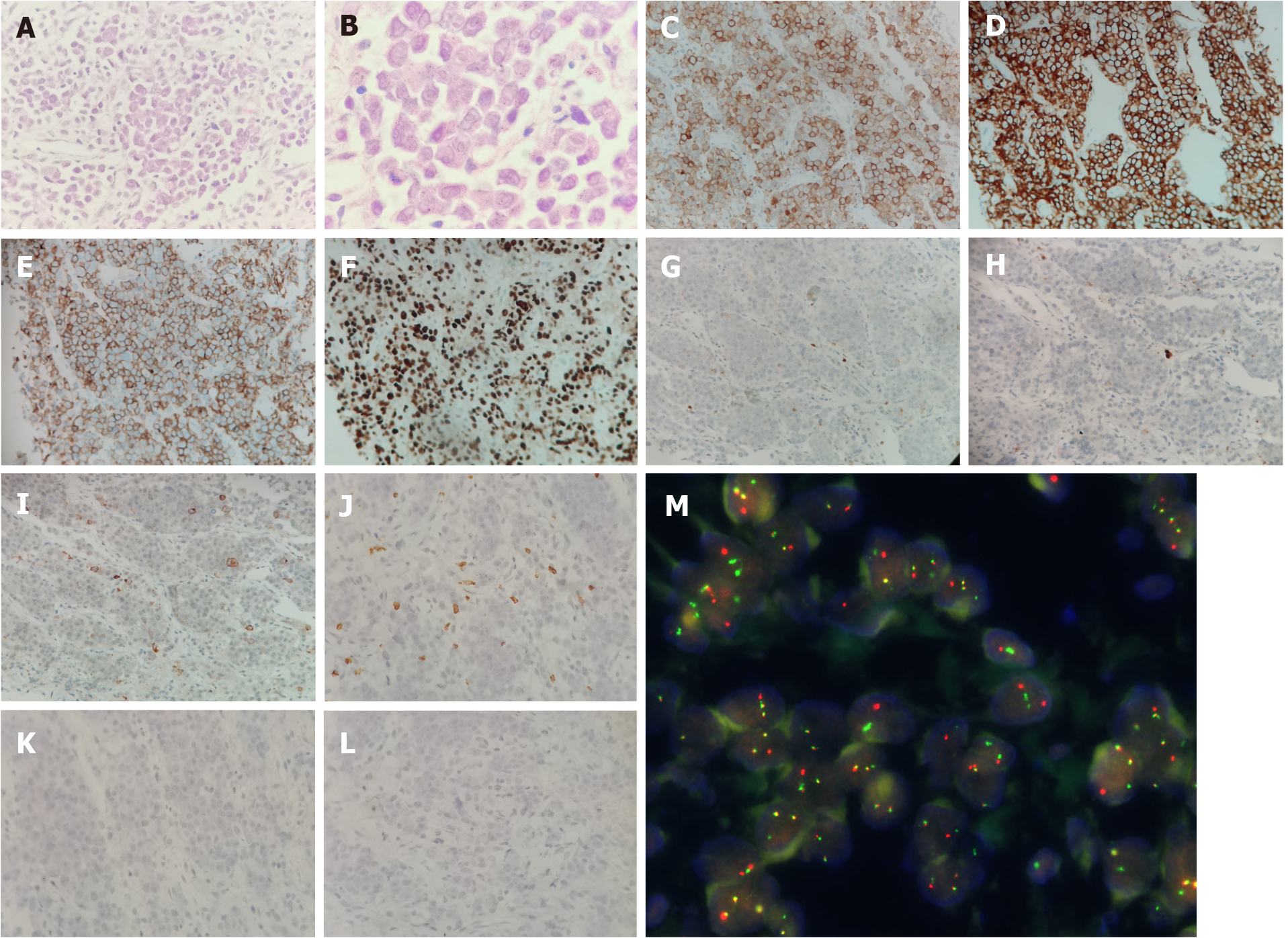
A computed tomography-guided biopsy of the mass revealed dense proliferation of relatively uniform medium-sized cells. The malignant cells had irregular nuclear contours with vesicular nuclei and prominent nucleoli. On immunohistochemical analysis, the tumor cells were positive for CD30, CD43, CD7, CD4, CD38, and leukocyte common antigen and negative for anaplastic lymphoma kinase, cytokeratin, carcinoembryonic antigen, thyroid transcription factor-1, napsin A, CDX2, CD2, CD3, CD5, CD20, CD56, TIA, GRB, CD117, CD138, myeloperoxidase, CD235a, and CD61; the Ki-67 index was 95% (Figure 2). The pathological features were consistent with a diagnosis of ALCL. Fluorescence in situ hybridization (FISH) with dual fusion BCR/ABL probes showed diffuse BCR/ABL rearrangement signals in the paraffin-embedded slide (Figure 2).
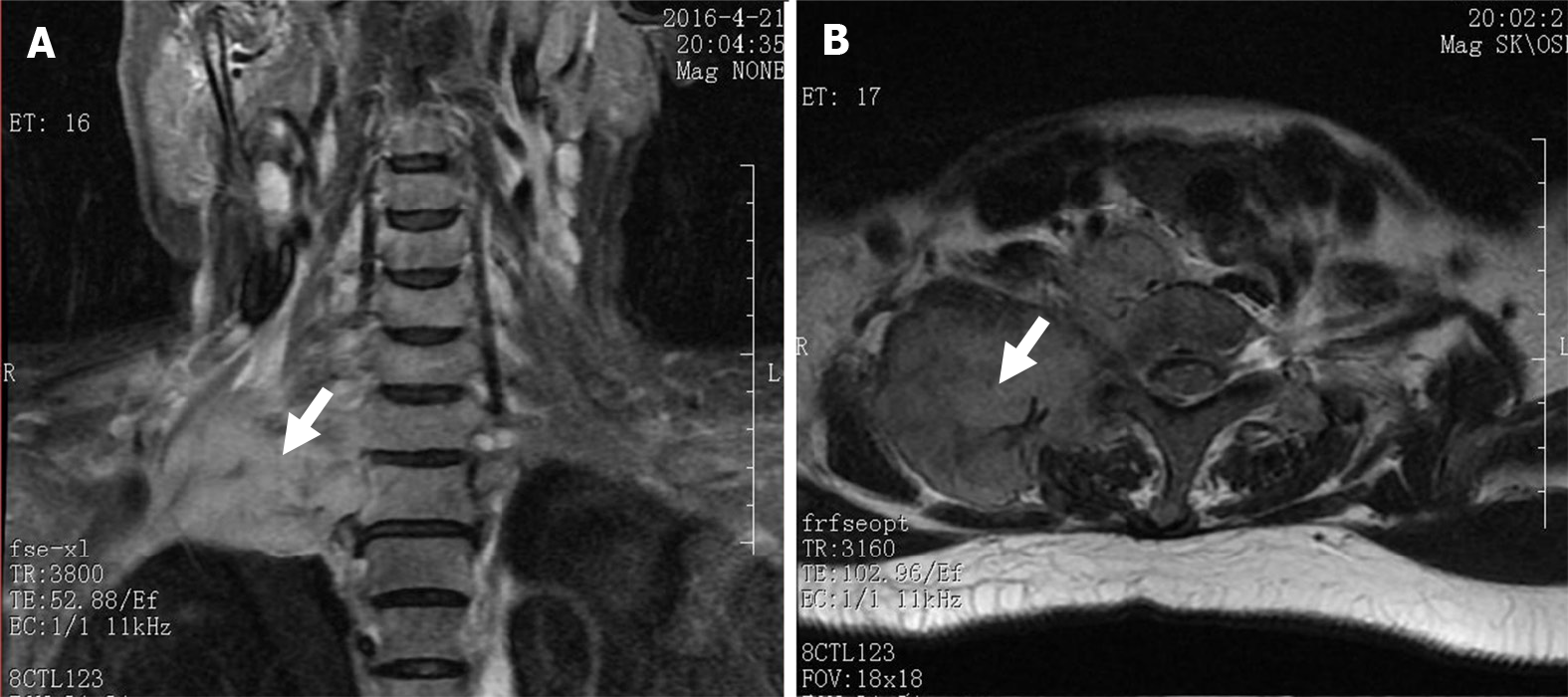
Magnetic resonance imaging showed a large soft tissue mass in the right cervical root and supraclavicular fossa with a maximum diameter of 8.5 cm × 6.8 cm (Figure 3). No enlarged lymph nodes were found in the mediastinum or retroperitoneum.
The patient was diagnosed with sudden extramedullary Ph-positive ALCL transformation and Horner’s syndrome.
The patient refused chemotherapy and was treated with nilotinib 400 mg twice a day. The pain gradually alleviated with treatment, the local neoplasm gradually shrunk, and the symptoms of Horner’s syndrome disappeared. By November 2016, the patient had achieved complete cytogenetic remission, and the BCR/ABL fusion gene was undetectable.
On the most recent follow-up visit in April 2022, the patient was in good condition with undetectable molecular residual disease.
Although the treatment of CML has improved tremendously in the era of TKIs, there remains a proportion of patients who progress to accelerated or blast phase. In clinical trials of imatinib and nilotinib, approximately 7% and 0.6% of patients entered the accelerated or blast phase, respectively[7,8]. In most cases, sudden blast transformation and extramedullary blast crisis usually occur in patients who are in the chronic or accelerated phase of CML. In one study of 213 patients with CML who were on first-line imatinib, the cumulative incidence of sudden blast phase was 5.9%[9]. The incidence rate of extramedullary blast crisis is unknown. Because the rate of secondary lymphoma in patients with CML treated with TKIs is higher than that in the general population[10], it is necessary to determine whether transformation is secondary to CML or arising from it. Reverse transcription PCR does not accurately identify the origin of lymphoma because BCR-ABL transcripts could be expressed by CML cells mixed in lymphoma tissues. Instead, FISH is used to confirm whether the lymphoma cells are transformed from CML cells given that it pinpoints the rearrangement of the BCR and ABL genes [11].
In our patient, the transformed lymphoma cells did not express myeloperoxidase, CD235a, CD61, or CD20, which suggests that the CML cells did not convert to a myeloid, erythroid, megakaryoid, or B-lymphocytic lineage when transformation occurred. The expression of CD4, CD7, and CD43 in the lymphoma cells indicated that this secondary ALCL was of a T-cell phenotype; the lack of T-cell-restricted intracellular antigen and granzyme B expression may thus differentiate transformed from primary ALCL.
Our patient’s course of events can be summarized as follows: (1) She experienced sudden extra
A review of the literature revealed that only 3 patients with ALCL secondary to CML have been reported[12-14]; however, none had BCR-ABL rearrangements. Such transformation of Ph-positive ALCL from CML is exceedingly rare; to the best of our knowledge, our patient is the first reported to have experienced such an event.
Patients with CML who experience sudden blast transformation have very poor prognoses; treatment options depend on the types of transformation, stage of lymphoma, phase of CML, and whether the transformed lymphoma cells are positive for the BCR-ABL translocation. There are no standard treatment protocols for patients with transformed CML, and the intervention strategy may involve combinations of chemotherapy, alternative TKIs, and hematopoietic stem cell transplantation. Our patient refused combination chemotherapy and was treated with the second-generation TKI nilotinib; she achieved complete cytogenetic remission and undetectable molecular residual disease in 6 months and remains in this status as of April 2022.
Although CML is a hematologic tumor with a good prognosis, a small percentage of patients still experience blast crisis. Moreover, extramedullary blast crisis is a very rare form of sudden blast crisis. When unexplained lymphadenopathy and/or an extramedullary mass are detected during the diagnosis and treatment of a patient with CML, the possibility of blast crisis or secondary lymphoma should be investigated. A pathological biopsy should be performed along with FISH to test for BCR-ABL fusion and determine the nature of the transformed cells. If these cells are Ph-positive, treatment with next-generation TKIs should be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA, Romania; Sorio C, Italy S-Editor: Chen YL L-Editor: A P-Editor: Wu RR
| 1. | Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ; IRIS Investigators. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 873] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 2. | Guilhot J, Baccarani M, Clark RE, Cervantes F, Guilhot F, Hochhaus A, Kulikov S, Mayer J, Petzer AL, Rosti G, Rousselot P, Saglio G, Saussele S, Simonsson B, Steegmann JL, Zaritskey A, Hehlmann R. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by the European LeukemiaNet. Blood. 2012;119:5963-5971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Kantarjian H, O'Brien S, Cortes J, Giles F, Thomas D, Kornblau S, Shan J, Beth Rios M, Keating M, Freireich E, Talpaz M. Sudden onset of the blastic phase of chronic myelogenous leukemia: patterns and implications. Cancer. 2003;98:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Jabbour E, Kantarjian H, O'Brien S, Rios MB, Abruzzo L, Verstovsek S, Garcia-Manero G, Cortes J. Sudden blastic transformation in patients with chronic myeloid leukemia treated with imatinib mesylate. Blood. 2006;107:480-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Breccia M, Nanni M, Mancini F, Russo E, Mecarocci S, Alimena G. Extramedullary blast crisis occurring in a Philadelphia-positive chronic myeloid leukemia patient with major cytogenetic response to imatinib. Haematologica. 2004;89:ECR11. [PubMed] |
| 6. | Rodler E, Welborn J, Hatcher S, Unger K, Larkin E, Gumerlock PH, Wun T, Richman C. Blastic mantle cell lymphoma developing concurrently in a patient with chronic myelogenous leukemia and a review of the literature. Am J Hematol. 2004;75:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2588] [Cited by in RCA: 2558] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 8. | Hochhaus A, Rosti G, Cross NC, Steegmann JL, le Coutre P, Ossenkoppele G, Petrov L, Masszi T, Hellmann A, Griskevicius L, Wiktor-Jedrzejczak W, Rea D, Coriu D, Brümmendorf TH, Porkka K, Saglio G, Gastl G, Müller MC, Schuld P, Di Matteo P, Pellegrino A, Dezzani L, Mahon FX, Baccarani M, Giles FJ. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia. 2016;30:57-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Tantiworawit A, Power MM, Barnett MJ, Hogge DE, Nantel SH, Nevill TJ, Shepherd JD, Song KW, Sutherland HJ, Toze CL, Abou-Mourad YR, Narayanan S, Broady RC, Forrest DL. Long-term follow-up of patients with chronic myeloid leukemia in chronic phase developing sudden blast phase on imatinib therapy. Leuk Lymphoma. 2012;53:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Miranda MB, Lauseker M, Kraus MP, Proetel U, Hanfstein B, Fabarius A, Baerlocher GM, Heim D, Hossfeld DK, Kolb HJ, Krause SW, Nerl C, Brümmendorf TH, Verbeek W, Fauser AA, Prümmer O, Neben K, Hess U, Mahlberg R, Plöger C, Flasshove M, Rendenbach B, Hofmann WK, Müller MC, Pfirrmann M, Hochhaus A, Hasford J, Hehlmann R, Saußele S. Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: long-term observation in CML Study IV. Leukemia. 2016;30:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Jin GN, Zou P, Chen WX, Ding ZY, Zhou H. Fluorescent in situ hybridization diagnosis of extramedullary nodal blast crisis. Diagn Cytopathol. 2013;41:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Montefusco E, Lo Coco F, Burgio VL, Rondinelli B, Di Giorgio G, Mancini M, Diverio D, Andriani A, Avvisati G, Alimena G. Occurrence of a Ki-1-positive anaplastic large-cell lymphoma in a patient with Ph' positive chronic myelogenous leukemia successfully treated by alpha-interferon. Leukemia. 1993;7:1896-1899. [PubMed] |
| 13. | Ichinohasama R, Miura I, Takahashi N, Sugawara T, Tamate E, Endoh K, Endoh F, Naganuma H, DeCoteau JF, Griffin JD, Kadin ME, Ooya K. Ph-negative non-Hodgkin's lymphoma occurring in chronic phase of Ph-positive chronic myelogenous leukemia is defined as a genetically different neoplasm from extramedullary localized blast crisis: report of two cases and review of the literature. Leukemia. 2000;14:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Charafeddine KM, Farchoukh LO, Khalifeh I. Primary cutaneous anaplastic large-cell lymphoma occurring in a case of chronic myeloid leukemia in remission. J Cutan Pathol. 2012;39:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |