Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10310
Peer-review started: May 23, 2022
First decision: June 16, 2022
Revised: July 3, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: October 6, 2022
Processing time: 127 Days and 5.7 Hours
Due to a slight rise in beta-human chorionic (β-hCG) levels that are undetectable, and vaginal bleeding that is similar to regular menstruation, ectopic pregnancy (EP) that occurs during the expected menstrual cycle prior to ovulation induction as part of in vitro fertilization (IVF) is likely to be undiagnosed. We present two cases of unexpected EP and emphasize the importance of the β-hCG assay when an unexplained increase in progesterone is present prior to the triggering of ovulation during controlled ovarian stimulation (COS).
A 26-year-old woman with primary infertility and a 31-year-old woman with secondary infertility. Both patients sought IVF treatment due to fallopian tube obstruction and underwent COS using the gonadotropin-releasing-hormone (GnRH)-antagonist protocol. In the late stage of COS, progesterone levels in both patients significantly increased, and luteinizing hormone levels decreased, followed by oocyte retrieval failure. A right salpingectomy was performed and tubal ectopic pregnancy was diagnosed by pathology in the first patient, and the second patients was diagnosed with a suspected EP abortion because her β-hCG levels declined to 12.5 mIU/mL. After full recovery for 2 mo, the first patient entered a new IVF treatment cycle with a GnRH-antagonist regimen and successfully achieved eight oocytes and three viable embryos. After 6 mo, the second patient received another COS treatment with a progestin-primed ovarian stimulation protocol and successfully achieved nine oocytes and five viable embryos.
β-hCG levels in the initial and midterm phases of COS must be considered in patients with unusual hormone dynamics.
Core Tip: In this report, we present two cases of undiagnosed ectopic pregnancies during controlled ovulation stimulation (COS) who sought in vitro fertilization treatment due to fallopian tube obstruction. In the late stage of COS, progesterone levels in both patients significantly increased and luteinizing hormone (LH) levels decreased, followed by oocyte retrieval failure. These cases emphasized the importance of vigilance about patients with unexplained increases in progesterone and decreases in LH during the COS process. Further, the examination of human chorionic gonadotropin in the initial and midterm phases of COS should be considered essential for patients with unusual hormone dynamics.
- Citation: Zhou WJ, Xu BF, Niu ZH. Ectopic pregnancy and failed oocyte retrieval during in vitro fertilization stimulation: Two case reports. World J Clin Cases 2022; 10(28): 10310-10316
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10310.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10310
The incidence of ectopic pregnancy (EP) in patients undergoing in vitro fertilization (IVF) is 1.5%-2.1%[1], which is higher than that in natural conception. The epidemiology and risk factors of EP after IVF have been widely investigated. Several hypotheses have been proposed to explain this difference, including high proportion of abnormal tubal function, hormonal environments, technical aspects of IVF procedures, and estimated embryo implantation potential[2]. Usually, doctors have high vigilance for EP after embryo transfer and most EPs can be diagnosed early by measuring beta-human chorionic gonadotropin (β-hCG) serum levels and through transvaginal ultrasound. However, the occurrence of EP during the controlled ovarian stimulation (COS) process is rarely reported. As almost one third of women with EP have no clinical signs and 9% have no symptoms, the early diagnosing of EP with β-hCG is important for clinicians, especially before the start of ovary stimulation. While, it was reported that about 1% of EPs will have a negative urine pregnancy test and a β-hCG level of less than 20 mIU/mL[3]. Herein, we present two cases of unexpected EP with initial very low serum β-hCG levels during COS and emphasize the importance of the β-hCG assay when an unexplained increase in progesterone is present prior to the triggering of ovulation during COS.
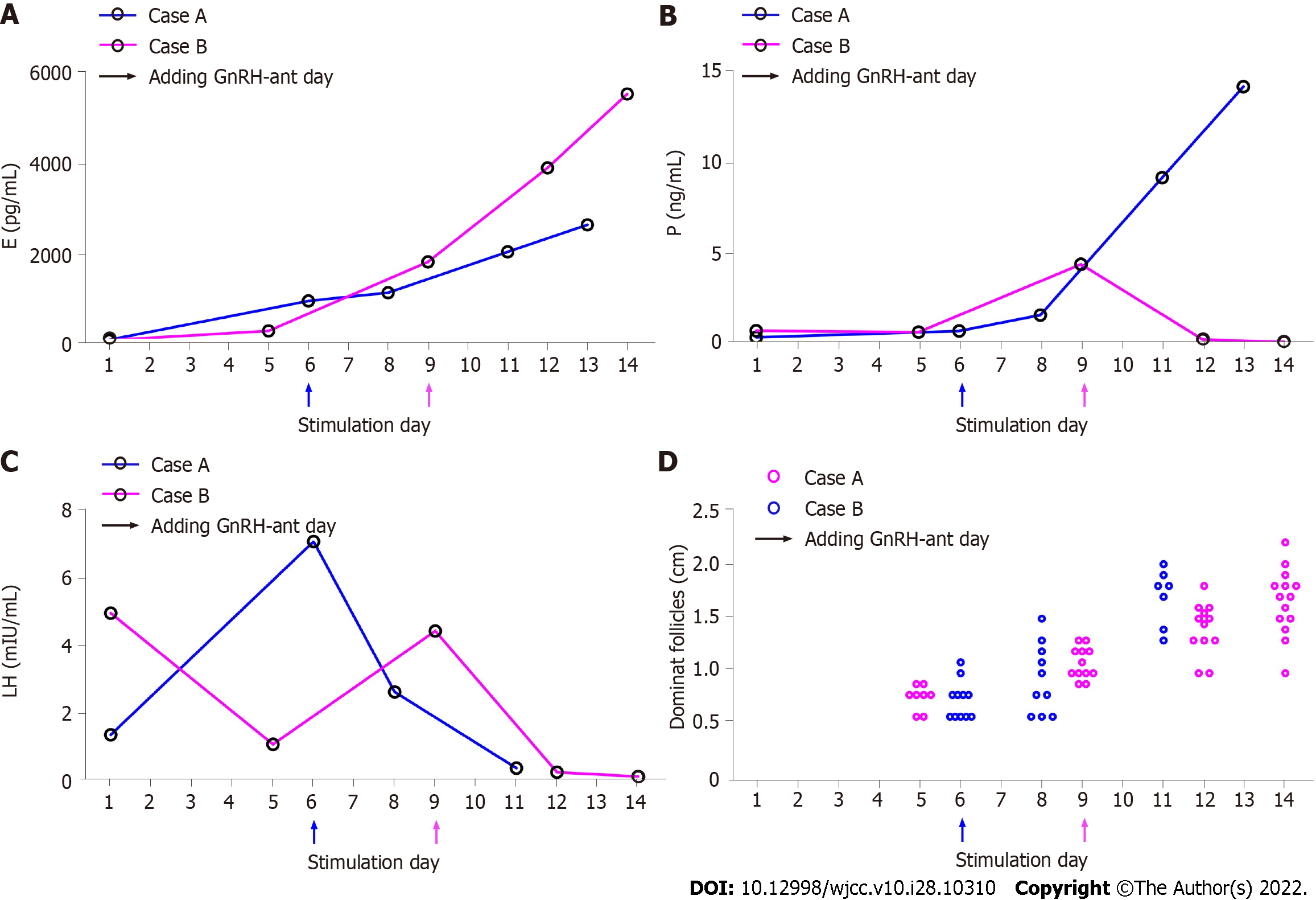
The basic characteristics of the two patients in COS cycle are displayed in Table 1 and the serial sex hormone levels and follicle sizes of the two patients during COS are shown in Figure 1. Both received IVF treatment because of fallopian tube factors, and neither had a history of EP.
| Case 1 | Case 2 | |
| Age (yr) | 26 | 30 |
| Indication for IVF | Bilateral fallopian tube obstruction | Right uterus cornual obstruction and partially obstruction of left fallopian tube |
| Primary/secondary infertility | Primary | Secondary |
| Hormone levels on day 2nd of COS cycle | ||
| FSH (mIU/ml) | 8.92 | 8.16 |
| LH (mIU/ml) | 1.37 | 4.95 |
| E2 (pg/ml) | 33 | 23 |
| P (ng/ml) | 0.39 | 0.75 |
| β-hCG (mIU/ml) | 2.77 | 2.36 |
| AMH (ng/ml) | 1.3 | 2.6 |
| COS description | ||
| Total quantity of Gn | 2325 IU | 2100 IU |
| Duration of COS | 10 d | 13 d |
| Numbers of retrieved oocytes | 0 | 0 |
Case 1: The patient was 26-year-old, gestation 0 parturition 0. She had a medical history of bilateral fallopian tube proximal obstruction diagnosed by hysterosalpingogram (HSG).
Case 2: The patient 2, 30-year-old and gestation 2 parturition 0, had already had one previous IVF cycle in other clinic (five oocytes retrieval and one embryo transfer, no pregnant).
Case 1: From 18th March, follicle stimulating hormone (FSH) (Gonal-F, Merck Serono, Darmstadt, Germany) was administered at 200 IU/d for 6 d. The dose was adjusted to 150 IU/d for another 5 d according to the ovarian response. A gonadotropin release hormone (GnRH)-antagonist (0.25 mg) (Cetrotide, Merck Serono, Darmstadt, Germany) was administered beginning on day 7 of stimulation and was combined with menotropin (75 IU) for injection (HMG, LIVZON Inc., China). On March 28th, hCG (7000 IU) (HCG; LIVZON Inc., China) was injected to trigger ovulation. Thirty six hours later, the presence of all the follicles were confirmed by transvaginal ultrasonography (TVS) before the performance of oocyte aspiration. No oocyte was retrieved despite the repeatedly flushing of all the follicles. We noticed the follicular fluid being deep yellow which suggested oocyte premature luteinized. On that day, the serum β-hCG, estrogen and progesterone serum concentrations measured just after the failed oocyte retrieval were 7401 mIU/mL, 2607 pg/mL and 14.21 ng/mL, respectively.
Case 2: Because of right uterus cornual obstruction and partially obstruction of left fallopian tube diagnosed by HSG she did not become pregnant. From the 14th of October, menotropin (300 IU) (HMG; LIVZON Inc., China) was injected per day for 12 d. GnRH-antagonist (0.25 mg) (Cetrotide; Merck Serono, Darmstadt, Germany) was started on day 9 and continued to the trigger day. The patient was given hCG (7000 IU) (HCG; LIVZON Inc., China) intramuscularly on October 27th to trigger ovulation. After 36 h, no sign of ovulation was observed through TVS before oocyte retrieval but no oocytes were retrieved despite repeated flushing.
Case 1: She had reported a regular menstrual cycle (4-5 d/30-31 d) and the last menstrual period (LMP) was on March 17th 2020 with normal vaginal bleeding. She declared the previous menstrual period as February 20th 2020 and an unprotected sexual behavior on March 9th. Her husband’s sperm test was normal (concentration of 65.1 × 106/mL and a + b = 23.3% + 25.2%).
Case 2: She had a regular menstrual cycle (4-5 d/28 d) with LMP reported as October 13th 2020 and normal vaginal bleeding as usual.
Normal vaginal bleeding as usual menstruation and negative abdominal pain.
Case 1: The day following oocyte retrieval, TVS indicated a pseudogestational sac in the right adnexa (16 mm × 14 mm × 12 mm), with an increased β-hCG level measuring 8432 mIU/mL.
Case 2: Her β-hCG level had declined 3 d later after the intervention to 327.8 mIU/mL. Five days later, her β-hCG level had further declined to 12.5 mIU/mL.
Case 1: Laparoscopy was performed right now. Intraoperative findings proved right fallopian tube ectopic pregnancy as well as approximately 500 cc of clotted fresh blood in the pelvis. Finally, tubal ectopic pregnancy was diagnosed by pathology.
Case 2: The serum β-hCG levels in patient 2 increased from 366.08 mIU/mL on oocyte retrieval day to 592 and 1286.7 mIU/mL on day 7 and 15 following oocyte retrieval, respectively. TVS performed 15 d following oocyte retrieval indicated that no pregnancy sac in the uterus and a suspicious mass (5 mm × 8 mm × 9 mm) located in the right adnexa. Three days later, the patient experienced vaginal bleeding and her serum β-hCG levels declined. Therefore, a suspected EP abortion was considered for patient 2.
Case 1: Right salpingectomy was performed.
Case 2: The blood values and ultrasound of the patients were followed up, and no other special treatment was given.
Case 1: After full recovery for 2 mo, the patient entered a new IVF treatment cycle with GnRH-antagonist regimen and successfully achieved eight oocytes and three viable embryos.
Case 2: After 6 mo, the patients received another COS treatment with a progestin-primed ovarian stimulation protocol and successfully achieved nine oocytes and five viable embryos.
Oocyte retrieval failure is an adverse outcome of IVF cycles, and early luteinization is one of the major reasons. However, to the best of our knowledge, oocyte retrieval failure caused by accidental EP during ovulation has not yet been reported.
The exclusion of pregnancy before ovary stimulation is necessary, either with the administration of oral or injection medicines. Usually, urine pregnancy test is affordable and convenient. However, EP before ovary stimulation might be misdiagnosed due to low β-hCG levels in early pregnancy. In a normal intrauterine pregnancy, β-hCG can be detected in the plasma as early as eight days after ovulation[4] and will reach 50-300 mIU/mL within two weeks of fertilization[5]. It has been shown that about 85% of women of with EP display lower β-hCG levels than those in normal pregnancies, and one retrospective study of 207 patients with EP reported that 2.6% of EPs had a low serum β-hCG level of under 20 mIU/mL and more than 1% of EPs have been reported to have undetectable levels of β-hCG[6,7].
Not all patients with EP displayed a classic triad of symptoms, including delayed menses, vaginal bleeding, and lower abdominal pain. Simultaneous measurement of serum estradiol (E2) and progesterone levels is helpful for the early diagnosis of EP when there are undetectable serum β-hCG levels. Usually, estradiol levels > 100 pg/mL or progesteronelevels > 3 ng/mL after 14 d of ovulation suggest sustained endogenous luteal activity[8]. However, the present two cases displayed normal estradiol and progesterone levels on the second day of the menstrual cycle, with β-hCG measuring 2.77 mIU/mL and 2.36 mIU/mL, respectively. Also, neither patient declared any of the typical EP symptoms throughout the COS procedure, resulting in failure to diagnose EP before oocyte retrieval.
During the COS procedures in these cases, both patients showed normal elevated estrogen concentration and follicle size, indicating that the follicles could develop under the stimulation of exogenous FSH, even during pregnancy. However, luteinizing hormone (LH) levels dropped and progesterone levels increased dramatically in the late COS phase. Follicular development during pregnancy is unusual because the low levels of circulating gonadotropins suppressed by high inhibin and P levels. However, the exogenous administration of gonadotropins can overcome this inhibition and induce follicular development[9-11]. A case of follicular growth in response to clomiphene citrate (CC) has been reported in the presence of an EP by Bayrak et al[12]. They observed multiple follicular development in a woman receiving superovulation with CC in spite of the presence of an undiagnosed ovarian EP. And more interested was follicular growth even occurred in the ovary which the EP was eventually diagnosed in. The women did not receive trigger medicine before intrauterine insemination as the ovulation kit was positive and no ovulation was confirmed by ultrasound. In 2017, Orvieto et al[13] reported a case of ovarian hyperstimulation syndrome (OHSS) following GnRH agonist trigger and freeze-all, masking EP. The 36 years old woman displayed normal estradiol (146 pmol/L) and progesterone (0.8 nmol/L) level on day 3. During 8 d of ovary stimulating, her E2 and P levels continued to rise and reached a peak E2 Level of 14533 pmol/L and P level of 12.8 nmol/L. She was triggered with GnRH agonist 0.3 mg, yielding 6 matured oocytes that were vitrified. Six days following ovum pick up, the woman was admitted to ward with typical OHSS signs and her serum β-hCG revealed of 2881 IU/L. The patient was diagnosed as right tubal EP through TVS and offered intramuscular methotrexate injection right now. Goeckenjan et al[14] also demonstrated the feasibility of triggering final follicular maturation by GnRH- antagonist after stimulating and recruiting ovarian follicles during pregnancy. However, the effect of gonadotrophin administration on the development of EP was still not reported.
Because of the homology between hCG and LH, hCG can be used as a surrogate for the LH surge[15]. In COH with antagonist protocol, both hCG and GnRH agonist, which evoke endogenous LH surge, could be used as trigger. Compared with LH, hCG has a longer half-life and may exert a more lasting effect on LH receptors[16]. In cases presented here, we failed to yield oocytes by triggering with hCG, which was different from the previously reported cases. What’s more, the character of follicular fluid indicated premature luteinization of follicles. Reviewing the reports by Orvieto et al[13] and Goeckenjan et al[14], the level of β-hCG of patients during late follicle phase was lower than those in our report. Therefore, it is questionable which factor resulted in the failure of oocyte retrieval, trigger medicine or premature luteinization induced by higher β-hCG?
Until now, the effect of high β-hCG on oocyte quality was absent. Several studies have reported that co-treatment with low dose hCG (200 IU/d) before or in the early stage of COS may enhance developmental competence of oocytes and provide an effective way to reduce rFSH use[16,17]. Other studies have also demonstrated that the addition of low-dose hCG (50-200 IU/d) in the late follicular stage during ovarian stimulation can effectively promote follicular development and maturation and improve implantation and ongoing pregnancy rates[18,19]. However, it should be noted the probability of premature luteinization of granulosa cells induced by a significant increase in β-hCG at the late follicular stage.
In conclusion, our cases illustrate the ongoing clinical diagnostic challenges associated with EP that occurs during COS, which is rare. When an unexplained increase in progesterone levels occurs during ovulation induction, apart from considering the early onset of LH surge, testing for serum β-hCG to exclude pregnancy is recommended, especially to rule out the potentially life-threatening diagnosis of EP. Whenever the pregnancy is proven, oocyte retrieval should be cancelled and the risk of OHSS be cautioned.
The authors are grateful to the patients for their kind permission for their cases to be reported in this study. The authors thank all the physicians and nurses during the diagnosis, treatment, and nursing of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: birge Ö, Sudan; Naem AA, Syria; Sabetian S, Iran S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Yoder N, Tal R, Martin JR. Abdominal ectopic pregnancy after in vitro fertilization and single embryo transfer: a case report and systematic review. Reprod Biol Endocrinol. 2016;14:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Cheng LY, Lin PY, Huang FJ, Kung FT, Chiang HJ, Lin YJ, Lan KC. Ectopic pregnancy following in vitro fertilization with embryo transfer: A single-center experience during 15 years. Taiwan J Obstet Gynecol. 2015;54:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Barash JH, Buchanan EM, Hillson C. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2014;90:34-40. [PubMed] |
| 4. | Visconti K, Zite N. hCG in ectopic pregnancy. Clin Obstet Gynecol. 2012;55:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Stewart BK, Nazar-Stewart V, Toivola B. Biochemical discrimination of pathologic pregnancy from early, normal intrauterine gestation in symptomatic patients. Am J Clin Pathol. 1995;103:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Daniilidis A, Pantelis A, Makris V, Balaouras D, Vrachnis N. A unique case of ruptured ectopic pregnancy in a patient with negative preg-nancy test - a case report and brief review of the literature. Hippokratia. 2014;18:282-284. [PubMed] |
| 7. | Sheele JM, Bernstein R, Counselman FL. A Ruptured Ectopic Pregnancy Presenting with a Negative Urine Pregnancy Test. Case Rep Emerg Med. 2016;2016:7154713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Shah NJ, Pereira N, Romanski PA, Wright C, Kligman I, Rosenwaks Z. Tubal Ectopic Pregnancy with Undetectable Initial Serum β-Human Chorionic Gonadotropin Level. J Minim Invasive Gynecol. 2021;28:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | diZerega G, Hodgen GD. Pregnancy-associated ovarian refractoriness to gonadotropin: a myth. Am J Obstet Gynecol. 1979;134:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Serafini P, Yee B, Vargyas J, Marrs RP. Development of multiple ovarian follicles for in vitro fertilization in a patient with an undiagnosed ectopic pregnancy. Fertil Steril. 1985;43:656-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Paulson RJ, Lobo RA. Ovarian hyperstimulation complicating the clinical presentation of a pre-existing ectopic pregnancy. Fertil Steril. 1988;50:670-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Bayrak A, Fogle RH, Paulson RJ. Clomiphene citrate-induced follicular development in the presence of an ovarian ectopic pregnancy. Fertil Steril. 2008;89:456.e1-456.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Orvieto R, Vanni VS. Ovarian hyperstimulation syndrome following GnRH agonist trigger-think ectopic. J Assist Reprod Genet. 2017;34:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Goeckenjan M, Rösner S, Toth B, Strowitzki T, Germeyer A. Successful controlled ovarian stimulation despite elevated hCG levels after first-trimester abortion in the context of fertility preservation. Gynecol Endocrinol. 2013;29:960-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Fischer RA, Nakajima ST, Gibson M, Brumsted JR. Ovulation after intravenous and intramuscular human chorionic gonadotropin. Fertil Steril. 1993;60:418-422. [PubMed] |
| 16. | Beretsos P, Partsinevelos GA, Arabatzi E, Drakakis P, Mavrogianni D, Anagnostou E, Stefanidis K, Antsaklis A, Loutradis D. "hCG priming" effect in controlled ovarian stimulation through a long protocol. Reprod Biol Endocrinol. 2009;7:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Drakakis P, Loutradis D, Beloukas A, Sypsa V, Anastasiadou V, Kalofolias G, Arabatzi H, Kiapekou E, Stefanidis K, Paraskevis D, Makrigiannakis A, Hatzakis A, Antsaklis A. Early hCG addition to rFSH for ovarian stimulation in IVF provides better results and the cDNA copies of the hCG receptor may be an indicator of successful stimulation. Reprod Biol Endocrinol. 2009;7:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Blockeel C, De Vos M, Verpoest W, Stoop D, Haentjens P, Devroey P. Can 200 IU of hCG replace recombinant FSH in the late follicular phase in a GnRH-antagonist cycle? Hum Reprod. 2009;24:2910-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Serafini P, Yadid I, Motta EL, Alegretti JR, Fioravanti J, Coslovsky M. Ovarian stimulation with daily late follicular phase administration of low-dose human chorionic gonadotropin for in vitro fertilization: a prospective, randomized trial. Fertil Steril. 2006;86:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |