Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10244
Peer-review started: May 23, 2022
First decision: June 16, 2022
Revised: July 1, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 129 Days and 5.5 Hours
Percutaneous coronary intervention (PCI) is extensively used to treat acute coron
An 81-year-old female presented with 3 d of lethargy and 1 d of dyspnea. On November 16, 2021, the patient developed a coma. Her oxygen saturation drop
The potential serious complications in patients undergoing PCI, especially the patients who are hemodynamically unstable and have severely calcified abdominal vessels, should all be cons
Core Tip: Acute mesenteric ischemia (AMI) is a life-threatening condition. The etiology is mostly the emboli from the heart or thrombosis of atherosclerotic vessel walls involving the superior mesenteric artery. To date, AMI after percutaneous coronary intervention (PCI) has rarely been reported. Here we report a case of AMI after PCI. For hemodynamically unstable patients with sudden abdominal pain after PCI, AMI should be considered.
- Citation: Ding P, Zhou Y, Long KL, Zhang S, Gao PY. Acute mesenteric ischemia due to percutaneous coronary intervention: A case report. World J Clin Cases 2022; 10(28): 10244-10251
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10244.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10244
Percutaneous coronary intervention (PCI) is currently indicated for the treatment of patients with acute coronary syndromes (ACS) and patients with stable angina pectoris who have failed to respond to optimal pharmacological therapy[1]. When patients present with ACS, approximately 60% will undergo PCI, with the remainder undergoing coronary artery bypass grafting or medication only. In these cases, invasive treatment may reduce myocardial infarction and death[2]. However, the potential complications of PCI treatment remain a concern.
Mesenteric ischemia (MI) is caused by insufficient blood flow to meet the metabolic demands of the internal organs. Although clinically rare, the mortality rate for acute attacks is 60% to 80%[3]. The types of MI include arterial thrombosis or embolism, mesenteric vein thrombosis, and non-occlusive me
The patient described in this report underwent urgent PCI due to possible ACS, but presented postoperatively with decreased bowel sounds, distended abdomen, and increased abdominal wall tension. The patient’s abdominal computed tomography (CT) findings showed extensive pneumatosis intestinalis within her hepatic portal system (part of the intestinal wall and mesenteric arteries), as well as extensive necrosis of the intestinal wall. Despite extensive treatment and close attention, the patient died. The purpose of this case report is to provide a reference for the clinical management of patients with acute intestinal ischemic necrosis resulting from PCI.
An 81-year-old woman attended a local hospital because of fatigue, lethargy, fever, chills, phlegm and wheezing.
The patient developed fatigue, lethargy, fever, chills, phlegm and wheezing three days before admission, but without treatment. On November 16, 2021, the patient developed a coma and was sent to the emergency room of our hospital. As her oxygen saturation dropped to 70%-80%, the patient was admitted to the intensive care units (ICUs) for further treatment after consultation. After admission to the ICU, the patient developed a progressive drop in blood pressure, requiring norepinephrine to maintain a mean arterial pressure > 65 mmHg.
The patient had a medical history of chronic obstructive pulmonary disease, and bronchial asthma for more than 50 years and received long-term home oxygen therapy. She had suffered from and chronic heart insufficiency for five years and was treated with oral bisoprolol. There was no history of abdominal pain and chronic renal failure.
The patient had no history of smoking or alcohol consumption. There was no relevant family history.
The patient's body temperature was 36 °C, the pulse was 85 beats/min, the respiratory rate was 18 breaths/min, and the blood pressure was 105/45 mmHg. Breath sounds were decreased in both lungs, and wet rales could be heard at the bottom of both lungs. Cardiac and abdominal examinations were unremarkable.
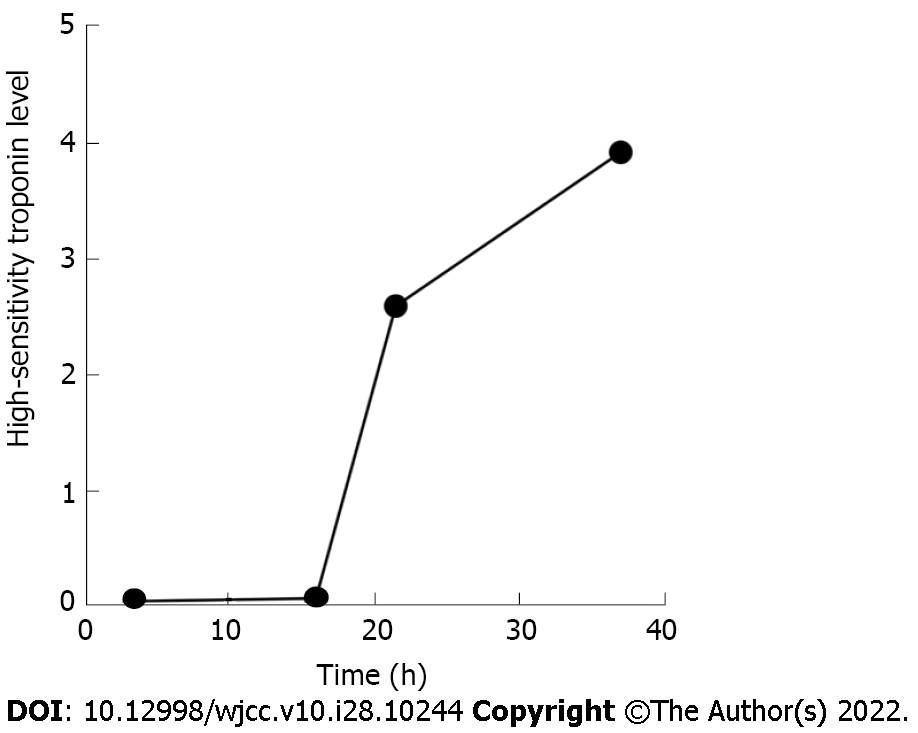
The initial troponin level was 0.05 ng/mL, and increased later (Figure 1). B-type natriuretic peptide was 4164 pg/mL. Results of arterial blood gas analysis were: Carbon dioxide partial pressure 58.7 mmHg, oxygen partial pressure 86.5 mmHg, and oxygenation index 172.97 mmHg; inflammatory indicators: White blood cell 8.12 × 109/L, neutrophil percentage 93.2%, C-reactive protein 298.38 mg/L, procalcitonin 6.26 ng/mL, and interleukin-6 > 5000 pg/mL. The polymerase chain reaction-severe acute respiratory syndrome coronavirus 2 test and multiple blood cultures were negative. Other blood tests, fecal examinations, and coagulation function were all normal.
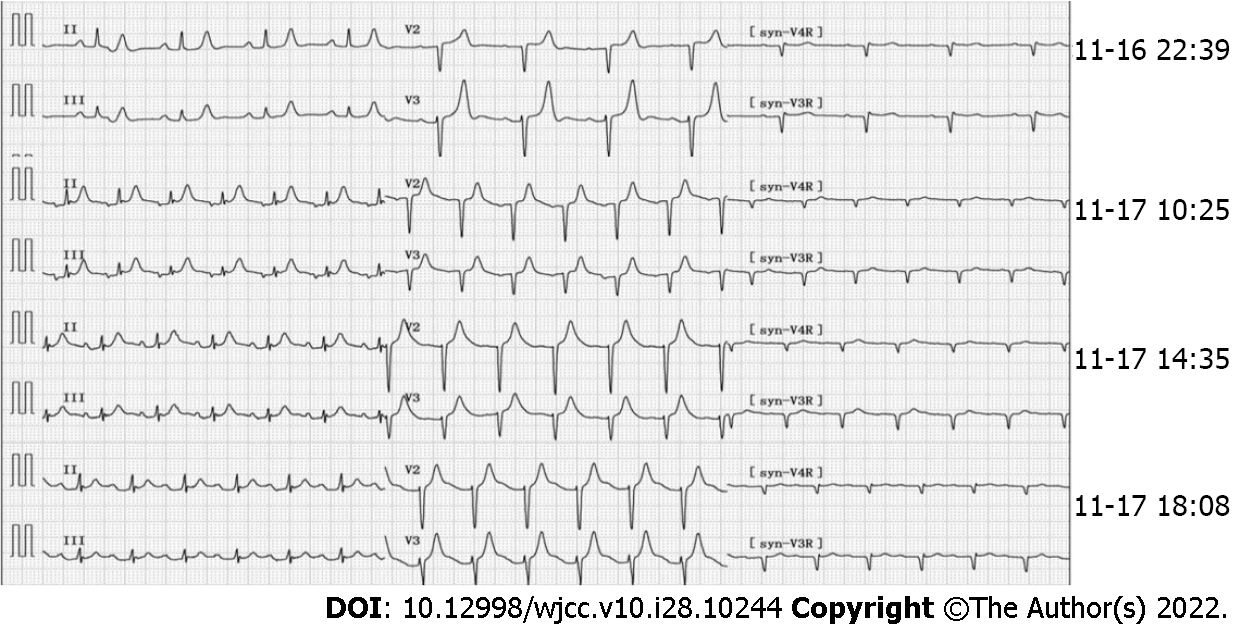
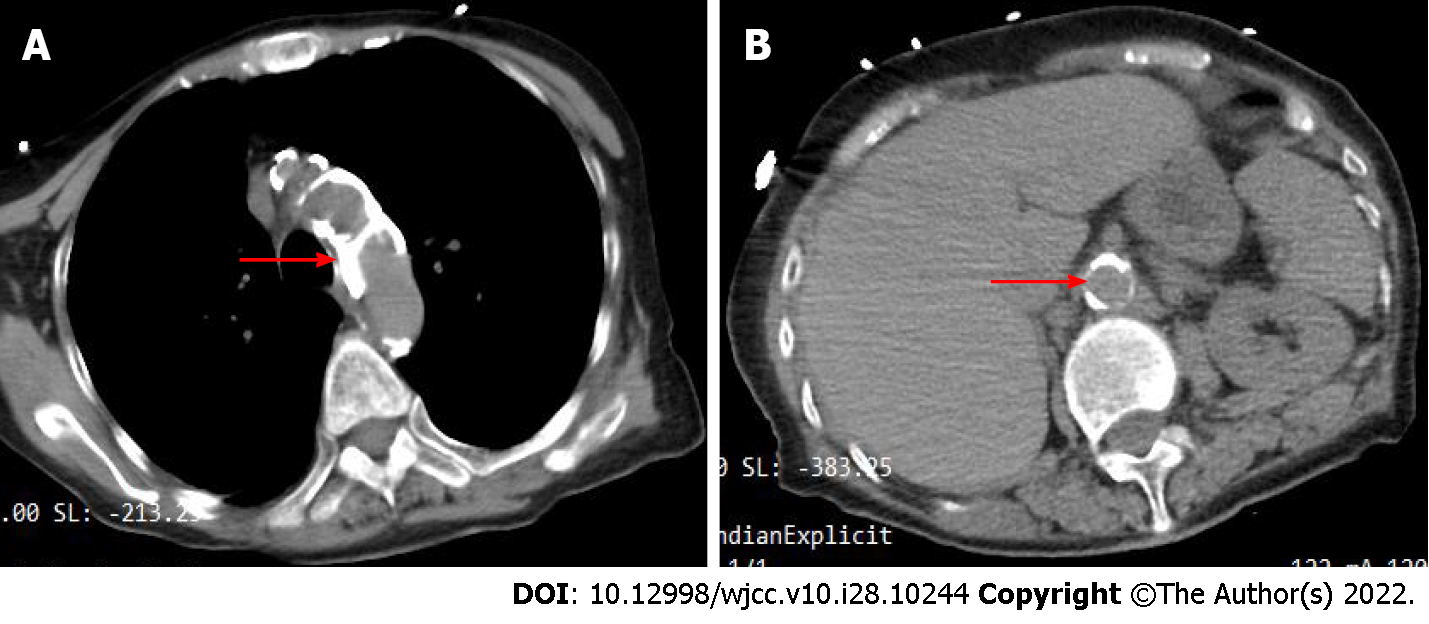
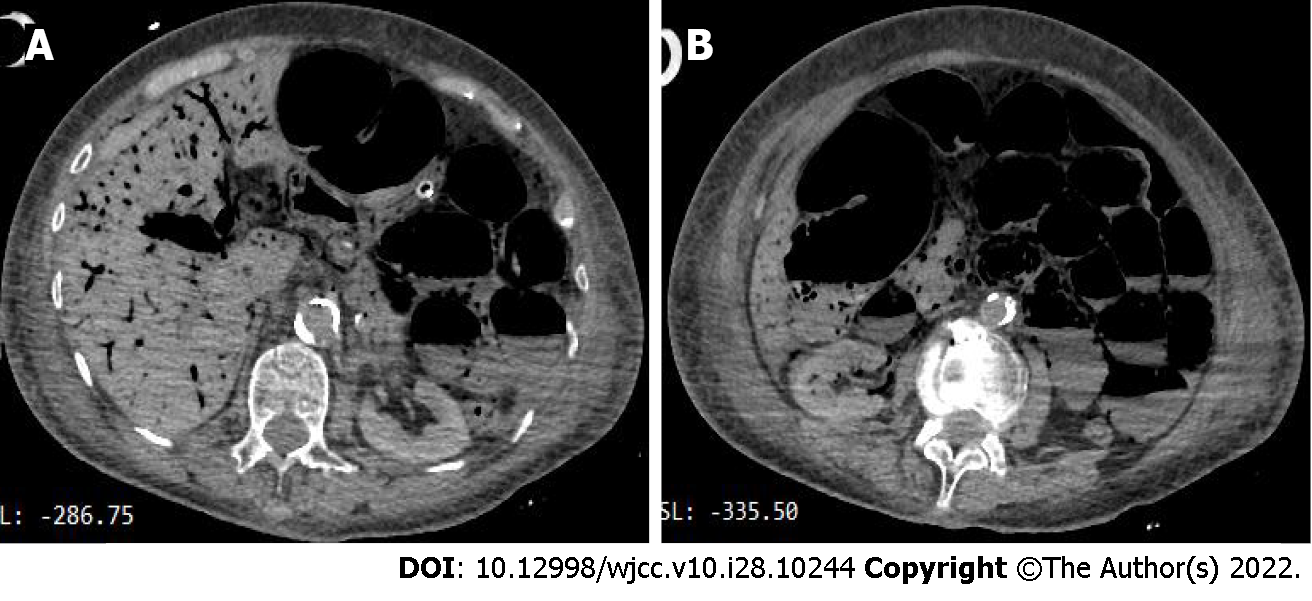
The initial electrocardiogram (ECG) findings: Sinus rhythm, T wave was high and sharp; R wave: V1-V4 progression was poor, V1 was Qr type, and subsequent re-examinations revealed ST-segment elevation (> 0.05 mv) (Figure 2). Echocardiography suggested left atrial enlargement, segmental abnormal motion of the left ventricular wall, left ventricular apical bulge (considered to be ventricular aneurysm formation), decreased left ventricular diastolic function, and lower limit of normal systolic function (LVEF) was 51%. Chest CT showed chronic bronchitis, emphysema, multiple infections in both lungs (mostly in the middle and lower lobe of the right lung), right pleural cavity small effusion, and obvious calcification of aortic arch and coronary artery. An abdominal CT scan (Figure 3) showed no abnormal changes, but obvious abdominal aorta calcification.
AMI and septic shock.
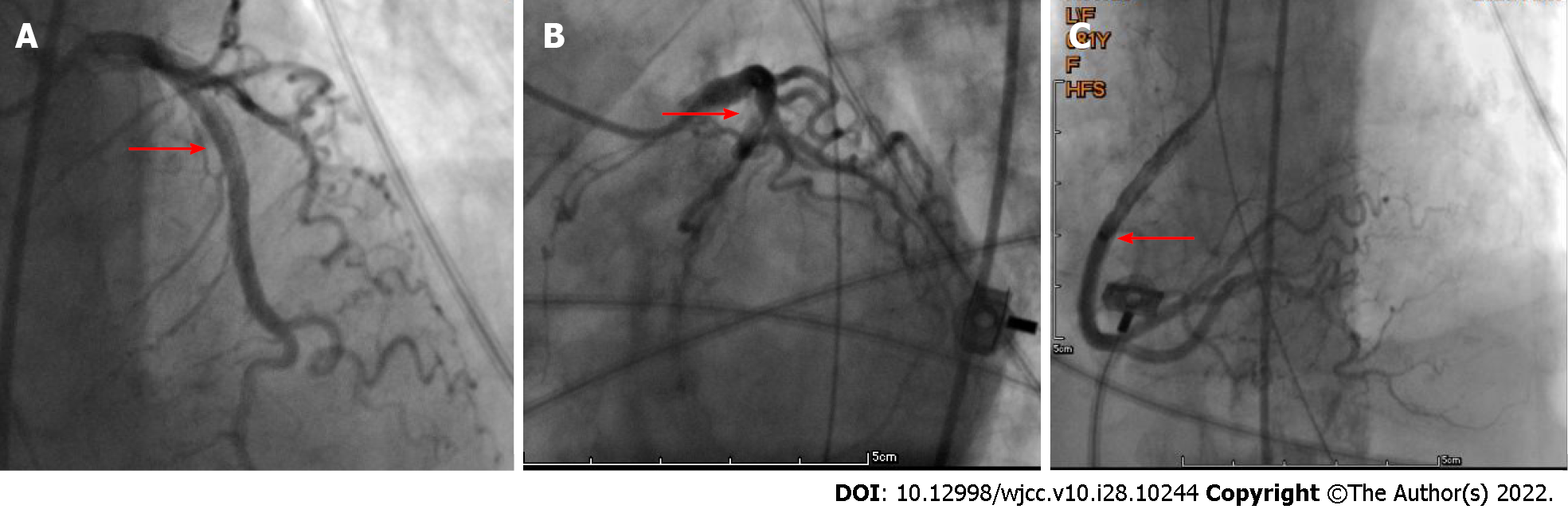
Following admission, the patient received assisted ventilation, vasoactive drugs to maintain blood pressure (the maximum dose of norepinephrine was 1 μg/kg, milrinone and amiodarone to control heart rate, as well as anti-infection and other treatments. However, it was impossible to determine whether she had chest pain as the patient was in a coma. The cardiologist assumed that the patient had developed ACS; therefore, she was scheduled for PCI. On the second day of admission (November 17, 2021), the patient underwent PCI via the left femoral artery approach, and the results showed (Figure 4) that there were no significant abnormalities in the left and right coronary arteries. On the second postoperative day (November 18, 2021), the patient developed abdominal distension and decreased bowel sounds (about 1-2/min) and, given that she was elderly, she was considered to have constipation and was prescribed a glycerin enema. On the 4th day (November 20, 2021), the patient suddenly lost consciousness, her blood pressure dropped, she developed abdominal wall swelling and increased tension, and bowel sounds disappeared. An urgent abdominal CT scan showed (Figure 5) extensive pneumatosis intestinalis within her hepatic portal system (part of the intestinal wall and mesenteric arteries), with a high probability of extensive intestinal wall necrosis.
The patient’s condition failed to reverse. Eventually, she died on day 5 after PCI due to refractory shock.
As the spectrum of indications for PCI continues to expand, directly performing PCI is the preferred reperfusion strategy within 12 h of the onset of symptoms in patients with ST-elevation myocardial infarction, in accordance with the clinical guidelines[5]. However, clinicians should be aware of the challenges associated with PCI. Common complications following PCI include coronary artery coarctation, coronary artery perforation, air embolism, coronary artery spasm, pseudo lesion formation, intramural hematoma, arterial thrombosis, and hemodynamic collapse[6,7]. Therefore, it is important for physicians to quickly identify complications and develop strategies to overcome them to minimize the risk of patient injury.
Although AMI is rare, when it occurs, patients are more likely to die. The pathophysiological mechanism of AMI is associated with insufficient or interrupted blood supply to the mesenteric arteries. This can be caused by arterial occlusion due to the emboli originating from the heart or acute thromboembolism secondary to atherosclerosis. AMI can be induced by any procedure involving vascular manipulation, even unintentionally, and may be induced when an atherosclerotic plaque is removed by coronary artery angiography[8]. Interventional therapy through the femoral artery approach may increase the risk of AMI, especially in patients with severe arterial calcification. Another risk is mesenteric vein occlusion, which is associated with thrombosis of the venous system. In addition, there is NOMI caused by severe splanchnic vasoconstriction. NOMI presumably is severe cerebral hypoperfusion caused by low cardiac output, the use of vasopressors and potential atherosclerosis. The key pathological mechanisms involved in the development of NOMI represent an exaggerated normal physiological response to maintain perfusion of vital organs at the expense of mesenteric perfusion. The development of persistent mesenteric vasoconstriction leads to a mismatch between intestinal supply and demand due to reduced intestinal blood flow and oxygen delivery, particularly to the fragile superficial mucosa. This mismatch can be exacerbated by increased intra-abdominal pressure, enteral nutrition, and the use of certain vasoactive drugs, ultimately leading to intestinal ischemia[9]. At the present time, there is no specific Lab test which can identify AMI. But evidence of hemoconcentration, elevated white cell count, high anion gap lactic acidosis and positive D dimer test (fibrinolytic marker) in the presence severe abdominal pain disproportionate to physical sign may indicate AMI[10]. We review published clinical studies related to AMI in recent years and summarize the types of studies, number of cases, characteristics and risk factors, and mortality (Table 1).
| No. | Ref. | Year | Type of study | Number of cases | Characteristics or risk factors | Mortality |
| 1 | Piton et al[13] | 2022 | Randomized controlled study | 24 | EN, dobutamine use, SAPS II score ≥ 62 and hemoglobin ≤ 10.9 g/dL | 58% |
| 2 | Del Val et al[14] | 2021 | Case report | 1 | Transfemoral interventional therapy, Vascular Calcification | 100% |
| 3 | Lareyre et al[15] | 2021 | Retrospective study | 86 | Vascular calcifications in the superior mesenteric artery | 63.90% |
| 4 | Sun et al[16] | 2020 | Retrospective study | 58 | APACHE II score and leukocytosis | 12.10% |
| 5 | Renaudier et al[17] | 2020 | Retrospective study | 38 | Renal replacement therapy and onset of a second shock within the first five days | 100% |
| 6 | Deng et al[18] | 2017 | Systematic review and meta-analysis | 547 | Age, cardiac shock, peripheral vascular disease, emergency surgery, atrial fibrillation, CK-MB level, serum creatinine > 200 μmol/L, blood loss, prolonged ventilation, need for IABP, inotropic treatment, blood transfusion, reoperation | 66.7%-94% |
| 7 | Guillaume et al[19] | 2017 | Retrospective observational study | 33 | Coronary artery bypass graft, need for blood transfusion during cardiopulmonary bypass, aspartate aminotransferase at least 100 UI/L, and Simplified Acute Physiology Score II at least 50 at ICU admission | 64%-83% |
| 8 | Mothes et al[20] | 2016 | Retrospective case-control study | 108 | The use of norepinephrine, epinephrine, and serum lactate levels > 3 mmol/L | 68% |
In our case, the patient received active therapy, and the shock was significantly improved, and blood pressure gradually stabilized. However, the serum troponin concentration of the patient continued to increase progressively, and bedside ultrasonography revealed left ventricular aneurysm, but no evidence of right ventricular enlargement. ECG indicated dynamic changes of ST segment and no atrial fibrillation was found. Therefore, the patient received PCI via femoral artery approach. But developed symptoms such as diminished bowel sounds and abdominal distension afterwards, and the dose of norepinephrine was increased [from 0.2 μg/(kg·min) to 0.8 μg/(kg·min)]. Due to her tracheal intubation status and past medical history of dementia, the patient was unable to describe her pain. Moreover, the use of analgesic medication trivialized the patient’s condition further, until abdominal CT showed hepatic portal gas and intestinal necrosis. As the patient died within a short period of time and no abdominal angiography was performed, it is difficult to determine the type of AMI in this patient. However, the patient was found to have extensive arterial calcification, and received PCI via the femoral artery approach. Therefore, the occurrence of AMI may be related to the arterial occlusion due to the shedding of calcified plaques during the interventional operation. In addition, although the patient's early shock was corrected, low-dose norepinephrine should be maintained. In case of hemodynamic instability, PCI may aggravate the AMI. Accordingly, any negative changes in the patient’s physiology, including new onset of organ failure, increase in vasoactive support and nutrition intolerance should raise the suspicion of AMI[11]. A retrospective study of 213 patients with AMI showed that the 30-d ICU mortality rate was 71%, the maximum dose of vasoactive drugs and arterial delta lactate were associated with poor prognosis, and anticoagulation therapy was associated with better survival[12]. Therefore, it is very important to detect patients at high risk of AMI as early as possible, and to provide timely diagnosis and treatment.
However, with the aim of offering some guidance and reference if such cases occur in the future, despite the sudden onset and short course of the disease in this patient, we believe our observations could be convincing if more randomized control trials were conducted as appropriate.
When PCI is performed in patients, especially in those who are hemodynamically unstable and have severe calcification of abdominal blood vessels, it is important to consider the serious complications of PCI. The possibility of AMI should be considered and investigated in all cases of unexplained abdominal pain after PCI.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mestrovic A, Croatia; Popovic DD, Serbia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Giannini F, Candilio L, Mitomo S, Ruparelia N, Chieffo A, Baldetti L, Ponticelli F, Latib A, Colombo A. A Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2018;11:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Banning A, Redwood S. Percutaneous coronary intervention for stable angina in ORBITA. Lancet. 2018;392:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Clair DG, Beach JM. Mesenteric Ischemia. N Engl J Med. 2016;374:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 329] [Article Influence: 36.6] [Reference Citation Analysis (1)] |
| 4. | Sakamoto T, Kubota T, Funakoshi H, Lefor AK. Multidisciplinary management of acute mesenteric ischemia: Surgery and endovascular intervention. World J Gastrointest Surg. 2021;13:806-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70:1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Kandan SR, Johnson TW. Management of percutaneous coronary intervention complications. Heart. 2019;105:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Doll JA, Hira RS, Kearney KE, Kandzari DE, Riley RF, Marso SP, Grantham JA, Thompson CA, McCabe JM, Karmpaliotis D, Kirtane AJ, Lombardi W. Management of Percutaneous Coronary Intervention Complications: Algorithms From the 2018 and 2019 Seattle Percutaneous Coronary Intervention Complications Conference. Circ Cardiovasc Interv. 2020;13:e008962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 8. | Tilsed JV, Casamassima A, Kurihara H, Mariani D, Martinez I, Pereira J, Ponchietti L, Shamiyeh A, Al-Ayoubi F, Barco LA, Ceolin M, D'Almeida AJ, Hilario S, Olavarria AL, Ozmen MM, Pinheiro LF, Poeze M, Triantos G, Fuentes FT, Sierra SU, Soreide K, Yanar H. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42:253-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Al-Diery H, Phillips A, Evennett N, Pandanaboyana S, Gilham M, Windsor JA. The Pathogenesis of Nonocclusive Mesenteric Ischemia: Implications for Research and Clinical Practice. J Intensive Care Med. 2019;34:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Ahmed M. Ischemic bowel disease in 2021. World J Gastroenterol. 2021;27:4746-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (4)] |
| 11. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 12. | Caluwaerts M, Castanares-Zapatero D, Laterre PF, Hantson P. Prognostic factors of acute mesenteric ischemia in ICU patients. BMC Gastroenterol. 2019;19:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 13. | Piton G, Le Gouge A, Boisramé-Helms J, Anguel N, Argaud L, Asfar P, Botoc V, Bretagnol A, Brisard L, Bui HN, Canet E, Chatelier D, Chauvelot L, Darmon M, Das V, Devaquet J, Djibré M, Ganster F, Garrouste-Orgeas M, Gaudry S, Gontier O, Groyer S, Guidet B, Herbrecht JE, Hourmant Y, Lacherade JC, Letocart P, Martino F, Maxime V, Mercier E, Mira JP, Nseir S, Quenot JP, Richecoeur J, Rigaud JP, Roux D, Schnell D, Schwebel C, Silva D, Sirodot M, Souweine B, Thieulot-Rolin N, Tinturier F, Tirot P, Thévenin D, Thiéry G, Lascarrou JB, Reignier J; Clinical Research in Intensive Care and Sepsis (CRICS) group. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: a post hoc analysis of the NUTRIREA2 trial. Intensive Care Med. 2022;48:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Del Val D, Rivero F, Cuesta J, Diego G, Antuña P, Alfonso F. Fatal acute mesenteric ischaemia following transcatheter aortic valve replacement. EuroIntervention. 2021;17:588-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lareyre F, Augène E, Massalou D, Chikande J, Guidi L, Jean-Baptiste E, Hassen-Khodja R, Raffort J. Vascular Calcifications are Associated with Increased Mortality in Patients with Acute Mesenteric Ischemia. Ann Vasc Surg. 2021;72:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Sun SL, Wang XY, Chu CN, Liu BC, Li QR, Ding WW. Predictors of irreversible intestinal resection in patients with acute mesenteric venous thrombosis. World J Gastroenterol. 2020;26:3625-3637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Renaudier M, de Roux Q, Bougouin W, Boccara J, Dubost B, Attias A, Fiore A, de'Angelis N, Folliguet T, Mulé S, Amiot A, Langeron O, Mongardon N. Acute mesenteric ischaemia in refractory shock on veno-arterial extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2020;10:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Deng QW, Tan WC, Zhao BC, Deng WT, Xu M, Liu WF, Liu KX. Risk factors for postoperative acute mesenteric ischemia among adult patients undergoing cardiac surgery: A systematic review and meta-analysis. J Crit Care. 2017;42:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Guillaume A, Pili-Floury S, Chocron S, Delabrousse E, De Parseval B, Koch S, Samain E, Capellier G, Piton G. Acute Mesenteric Ischemia Among Postcardiac Surgery Patients Presenting with Multiple Organ Failure. Shock. 2017;47:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Mothes H, Koeppen J, Bayer O, Richter M, Kabisch B, Schwarzkopf D, Hein HA, Zanow J, Doenst T, Settmacher U. Acute mesenteric ischemia following cardiovascular surgery--A nested case-control study. Int J Surg. 2016;26:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |