Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10301
Peer-review started: May 22, 2022
First decision: June 16, 2022
Revised: July 1, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 6, 2022
Processing time: 127 Days and 20.2 Hours
The incidence of multiple primary lung cancer (MPLC) in China is 0.52%-2.45%. Most primary lung cancer cases have reported two lesions or three in rare cases. We report a rare case of bilateral simultaneous multiple primary lung adenocarcinoma of four different genotypes.
A 58-year-old woman was admitted to our hospital on June 29, 2021, and upon physical examination, four multiple pulmonary nodules were identified in both lungs. Further computed tomography (CT) images revealed the presence of gro
The surgical plan for multiple pulmonary nodules should be carefully considered. For carefully selected patients with concurrently occurring multiple lung nodules in both lungs, sublobectomy is a safe and feasible plan for concurrent bilateral resection of the lesions. Genetic sequencing is necessary for MPLC diagnosis and treatment.
Core Tip: In this case, nodules in bilateral four lobes were resected simultaneously via video-assisted thoracic surgery. Postoperative pathological and genetic analyses revealed that the types of nodules were left upper lobe adenocarcinoma in situ [epidermal growth factor receptor (EGFR) exon 21], left lower lobe invasive adenocarcinoma (Erb-B2 receptor tyrosine kinase 2 exon 20 and tumor protein p53 exon 8), and right upper lobe microinvasive adenocarcinoma (EGFR exon 19), and right lower lobe microinvasive adenocarcinoma (EGFR exon 18). Genetic examination is of decisive significance in the identification of multiple lung cancers and metastatic cancers.
- Citation: Zhang DY, Liu J, Zhang Y, Ye JY, Hu S, Zhang WX, Yu DL, Wei YP. One-stage resection of four genotypes of bilateral multiple primary lung adenocarcinoma: A case report. World J Clin Cases 2022; 10(28): 10301-10309
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10301.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10301
Lung cancer has a high incidence and mortality rate, and is one of the major malignant tumors that pose a threat to human health and life. In the past 30 years, lung cancer has ranked first among malignant tumors in China with regard to its incidence and mortality rates[1]. With the development of multilayer spiral computer scanning technology and the popularization of lung cancer screening, a greater number of lung nodules are being discovered, many of which are multiple pulmonary nodules. Some of these nodules are pathologically confirmed as multiple primary lung cancers after surgery. Multiple primary lung cancer (MPLC) refers to the concurrent or successive occurrence of primary lung cancer lesions of at least two different sources at different sites of a patient’s lungs. These lesions can develop in one or two lungs. Two types of MPLCs have been described: simultaneous MPLC (sMPLC) and metachronous MPLC (mMPLC). At 0.52%-2.45%[2], the incidence of MPLC in China is lower than that of primary lung cancer.
The prognosis and treatment of primary lung cancer and lung cancer with intrapulmonary metastasis (IM) are significantly different[3]. Therefore, it is important to accurately distinguish between MPLCs, especially sMPLCs and IMs. The traditional methods used for differential diagnosis have many li
Here, we report a rare case of primary lung adenocarcinoma of four different genotypes that was treated at the Second Affiliated Hospital of Nanchang University.
A 58-year-old woman was admitted to the hospital with a 10-mo history of pulmonary sarcoidosis.
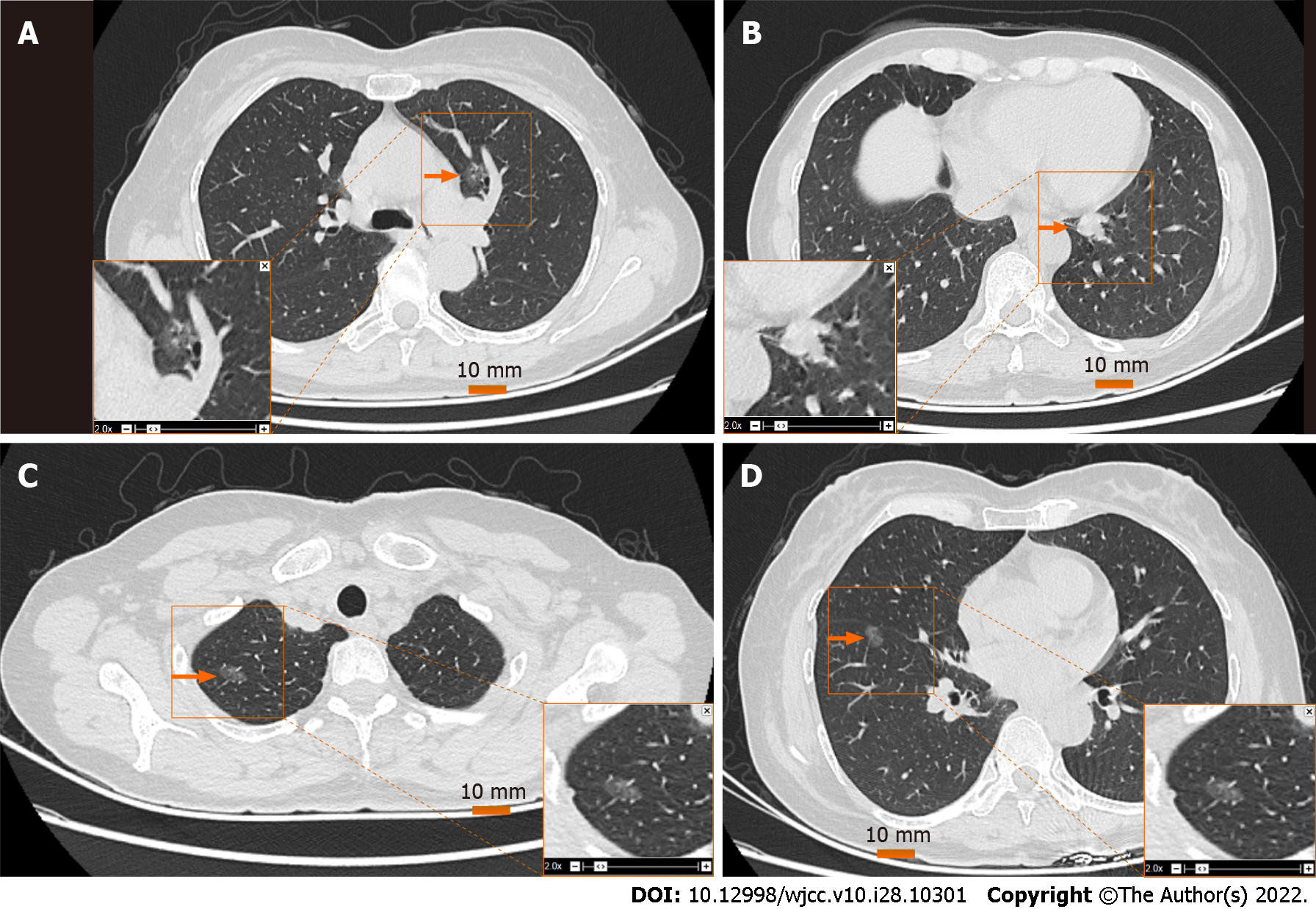
Pulmonary sarcoidosis was first discovered accidentally during a chest computed tomography (CT) scan 10 mo prior. Chest CT examination revealed tiny nodules in both lungs; the largest nodule measured 4.6 mm. No symptoms such as cough and expectoration, chest pain, dyspnea, fever, night sweats, fatigue, or anorexia were present. Upon further follow-up and clinical evaluations, enhanced chest CT findings in the hospital’s outpatient department indicated the presence of multiple ground glass nodules in both lungs (Figure 1). These nodules were predicted to be high-risk nodules by artificial intelligence.
She had a previous history of breast nodules and thyroid nodules, which were not indicative of a history of diseases such as tuberculosis and diabetes.
The patient denied having a family history of malignant tumors. The patient had no smoking or drinking history.
No obvious abnormalities were found after a cardiopulmonary examination.
No obvious abnormality was found in the blood routine examination results, liver and kidney function, or electrolyte and serum tumor markers (lung cancer marker combination: carcinoembryonic antigen, carbohydrate antigen-199, squamous cell carcinoma associated antigen, neuron-specific enolase, and cytokeratin-19 fragment).
Pulmonary function was uneventful, forced vital capacity (FVC) was 2.27 L, and forced expiratory volume (FEV1) was 2.23 L or 78.41% of the predicted value. The enhanced chest CT (Figure 1) findings showed that the bilateral lung markings were enhanced. Density shadows of nodular soft tissues were found in the lower lobe of the left lung near the hilum; the density was uniform. No calcifications were present. The diameter of the nodule was about 12 mm. The nodule had clear boundaries. The enhanced scan showed obvious and uniform enhancement. Scattered ground glass nodules were present in both lungs, especially in the right lung. The diameter of the larger nodule was about 9 mm; enhanced scanning showed no obvious enhancement. The trachea and bronchus were unobstructed, and no clear enlarged lymph nodes were present in both the hila and mediastinum. No obvious thickening of bilateral pleura and no effusion in pleural cavity were observed.
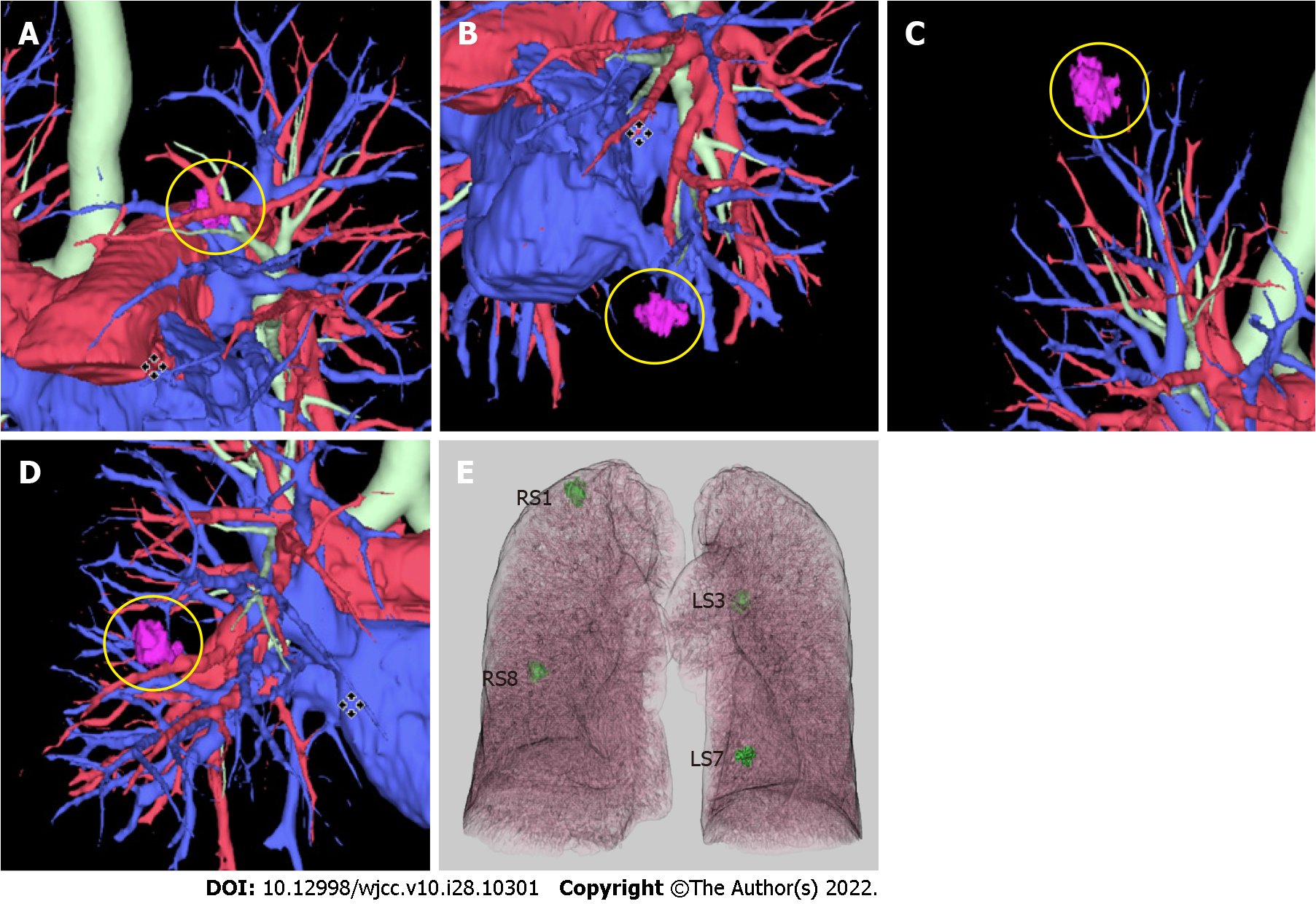
After excluding surgical contraindications, three-dimensional reconstructions of CT images were per
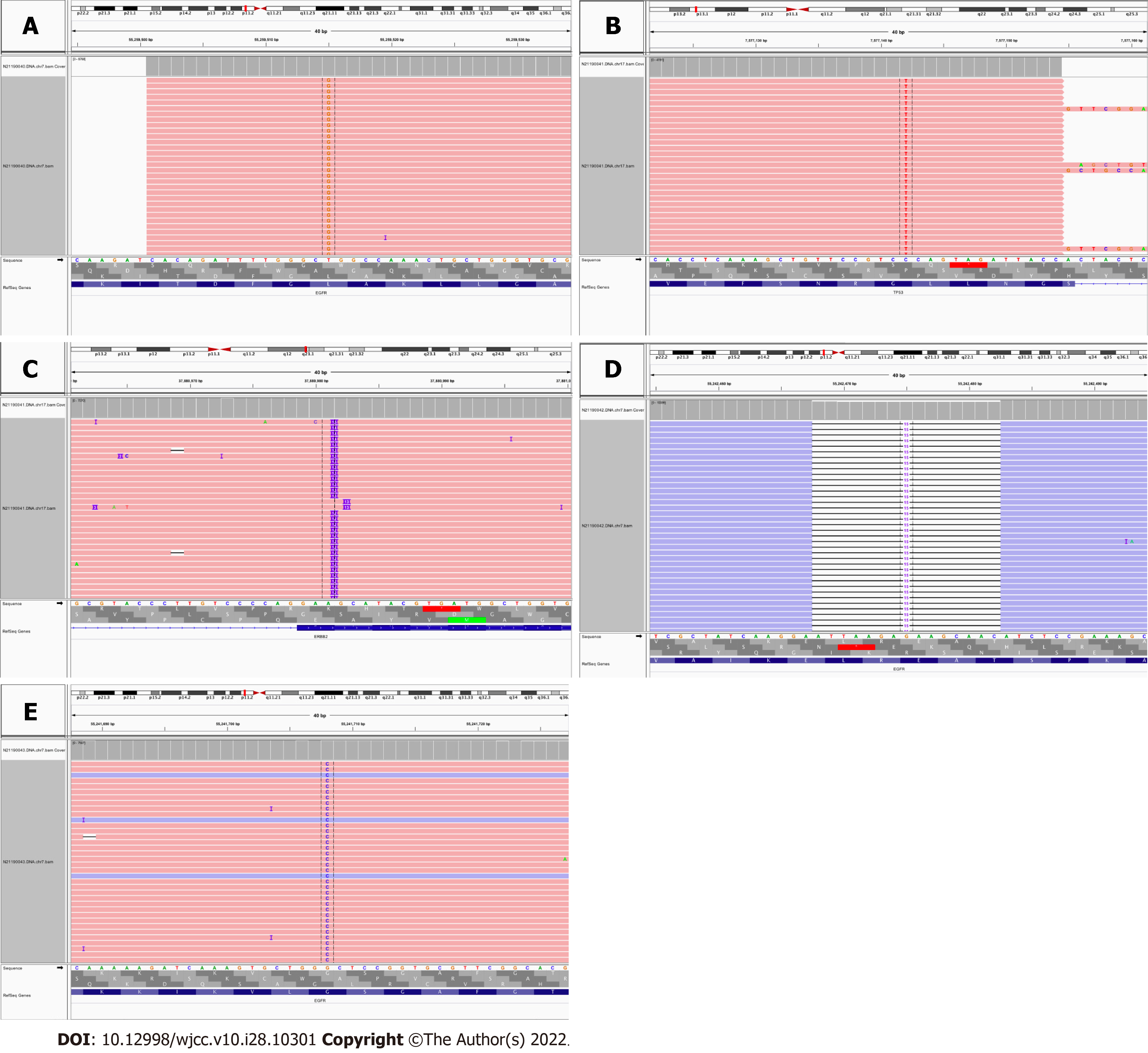
| Nodule location | Mutant gene | Detection result | Abundance/NDF, % |
| Upper lobe of left lung | EGFR | Exon 21 c.2573T>G p.L858R | 4.05 |
| Lower lobe of left lung | ERBB2 | Exon 20 c.2310_2311i nsGCATAC GTGATG p.E770_A77 1insAYVM | 25.40 |
| TP53 | exon 8 c.796G>A p.G266R | 2.84 | |
| Upper lobe of right lung | EGFR | Exon 19 c.2238_2252 delATTAAG AGAAGCA AC p.L747_T751 del | 10.24 |
| Lower lobe of right lung | EGFR | Exon 18 c.2156G>C p.G719A | 8.89 |
The patient was eventually diagnosed with sMPLC.
The surgical plan was left anterior lobe resection + left lower lobe wedge resection + right upper lobe apical resection + right lower lobe wedge resection. For an invasive cancer with a predicted FEV1 value of 78.41%, which is less than 80%, no further lobectomy is required, according to previously published research[6]. After the surgical procedure, the patient was treated with antibiotics and analgesics. Other supportive treatments, such as appropriate nutrition and symptomatic treatment, were also administered.
The patient recovered and was discharged on postoperative day 8. Postoperative evaluation of pulmonary function showed that her FVC was 1.40 L and her FEV1 was 1.24 L or 57% of the predicted value.
MPLC refers to the concurrent or successive occurrence of primary lung cancer of at least two different sources at different sites of a patient’s lungs. Two types of MPLC have been described: sMPLC and mMPLC. According to the criteria proposed by Martin and Melamed[7] in 1975 for the diagnosis of MPLC, the lesions must be of different tissue types or originate from different carcinoma in situ, if the types are the same. Each lesion must also be located in different anatomical sites with no common lymphatic or extrapulmonary metastatic paths at the time of diagnosis. For mMPLC, an additional tumor-free interval of at least 2 years is required. Compared to the incidence rate of lung cancer, the incidence rate of MPLC is lower at 0.52%-2.45%[2]. Most cases of primary lung cancer have been reported to have two lesions and three in rare cases. In this case, four different lesions were identified. After a comprehensive preoperative evaluation, this case was considered to be a case of high-risk nodules.
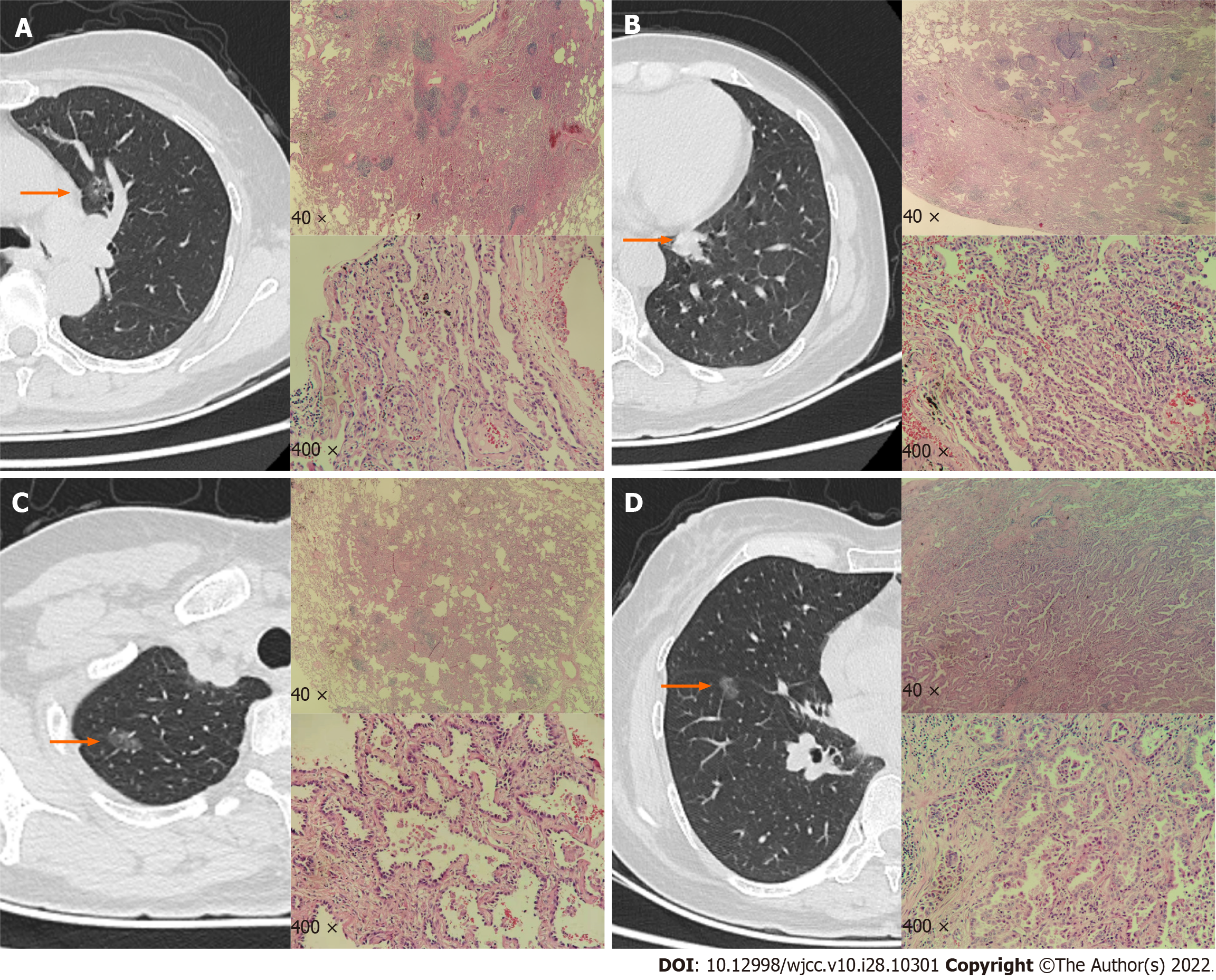
Chest CT and other imaging examinations did not indicate intrapulmonary metastasis, and the hilar and mediastinal lymph nodes were not enlarged. However, some nodules were indicative of inflammation in the left lower lung. As percutaneous lung biopsy carries the risk of false-negative results due to the small amount of sampling tissue, as well as the risk of pleural implant transfer, we determined that the most appropriate approach was to surgically resect all the lesions simultaneously. Therefore, single-hole thoracoscopic surgery was indicated. Four nodules in both lungs were also resected. Postoperative pathological examination confirmed our suspicion.
With the increasing use of high-resolution low-dose spiral CT in lung cancer screening, the discovery of pulmonary nodules is more common, with 50% of screened patients having multiple pulmonary nodules[8]. MPLC is mostly diagnosed early during physical examination by the identification of pulmonary nodules. Currently, no guidelines have been established for the diagnosis and treatment of MPLC. Therefore, treatment regimen for multiple pulmonary nodules can be adopted for the treatment of MPLC. In National Comprehensive Cancer Network (NCCN) and China’s guidelines for the diagnosis and treatment of lung nodules, the treatment of multiple lung nodules is mentioned, but no specific guidelines for the diagnosis and treatment of multiple lung nodules were found in the literature.
However, we decided on a plan based on clinically relevant suggestions in the literature, domestic expert views, and relevant foreign guidelines. With respect to the timing of surgical intervention, from the 2018 version of the guidelines on diagnosis and treatment of pulmonary nodules in China[9] and those on the diagnosis and treatment of early lung adenocarcinoma of ground glass nodules[10] at Shanghai Pulmonary Hospital, experts recommend that each nodule should be evaluated separately. They also recommend that major lesions should be prioritized over secondary lesions. Comprehensive evaluation of the lesion’s location, size, and effect on lung function should be conducted. Selective local resection of lesions is also recommended. For suspected MPLC, the mediastinal lymph node status should be evaluated. Surgical treatment is not recommended, if mediastinal lymph node metastasis is present. If mediastinal lymph node metastasis is negative, a specific surgical plan, as to whether to remove the main or all lesions, can be formulated according to lesion status and systemic conditions. The NCCN 2020 guidelines, Fleischner Society 2017 guidelines[11], and American Association of Chest Physicians 2013 guidelines[12] recommend regular follow-up of patients with high-risk nodules. The follow-up frequency and duration are based on the main lesions, and the timing of surgical intervention is subject to the specific evaluation of clinicians, as no specific timing recommendations for surgical intervention are available.
In terms of the surgical plan, surgery is still the first choice for multiple pulmonary nodules with clear pathological results or high-risk nodules. The operation plan is determined according to the number, location, and size of the lesions; pulmonary function; and physical condition of the patient. At present, there is consensus among surgeons with regard to the scope of surgical resection of multiple pulmonary nodules, and these are the general guidelines that are typically followed: (1) If multiple pulmonary nodules are located in the same lobe, lobectomy is preferred; (2) If the nodule is located in different lobes of the ipsilateral lung and the nodule diameter is greater than or equal to 2 cm, lobectomy is recommended. If the diameter is less than 2 cm, wedge-shaped, segmental, or combined segmental resection is recommended according to the location and relationship with adjacent blood vessels[13]. Total pneumonectomy is not recommended for multiple pulmonary nodules; it has a poor prognosis[14]; and (3) If the nodules are located in different lobes of bilateral lungs, the choice of simultaneous surgical resection or secondary surgical resection is still controversial. If the patient has good cardiopulmonary function reserve, bilateral simultaneous surgery can be considered on the premise of strictly grasping the surgical indications. The surgery is safe and feasible, with less trauma, less cost, and faster postoperative recovery than second stage surgery[15,16]. Chen et al[17] believe that simultaneous resection and staged resection have a similar prognosis. For patients who cannot tolerate concurrent bilateral surgery, priority can be given to the treatment of the main lesions. The other lesions can be treated during the second stage. Some physicians believe that second stage surgery is safer, more reasonable, and more effective than simultaneous surgery in the treatment of bilateral multiple pulmonary nodules[18]. Multidisciplinary consultation is recommended for the discussion and decision on the timing of surgical intervention and concurrent or secondary surgery. In this case, the patient's bilateral pulmonary nodules were small, and pulmonary function and general condition were acceptable. According to the preoperative plan, double sublobar resection was executed at the same time. Romaszko et al[19] believe that the application of a molecular method to accurately determine the cloning source of MPLC may help determine the appropriate therapy and improve the prognosis of patients. In the present study, high-throughput sequencing (semiconductor sequencing) of 26 lung cancer genes in the four nodules was performed after surgical resection, with the human epidermal growth factor receptor, KRAS, BRAF, phosphoinositide 3-kinase, catalytic subunit alpha, ALK, and ROS1 gene mutation detection kit from Tianjin Novogene Bioinformatics Technology Co. Ltd. (Tianjin, China). The experimental method was multiplex PCR capture and semiconductor sequencing. First, DNA and RNA was extracted and purified from paraffin-embedded pathological tissue specimens of the four nodules. Then the DNA and cDNA fragments in the target area were enriched by multiplex PCR, and the enriched library was quantified and subjected to quality control. Finally, the quantified library was sequenced with a gene sequencer (model DA8600; Daan Gene Co. Ltd., Sun Yat-Sen University, Guangzhou, China) for high-throughput sequencing to obtain the DNA and RNA sequence information of the target region, and the supporting software was used to communicate with the human genome. The information obtained was checked against the Human Genome Database to determine whether mutation or fusion occurs. The results of genetic testing and imaging of the mutations are shown in Table 1 and Figure 4.
Under the guidance of three-dimensional reconstruction, Chu et al[20] used a combination of thoracoscopic wedge resection and segmental resection to resect five nodules in different lobes of both lungs. The patients were discharged on postoperative day 8 without complications. Yu et al[21] chose video-assisted wedge lung resection, when there were four nodules in different lobes of both lungs. Since the nodules in the right upper lobe were invasive adenocarcinoma, and those in the right middle lobe were micro-invasive adenocarcinoma, further lobectomy with lymph node dissection was performed. Postoperative recovery was acceptable. Sublobectomy has been proven to be an alternative to lobectomy for early lung cancer[22]. Additionally, the development of minimally invasive surgery makes simul
On the premise of the careful selection of patients, when considering the diagnosis of multiple primary lung adenocarcinomas, concurrent sublobectomy on both sides is a safer and more feasible option. Molecular sequencing and further genetic analysis of mutations in each nodule are necessary in the investigation of nodules of multiple sources.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tangsuwanaruk T, Thailand; Wang P, China S-Editor: Wang DM L-Editor: Filipodia P-Editor: Wang DM
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15314] [Article Influence: 3062.8] [Reference Citation Analysis (4)] |
| 2. | Zhang YX, Xie Qiang, Gu CD. Progress in diagnosis and treatment strategies for multiple primary lung cancer. Chin J Clin Thorac Cardiov Surg. 2021;28:609-614. (In Chinese with English abstract). [DOI] [Full Text] |
| 3. | Stella F, Luciano G, Dell'Amore A, Greco D, Ammari C, Giunta D, Bini A. Pulmonary Metastases from NSCLC and MPLC (Multiple Primary Lung Cancers): Management and Outcome in a Single Centre Experience. Heart Lung Circ. 2016;25:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Hong H, Hahn S, Matsuguma H, Inoue M, Shintani Y, Honda O, Izumi Y, Asakura K, Asamura H, Isaka T, Lee K, Choi YS, Kim YT, Park CM, Goo JM, Yoon SH. Pleural recurrence after transthoracic needle lung biopsy in stage I lung cancer: a systematic review and individual patient-level meta-analysis. Thorax. 2021;76:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Chen D, Mei L, Zhou Y, Shen C, Xu H, Niu Z, Che G. A novel differential diagnostic model for multiple primary lung cancer: Differentially-expressed gene analysis of multiple primary lung cancer and intrapulmonary metastasis. Oncol Lett. 2015;9:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT; American College of Chest Physicians. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132:161S-177S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 294] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606-612. [PubMed] |
| 8. | Heuvelmans MA, Walter JE, Peters RB, Bock GH, Yousaf-Khan U, Aalst CMV, Groen HJM, Nackaerts K, Ooijen PMV, Koning HJ, Oudkerk M, Vliegenthart R. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer. 2017;113:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Lung cancer group of respiratory branch of Chinese Medical Association; Expert group of China lung cancer prevention and control Alliance. Consensus of Chinese experts on diagnosis and treatment of pulmonary nodules (2018 Edition). Zhonghua Jiehe He Huxi Zazhi. 2018;41:763-771. [DOI] [Full Text] |
| 10. | Jiang GN, Chen C, Zhu YM, Xie D, Dai J, Jin KQ, Shen YR, Wang HF, Li H, Zhang LJ. Shanghai Pulmonary Hospital Experts Consensus on the Management of GroundGlass Nodules Suspected as Lung Adenocarcinoma (Version 1). Zhong Guo Fei Ai Za Zhi. 2018;21:147-159. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 11. | MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, Ohno Y, Powell CA, Prokop M, Rubin GD, Schaefer-Prokop CM, Travis WD, Van Schil PE, Bankier AA. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284:228-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 1551] [Article Influence: 193.9] [Reference Citation Analysis (0)] |
| 12. | Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, Wiener RS. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest. 2013;143:e93S-e120S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 992] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 13. | Fabian T, Bryant AS, Mouhlas AL, Federico JA, Cerfolio RJ. Survival after resection of synchronous non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Kocaturk CI, Gunluoglu MZ, Cansever L, Demir A, Cinar U, Dincer SI, Bedirhan MA. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J Cardiothorac Surg. 2011;39:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Ge TF, Xu N, Zhu F, Tang L, Liu D, Wang L, Qian P, Guo H, Hua CS, Chen H. Analysis of a surgical series of patients with synchronous multiple ground-glass nodules. Zhongguo Xiongxinxueguan Waike Linchuang Zazhi. 2021;28:830-836. [DOI] [Full Text] |
| 16. | Yao F, Yang H, Zhao H. Single-stage bilateral pulmonary resections by video-assisted thoracic surgery for multiple small nodules. J Thorac Dis. 2016;8:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Chen TF, Xie CY, Rao BY, Shan SC, Zhang X, Zeng B, Lei YY, Luo HH. Surgical treatment to multiple primary lung cancer patients: a systematic review and meta-analysis. BMC Surg. 2019;19:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Zhang ZR, Mao YS, Gao SG, Mu JW, Xue Q, Wang DL, Gao YS, Zhao J, He J. [Survival after surgical treatment of bilateral synchronous multiple primary non-small cell lung cancers]. Zhonghua Zhong Liu Za Zhi. 2016;38:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Romaszko AM, Doboszyńska A. Multiple primary lung cancer: A literature review. Adv Clin Exp Med. 2018;27:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Chu XP, Chen ZH, Lin SM, Tang WF, Zhang JT, Lai YM, Fu R, Qiu ZB, Lin JT, Nie Q, Yang XN, Wu YL, Zhong WZ. Precise resection of multiple pulmonary nodules using a three-dimensional reconstruction model: A case report. Thorac Cancer. 2021;12:970-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Yu G, Jiang X, Cao H, Huang B. Bilateral synchronous multiple lung nodules: Surgical experience from two cases. Saudi J Biol Sci. 2018;25:971-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Altorki NK, Yip R, Hanaoka T, Bauer T, Aye R, Kohman L, Sheppard B, Thurer R, Andaz S, Smith M, Mayfield W, Grannis F, Korst R, Pass H, Straznicka M, Flores R, Henschke CI; I-ELCAP Investigators. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. 2014;147:754-62; Discussion 762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |