Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10260
Peer-review started: April 30, 2022
First decision: June 19, 2022
Revised: July 2, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 150 Days and 7.4 Hours
Solitary splenic tuberculosis (TB) is unusual and rarely reported. Whether splenic TB is best treated surgically is still controversial. We describe a 73-year-old man with solitary splenic TB and no extrapulmonary TB.
We report the case of a 73-year-old man with solitary splenic TB who complained of emaciation and fatigue. Abdominal computed tomography (CT) images suggested a splenic space-occupying lesion. We then performed a CT-guided splenic biopsy. The postoperative pathological examination revealed splenic TB. The patient took quadruple anti-TB medication. After 1 year, the patient recovered his normal weight and had no feeling of fatigue, and the splenic lesion had shrunk significantly.
If patients receive combined, appropriate, regular, full-time anti-TB treatment, solitary splenic TB may be cured.
Core Tip: Solitary splenic tuberculosis (TB) is unusual and rarely reported in the literature internationally. Whether splenic TB is best treated surgically is still controversial. We report a rare case of solitary splenic TB. The patient took quadruple anti-TB medication. This case provides a basis for diagnosis and treatment of splenic TB.
- Citation: Guo HW, Liu XQ, Cheng YL. Solitary splenic tuberculosis: A case report. World J Clin Cases 2022; 10(28): 10260-10265
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10260.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10260
Tuberculosis (TB) is an important health problem in developing countries. Despite medical advances, TB remains one of the world’s most prevalent and fatal infectious diseases[1,2]. Pulmonary TB is the commonest form of infection but it may also involve many extrapulmonary sites. Splenic TB is a rare form of infection. It was first reported by Coley in 1846[3]. The spleen can be involved in two forms. Primary infection is an isolated focus of infection in the spleen without any other organ involvement, but it is a rare occurrence[4]. Splenic TB presents with multifarious clinical symptoms. We describe a 73-year-old man with solitary splenic TB and no extrapulmonary TB.
A 73-year-old man presented to the gastroenterology clinic of the first affiliated hospital of Tsinghua University complaining of weight loss and pronounced fatigue over the past two months.
The patient complained of weight loss and pronounced fatigue over the past 2 mo, but no other symptoms, such as nausea, vomiting, abdominal pain, hematemesis, black stool, cutaneous or sclera icter, night sweats, and unexplained fever.
The patient had cerebral infarction 2 years ago. He denied a history of TB and TB exposure. He had never taken hormones or immunosuppressive agents and never been infected with human immunodeficiency virus (HIV).
His medical history did not include any family history of TB.
The vital signs were as follows: Body temperature, 36.3 °C; heart rate, 82 beats/min; respiratory rate, 18 breaths/min; blood pressure, 127/65 mmHg. Palpation of the abdomen revealed no epigastric tenderness or hepatosplenomegaly.
No abnormality was found in sputum smear. Tubercle bacillus antibody was negative, but a whole blood interferon-ã release assay was positive.
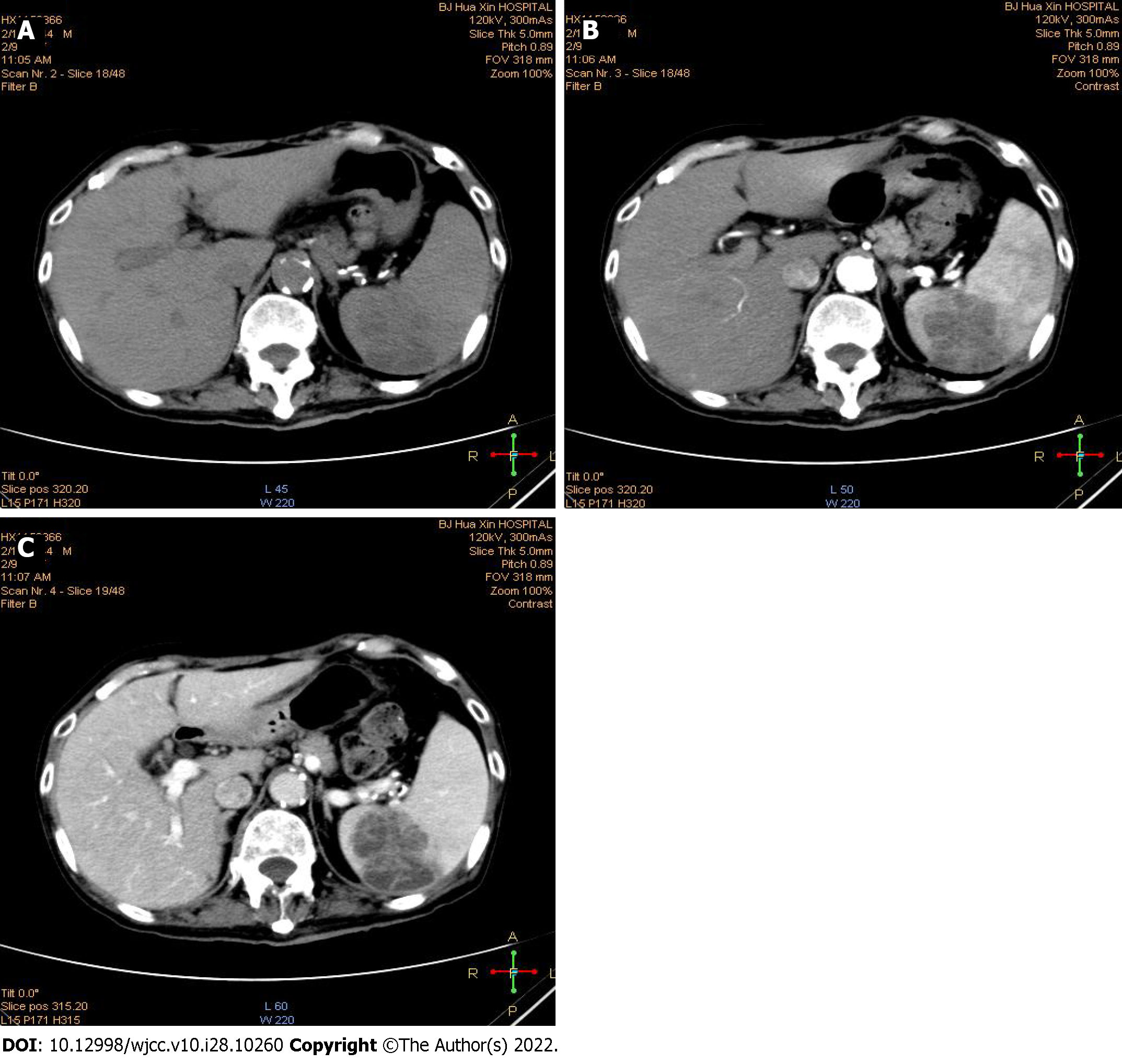
No tuberculous lesions were found on chest computed tomography (CT). Abdominal CT showed a large circular lesion of mixed density (4.4 cm × 5.5 cm) in the spleen. The CT value of plain scan was 19-25 HU. The CT value of the enhanced scan was 36 HU in the arterial phase and 48 HU in the portal phase. There were small patches of low-density liquefaction necrosis in the splenic parenchyma (Figure 1). Abdominal magnetic resonance imaging showed a large lesion (5.7 cm) on the spleen with high T2 signal, equal or slightly high heterogeneous T1-weighted signal, and visibly high diffusion-weighted signal.
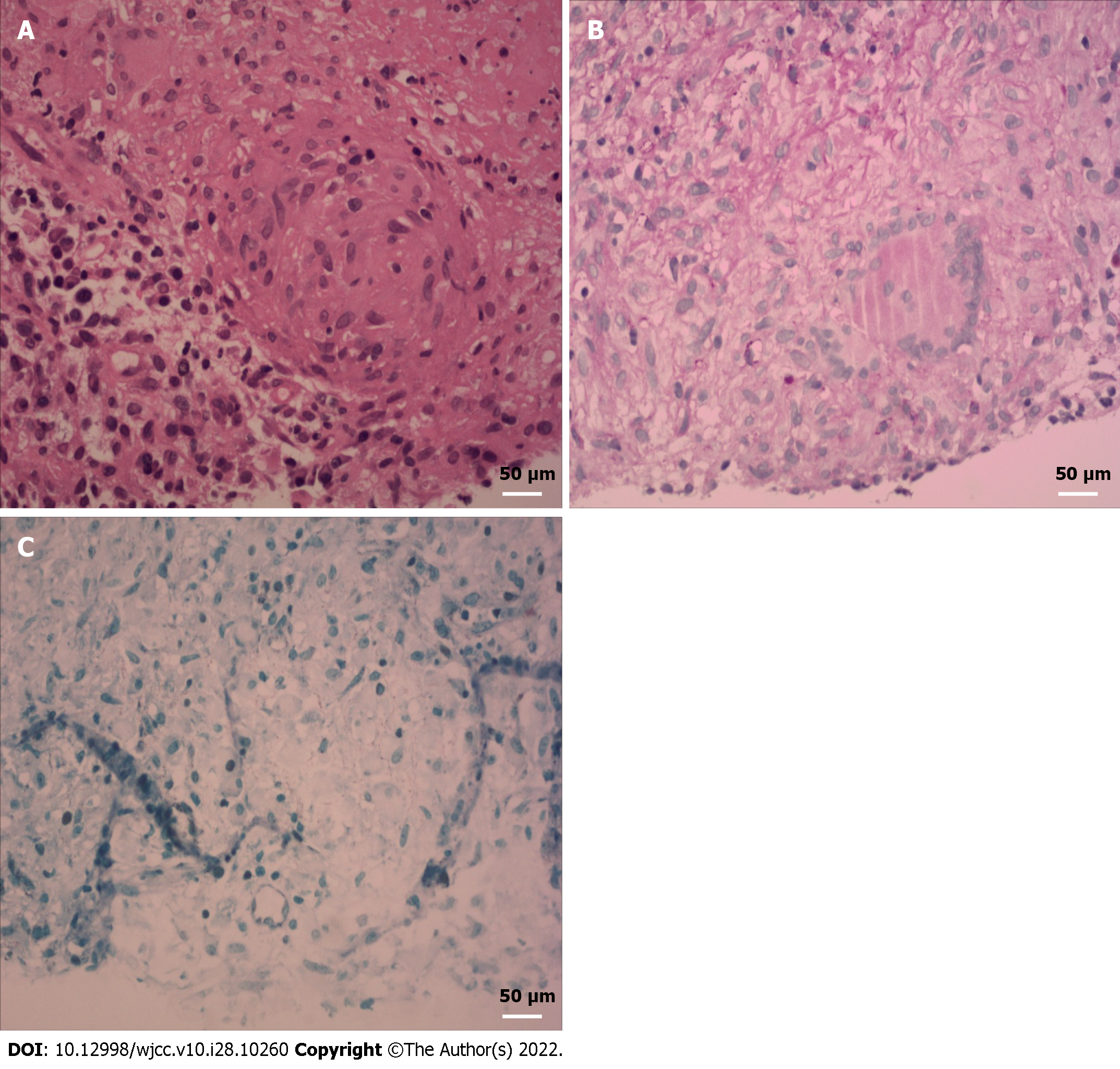
With the consent of the patient, CT-guided puncture biopsy was performed for splenic lesions. To our surprise, immunohistochemical analysis showed splenic granulomatous inflammation with large inflammatory necrosis and granulation tissue (Figure 2A and B), Mycobacterium tuberculosis acid fast staining was positive (Figure 2C), and M. tuberculosis DNA was positive.
According to the patient’s medical history, auxiliary examination and pathology, the final diagnosis was splenic TB.
The patient was started on quadruple anti-TB therapy, which included streptomycin, rifampin, pyrazinamide and ethambutol.
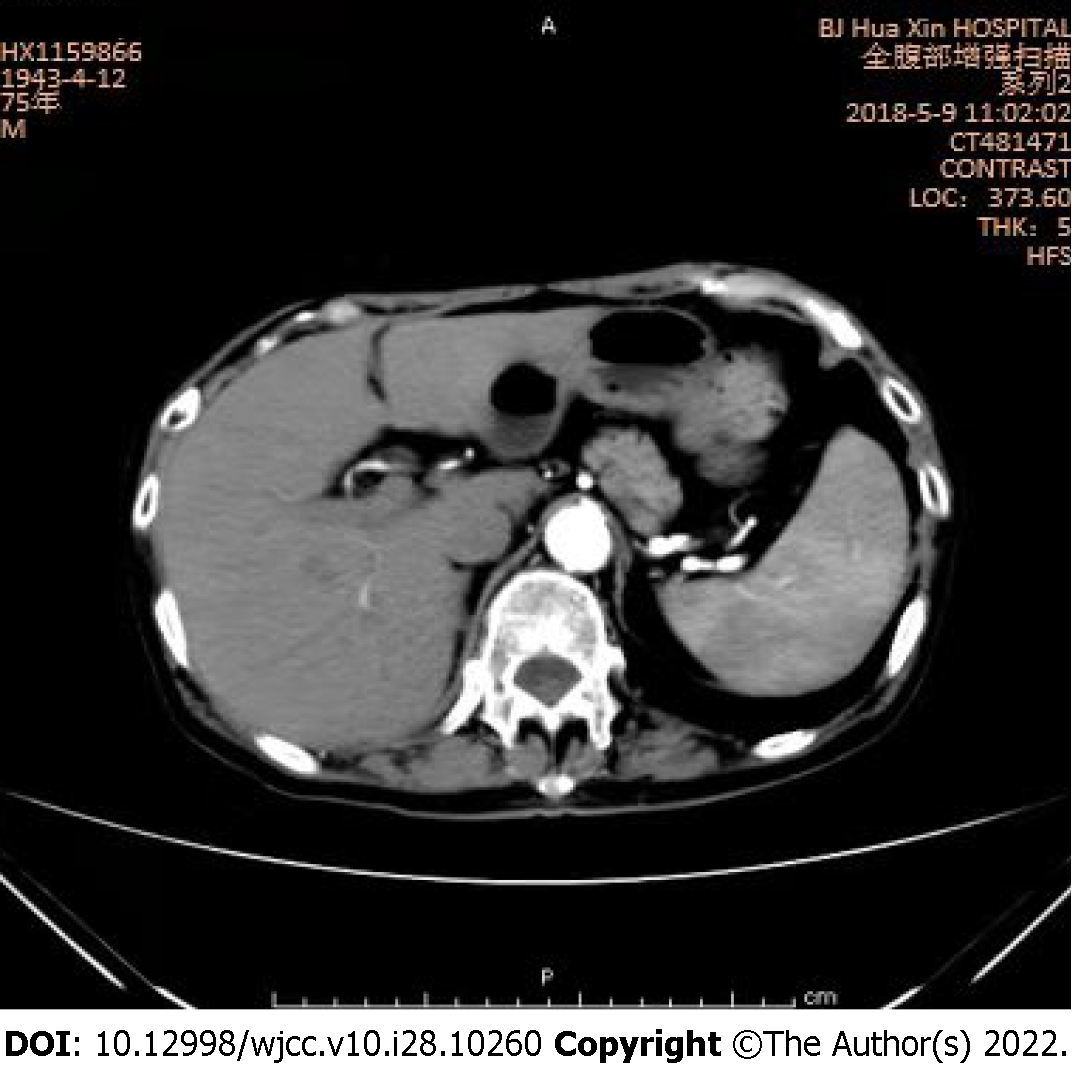
After 1 year, the patient recovered his normal weight and had no fatigue. He presented to our outpatient service for abdominal CT. Abdominal CT revealed that the lesion had shrunk significantly (Figure 3), so the patient was advised to continue taking medication for at least 6 mo.
The most common anatomical sites affected by extrapulmonary TB are lymph nodes, pleura, bone and joints, urogenital tract, and meninges[5]. Splenic TB is typically associated with serious systemic illnesses such as immunosuppression, bacterial endocarditis, or sepsis as a result of hematogenous spread[3]. The possible forms of involvement of splenic TB include primary and secondary splenic TB. Secondary splenic TB is more common in clinical practice as part of miliary TB. It is often accompanied by tuberculous lesions in other parts of the body[4]. Primary splenic TB is rarer than secondary splenic TB in clinical practice. It is also known as isolated splenic TB, which means tuberculous lesions only in the spleen. Cases of isolated splenic TB accompanied by acquired immunodeficiency syndrome (AIDS), diabetes, or use of hormonal drugs are more commonly reported[6,7]. In our case, we identified isolated splenic TB in an immunocompetent patient, which is an infrequent finding.
Splenic TB is mainly characterized by fever, anorexia, body mass loss, and left upper abdominal pain[8]. In this case, the patient had nonspecific symptoms of TB, and only a feeling of general malaise. Splenic TB appears as four types on CT due to pathological changes and different courses of disease: Miliary, abscess, nodular, fibrosis, and nodular lesions are the most common in isolated splenic TB[9]. In this case, the type of splenic TB was nodular. Whether splenic TB is best treated surgically is still controversial. Xia et al[10] suggested that splenectomy is the only effective treatment for splenic TB, but Deng et al[11] maintained that it should be treated on a case-by-case basis. If there are lesions indicating tuberculous activity in other parts of the body, as in secondary splenic TB, systemic treatment should be recommended. The injury inherent in even careful surgery may cause TB to spread, but splenectomy should be considered in the following cases: (1) Giant tuberculous masses in the spleen; (2) splenic TB combined with abscess; (3) possibility of malignant tumor; (4) severe splenic hyperfunction; (5) esophageal varices or bleeding; (6) no reduction in splenic lesions after regular anti-TB treatment; and (7) pancreatic tail TB or abdominal abscess. Bi et al[12] suggested that splenectomy should be recommended for primary splenic TB. However, five cases of splenic TB recovered after simple anti-TB treatment, which indicates that splenectomy is not the only means of treating splenic TB, and standard anti-TB drug treatment often can achieve the desired effect. In recent years, with the improvement of the understanding of the spleen as an immune organ and the recognition of dangerous infection after splenectomy[13], greater requirements have been placed on the preservation of the spleen. Therefore, the diagnosis of splenic TB and regular medical treatment are particularly important. Some studies[14] have proposed different treatments according to different pathological types of splenic TB. It is considered that patients with miliary type should receive complementary conventional anti-TB treatment. For patients with necrotic TB, anti-TB drugs do not always work, and surgery should be performed. For patients who have TB with calcification, short-term anti-TB treatment can be implemented, followed by clinical observation. Splenic TB is usually a local manifestation of systemic TB, and the decision of whether to perform surgery should be made according to the principle of systemic treatment. If surgery is performed, regular anti-TB treatment should be administered before and after surgery[15]. Antituberculosis treatment should follow the principles of combined, appropriate, regular, full-time medication with a 6- to 9-mo course of treatment.
Because solitary TB is rare and the patient has no typical symptoms, CT-guided puncture biopsy is a reliable method to help to make a definite diagnosis. Although surgical splenectomy is a recommended method, anti-TB drug treatment alone may be help to achieve the goal of cure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arteaga-Livias K, Peru; Velmurugan R, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Zenteno-Cuevas R, Munro-Rojas D, Pérez-Martínez D, Fernandez-Morales E, Jimenez-Ruano AC, Montero H, Escobar L, de Igartua E, Trigos Á, Fuentes-Dominguez J. Genetic diversity and drug susceptibility of Mycobacterium tuberculosis in a city with a high prevalence of drug resistant tuberculosis from Southeast of Mexico. BMC Infect Dis. 2021;21:1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Pop M, Pop C, Homorodean D, Itu C, Man M, Goron M, Gherasim R, Coroiu G. Abdominal miliary tuberculosis in a patient with AIDS: a case report. Rom J Gastroenterol. 2003;12:231-234. [PubMed] |
| 3. | Lin SF, Zheng L, Zhou L. Solitary splenic tuberculosis: a case report and review of the literature. World J Surg Oncol. 2016;14:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Gupta A. Splenic tuberculosis: a comprehensive review of literature. Pol Przegl Chir. 2018;90:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect. 2018;7:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Vanhoenacker FM, De Backer AI, Op de BB, Maes M, Van Altena R, Van Beckevoort D, Kersemans P, De Schepper AM. Imaging of gastrointestinal and abdominal tuberculosis. Eur Radiol. 2004;14 Suppl 3:E103-E115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Kumar S, Pai AG, Tungenwar PN, Bhandarwar AH. Isolated primary tuberculosis of spleen-A rare entity in the immuno-competent patient. Int J Surg Case Rep. 2017;30:93-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Pottakkat B, Kumar A, Rastogi A, Krishnani N, Kapoor VK, Saxena R. Tuberculosis of the spleen as a cause of Fever of unknown origin and splenomegaly. Gut Liver. 2010;4:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Pereira JM, Madureira AJ, Vieira A, Ramos I. Abdominal tuberculosis: imaging features. Eur J Radiol. 2005;55:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Xia HS, Cao XF, Jiang HC. Modern Spleen Surgery. Jiangsu: Jiangsu Science and Technology Press, 2000: 91-93. [DOI] [Full Text] |
| 11. | Deng YS, Chen P, He ZP. Clinical characteristics and treatment of splenic tuberculosis. Hanjian Jibing Zazhi. 2003;10:14-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Bi YT, Wang YL, Hong X, Liang XW, Cao GQ, Cui SH. Clinical features of spleen tuberculosis. Chongqing Yixue Zazhi. 2003;32:858-860. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Altamura M, Caradonna L, Amati L, Pellegrino NM, Urgesi G, Miniello S. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol Immunotoxicol. 2001;23:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Luo SK, He WX, Wende Hong. Diagnosis and treatment of 13 cases of spleen tuberculosis. Zhonghua Neike Zazhi. 1996;35:832-833. [DOI] [Full Text] |
| 15. | Zhen YY, Tang D, Liang LJ. Diagnosis and treatment experience of spleen tuberculosis. New medicine. 2000;31:714-715. [DOI] [Full Text] |