Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10214
Peer-review started: April 21, 2022
First decision: May 30, 2022
Revised: June 9, 2022
Accepted: August 23, 2022
Article in press: August 23, 2022
Published online: October 6, 2022
Processing time: 158 Days and 20.9 Hours
Twin reversed arterial perfusion (TRAP) sequence is an extremely rare congenital anomaly in monochorionic (MC) twins. The condition is characterized by a malformed fetus (acardiac twin) without cardiac activities being perfused by a structurally normal one (pump twin) via an artery-to-artery anastomosis in a reverse direction.
We described the first case of TRAP to receive laser surgery in Vietnam. The 26-wk pregnancy was originally misdiagnosed in another hospital as MC twins with single intrauterine fetal death. Following admission to our center, the diagnosis was amended to a 26-wk TRAP sequence stage IIb. The acardiac twin was 7.5 cm at the longest length, the ratio of the weight of the acardiac twin to the weight of the pump twin was more than 90%, the pump twin showed fetal distress with absent diastolic flow in umbilical artery of pump twin, and the peak systolic velocity in the middle cerebral artery = 1.6 MoM. We performed emergency laser photocoagulation of the acardiac twin’s umbilical cord. After surgery, we suc
TRAP should be appropriately diagnosed and treated early to avoid complications of the pump twin. Fetoscopic laser photocoagulation is a new and effective treatment for this condition.
Core Tip: It is an easy mistake to misdiagnose twin reversed arterial perfusion as a single intrauterine fetal death, especially in our case, as the acardiac twin has a head, body, and full limbs. Intrauterine fetal intervention is necessary when the pump twin shows signs of fetal distress. After surgery, the problem of premature rupture of membranes and delivery should be noted. The indication to terminate the pregnancy depends on the mother’s condition, fetal condition, and the acardiac twin’s longest size.
- Citation: Anh ND, Thu Ha NT, Sim NT, Toan NK, Thuong PTH, Duc NM. Twin reversed arterial perfusion sequence-a rare and dangerous complication form of monochorionic twins: A case report. World J Clin Cases 2022; 10(28): 10214-10219
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10214.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10214
Twin reversed arterial perfusion (TRAP) sequence only occurs in monochorionic (MC) twins and has a bad prognosis. The incidence is 1/35000 pregnancies and 1/100 MC twins[1]. In this condition, one fetus has partial or complete lack of cardiac development (called an “acardiac twin”) and is perfused with blood by a normal co-twin (called a “pump twin”) through abnormal vascular anastomosis connections. As a result, the pump twin shows signs of anemia, heart failure, hydrops fetalis, and the risk of stillbirth. The pump twin has a mortality rate of up to 55%[2-4]. The acardiac twin receives blood from the pump twin, so it continues to grow even larger than the normal co-twin, and postpartum mortality is 100%[2,5]. Early diagnosis by Doppler examination of the umbilical arteries of the two fetuses is necessary for appropriate antenatal management and treatment planning[6,7]. Today, many countries worldwide have successfully intervened in the amniotic cavity to treat TRAP complications, saving 80% of pump twin's lives[6,7].
This report describes the first TRAP sequence case with successful fetal intervention by intrauterine laser therapy in Vietnam.
A 28-year-old pregnant woman was transferred to our fetal medicine center because of polyhydramnios and was diagnosed with stage IIb TRAP sequence from ultrasound images.
This patient at 26 wk of gestation was diagnosed with a MC diamniotic (MCDA) twin with single intrauterine fetal death (sIUD) in another hospital.
Medical records were normal.
There was no personal or family history of other diseases.
Physical examination was not special.
Blood tests at presentation was in normal range.
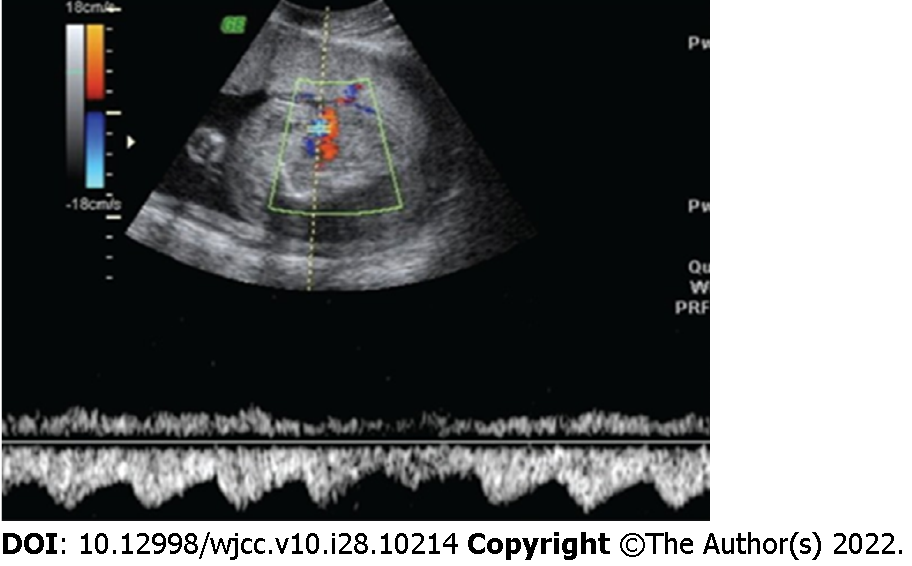
The pump twin had normal morphology with a three-vessel umbilical cord, polyhydramnios, a maximum vertical pocket (MVP) of 100 mm and an estimated weight of 700 g. Doppler signs of anemia were RI = 1, absent diastolic flow in umbilical artery of pump twin, peak systolic velocity in the middle cerebral artery = 1.6 MoM. The acardiac twin mass was edematous in the whole body; its brain and heart structures were not observed, but it had a torso, four limbs, and a two-vessel umbilical cord. The acardiac twin’s longest length was 7.5 cm and it had an estimated weight of 650 g following formula has been proposed to estimate the weight of acardiac fetus: Weight (g) = 1.2 × (longest length in cm)2 – (1.7 × longest length in cm) of Moore et al[8]. The ratio of the weight of the acardiac twin to the weight of the pump twin was over 90%. Doppler imaging showed reversed blood flow from the pump twin to the acardiac twin (Figure 1).
MC twins with stage IIb TRAP sequence.
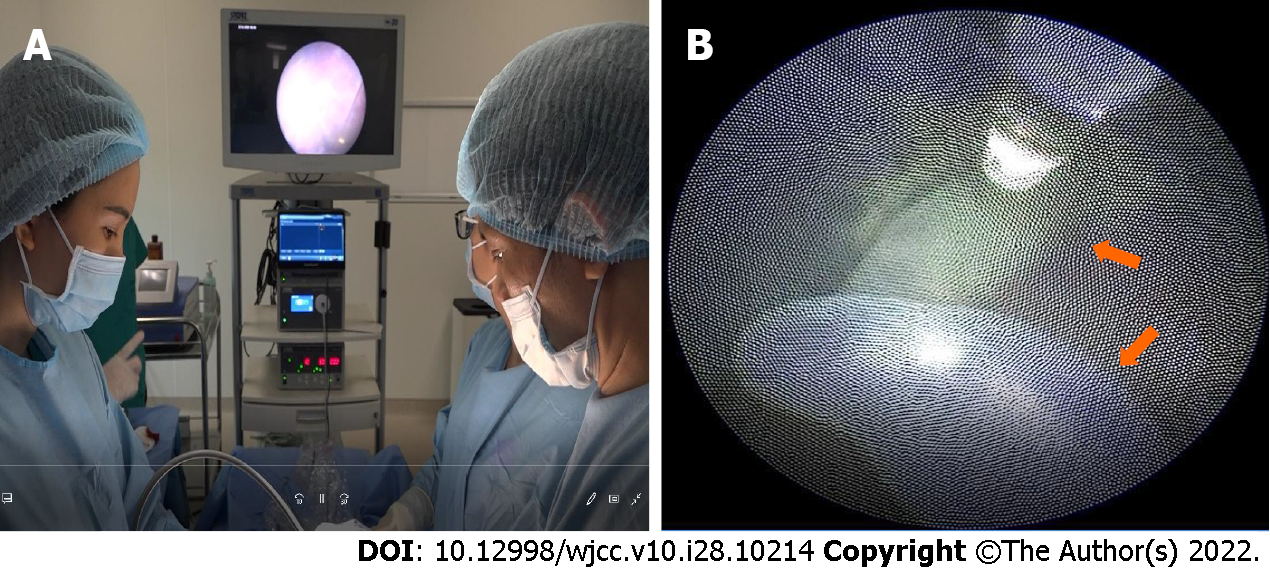
The patient was consulted and indicated for emergency surgery of the uterus, using laser photocoagulation to coagulate the umbilical cord of the acardiac twin and block the circulation from the pump twin to the acardiac twin (Figure 2). Combining general anesthesia (intravenous sedation) and local anesthesia was used for the anesthesia procedure (using Lidocaine 1 percent). The procedure was carried out in a chamber designated specifically for fetal surgery. For cord occlusion, a diode laser with a 600 fiber was utilized. The fetoscopic Image IS 4U by Karl Storz (Tuttlingen, Germany), the Medilas D MultiBeam by Dornier, and the Laser Light Guide by Dornier Medtech were utilized. Under ultrasound guidance, the Seldinger method was utilized to access the polyhydramnios cavity. The scope had a 2 mm diameter. The acardiac twin’s umbilical cord was reduced using laser photocoagulation. Under direct view, the umbilical cord vessels were ablated; full blockage required power levels between 50 and 60 W. When the whole umbilical cord blood artery was blocked in the acardiac twin, the procedure was deemed successful. The lack of umbilical cord flow at the ablation location as shown by a doppler ultrasonography examination served as proof of that. Then the MVP was emptied until it was within the typical range (MVP 5 or 6 cm).
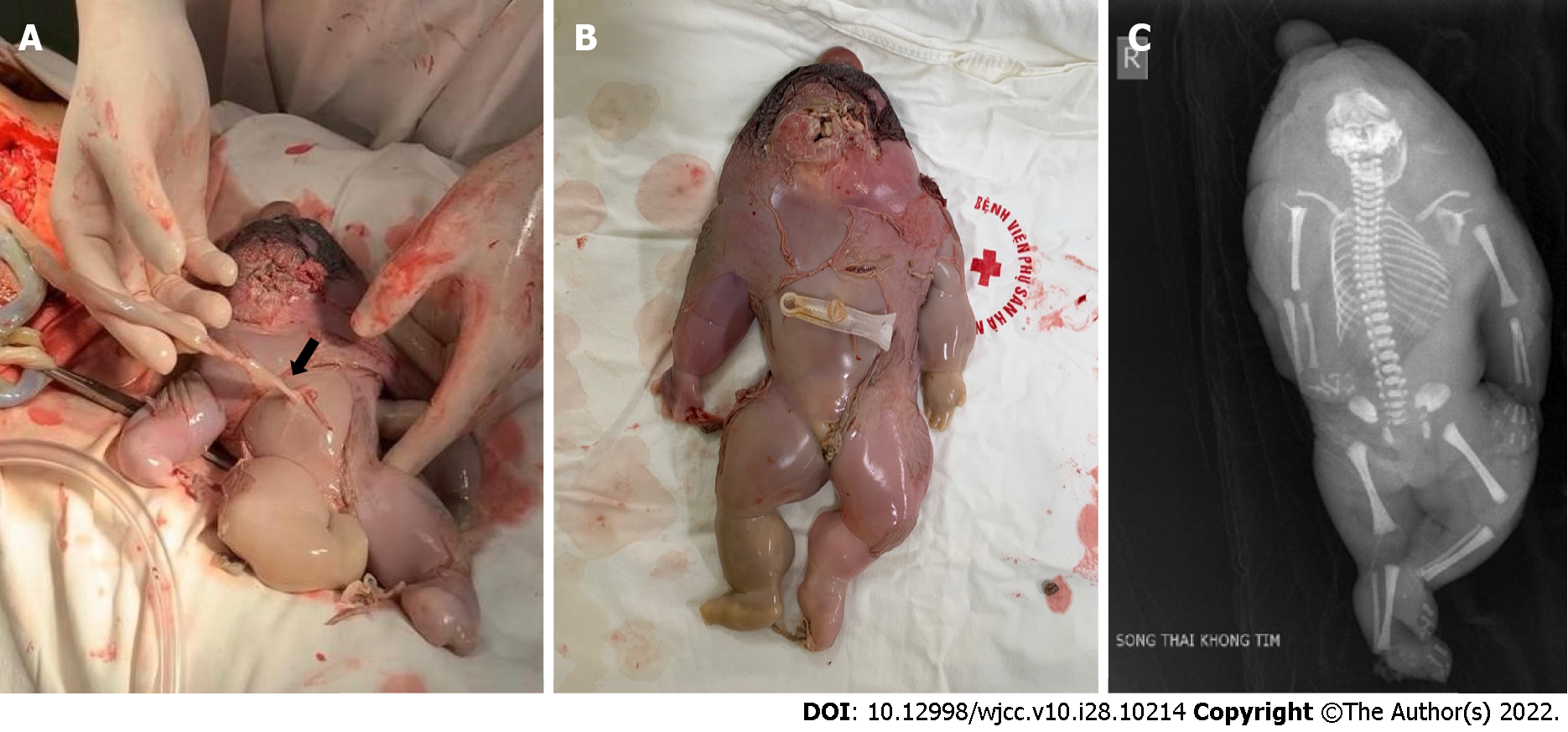
Following surgery, expectant mothers underwent examinations, ultrasounds, and corticosteroid medication at 28 wk of gestation, 48 h, 7 d, and every 2 wk until delivery. Continued monitoring did not show any abnormal signs. After 8 wk and at 34 wk pregnant, the patient suffered from a ruptured membrane and had emergency surgery at Hanoi Obstetrics and Gynecology Hospital to remove the female baby (1400 g, apgar 8-9). The acardiac twin had edema, a small head, torso, all limbs, a weight of 780 g, and normal chromosomes (Figure 3).
An uncommon problem with MC twins is a sequence known as TRAP. The actual etiology, phy
Due to the development of ultrasound, TRAP syndrome can be diagnosed as early as 11 wk of gestation[7]. The characteristic ultrasound sign that helps diagnose TRAP syndrome is MC twins with one morphologically normal fetus and one structurally highly abnormal fetus without a heart or cardiac activity. Doppler imaging of the umbilical artery shows reverse arterial blood flow to the acardiac twin[6,7,10,11]. Our case was diagnosed late due to being previously misdiagnosed as MCDA twins with sIUD. It was an easy mistake to misdiagnose TRAP, especially in our clinical case, as the acardiac twin had a complete structure, including the head, torso, four limbs, and no fetal cardiac activity. The acardiac twin in our case was of the acardius anceps type, accounting for approximately only 10% of TRAP cases (Figure 3)[12]. Therefore, it is essential in clinical practice to closely monitor twins to diagnose the number of placentas, especially in the first trimester. Both MC twin fetuses should be checked for fetal structure, cardiac activity and monitored for size progression. Furthermore, caution should be exercised in diagnosing stillbirth when the stillbirth mass is still increasing in size. Doppler ultrasound was deemed essential in MC twins to detect abnormalities and for early diagnosis of TRAP syndrome.
The survival of the pump twin and achieving term for delivery are the major objectives in the management of the TRAP sequence[11]. Indications for treatment, including pregnancy management and surgical intervention, depend on the condition of the pump twin[11]. Wong and Sepulveda[11] proposed a classification to help predict the management of the acardiac twin. In our case, despite the delayed diagnosis, it was fortunate that the pump twin had not previously shown signs of anemia or fetal distress. The two fetuses could still develop in the uterus[11]. Fortunately, the patient transferred to our center was diagnosed with TRAP sequence stage IIB, according to Wong and Sepulveda's classification[11]. The pump twin showed signs of anemia and fetal distress, requiring timely intervention. In the following years, the acardius's umbilical cord ablation was identified as a principal target of therapeutic techniques. The most popular methods for treating TRAP sequence appear to be intrauterine laser therapy and radiofrequency ablation, which are both safe and dependable. Our case was treated with laser photocoagulation of the acardiac twin's umbilical cord.
Following surgery, we maintained the pregnancy for a further 8 wk for delivery at 34 wk. Premature rupture of membranes has been one of the most common complications following preterm surgery because laparoscopic laser amniotomy is an interventional procedure. Therefore, it is important to pay attention to treating threatened preterm labor after surgery to increase its success rate. In our case, maintaining the pregnancy for a further 8 wk and increasing the gestational age to 34 wk improved the newborn’s survival odds. An elective cesarean section was performed in our clinical case, due to the enormous cardiac fetus, with a diameter of 12 cm at the widest point. Removing the heartless fetus from the uterus was more problematic, despite the elective cesarean section. The indication for pregnancy termination by cesarean section or vaginal delivery depended not only on the mother and fetus’ situation but also on the size of the non-cardiac mass. There had been cases of threatened uterine rupture during vaginal deliveries due to large cardiac fetus size[4-6,11]. Thus, the prognosis of delivery in the TRAP sequence depended on the mother's condition, the pump twin, and the maximum diameter of the acardiac twin.
TRAP is diagnosed using ultrasound; it is necessary to differentiate it from malformation or sIUD, especially when of the acardius anceps type. Furthermore, it is vital to carefully monitor both fetuses with a weekly Doppler ultrasound to detect complications early, especially signs of heart failure and anemia in the pump twin. If the fetus has signs of poor prognosis requiring interventional treatment, it should be transferred to a fetal intervention center for treatment. The delivery prognosis is based on three factors: The mother’s condition, the pump twin’s condition, and the acardiac twin's size.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Viet Nam
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aldiansyah D, Indonesia; Singhal A, India S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Hecher K, Ville Y, Nicolaides KH. Color Doppler ultrasonography in the identification of communicating vessels in twin-twin transfusion syndrome and acardiac twins. J Ultrasound Med. 1995;14:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Emery SP, Bahtiyar MO, Dashe JS, Wilkins-Haug LE, Johnson A, Paek BW, Moon-Grady AJ, Skupski DW, O'Brien BM, Harman CR, Simpson LL. The North American Fetal Therapy Network Consensus Statement: prenatal management of uncomplicated monochorionic gestations. Obstet Gynecol. 2015;125:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Van Allen MI, Smith DW, Shepard TH. Twin reversed arterial perfusion (TRAP) sequence: a study of 14 twin pregnancies with acardius. Semin Perinatol. 1983;7:285-293. [PubMed] |
| 4. | Barré M, Le Vaillant C, Boog G, Joubert M, Winer N, Philippe H. [Acardiac twins: pronostics markers' study]. Gynecol Obstet Fertil. 2012;40:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Bharadwaj MK, Mohana Priya N. A rare complication of monochorionic twin pregnancy: Twin-reversed arterial perfusion (TRAP) sequence. Med J Armed Forces India. 2015;71:S114-S115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Pagani G, D'Antonio F, Khalil A, Papageorghiou A, Bhide A, Thilaganathan B. Intrafetal laser treatment for twin reversed arterial perfusion sequence: cohort study and meta-analysis. Ultrasound Obstet Gynecol. 2013;42:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Bornstein E, Monteagudo A, Dong R, Schwartz N, Timor-Tritsch IE. Detection of twin reversed arterial perfusion sequence at the time of first-trimester screening: the added value of 3-dimensional volume and color Doppler sonography. J Ultrasound Med. 2008;27:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 257] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Peyvandi S, Feldstein VA, Hirose S, Rand L, Brook MM, Moon-Grady AJ. Twin-reversed arterial perfusion sequence associated with decreased fetal cerebral vascular impedance. Ultrasound Obstet Gynecol. 2015;45:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Sepúlveda WH, Quiroz VH, Giuliano A, Henríquez R. Prenatal ultrasonographic diagnosis of acardiac twin. J Perinat Med. 1993;21:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Wong AE, Sepulveda W. Acardiac anomaly: current issues in prenatal assessment and treatment. Prenat Diagn. 2005;25:796-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Napolitani FD, Schreiber I. The acardiac monster. A review of the world literature and presentation of 2 cases. Am J Obstet Gynecol. 1960;80:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 1.5] [Reference Citation Analysis (0)] |