Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10193
Peer-review started: April 9, 2022
First decision: June 16, 2022
Revised: June 24, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 167 Days and 4.5 Hours
Extranodal natural killer/T cell lymphoma, nasal type (ENKL) is a highly aggressive malignancy characterized by its association with Epstein-Barr virus (EBV) and extranodal involvement, which shows a poor clinical outcome. Although L-asparaginase-based chemotherapy has improved the response rates of relapsed/refractory (R/R) ENKL, relapse occurs in up to 50% of patients with disseminated disease.
Immune evasion has emerged as a critical pathway for survival in ENKL and may be effectuated via STAT3-driven upregulation of programmed cell death ligand 1 (PD-L1) or other molecular pathways. Anti-PD-1 is effective for R/R ENKL with EBV-driven upregulation of PD-L1 expression. Anti-PD-1 combined with decitabine showed positive preliminary results in a patient with R/R ENKL and resistance to anti-PD-1.
The treatment experience, in this case, demonstrated the potential ability of decitabine combined with PD-1 inhibitor to treat R/R ENKL, thus providing a new treatment strategy for this tumor.
Core Tip: Extranodal natural killer/T cell lymphoma nasal type is a highly aggressive malignancy characterized by its association with Epstein-Barr virus and extranodal involvement, which shows a poor clinical outcome. Now, we report a rare case of relapsed/refractory classic Hodgkin lymphoma with resistance to anti-PD-1 in which anti-PD-1 combined with decitabine showed positive preliminary results. Our findings support the potential benefit of anti-PD-1 combined with decitabine in this type of refractory T-cell lymphoma.
- Citation: Li LJ, Zhang JY. Treatment of refractory/relapsed extranodal NK/T cell lymphoma with decitabine plus anti-PD-1: A case report. World J Clin Cases 2022; 10(28): 10193-10200
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10193.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10193
Extranodal natural killer/T cell lymphoma (ENKL) is a rare T-cell lymphoma with a poor prognosis and poor response to conventional chemotherapy. ENKL is more prevalent in China than in Western countries[1,2]. It is a highly aggressive lymphoma with poor efficacy and prognosis by traditional treatments[3,4]. The treatment options for relapsed/refractory (R/R) ENKL are still limited, and improving the treatment is an urgent requirement. Some studies have shown that ENKL cells avoid immune surveillance and the consequent killing of ENKL, resulting in a poor outcome[5].
A 54-year-old male patient was admitted to our hospital with a diagnosis of ENKL for > 4 years.
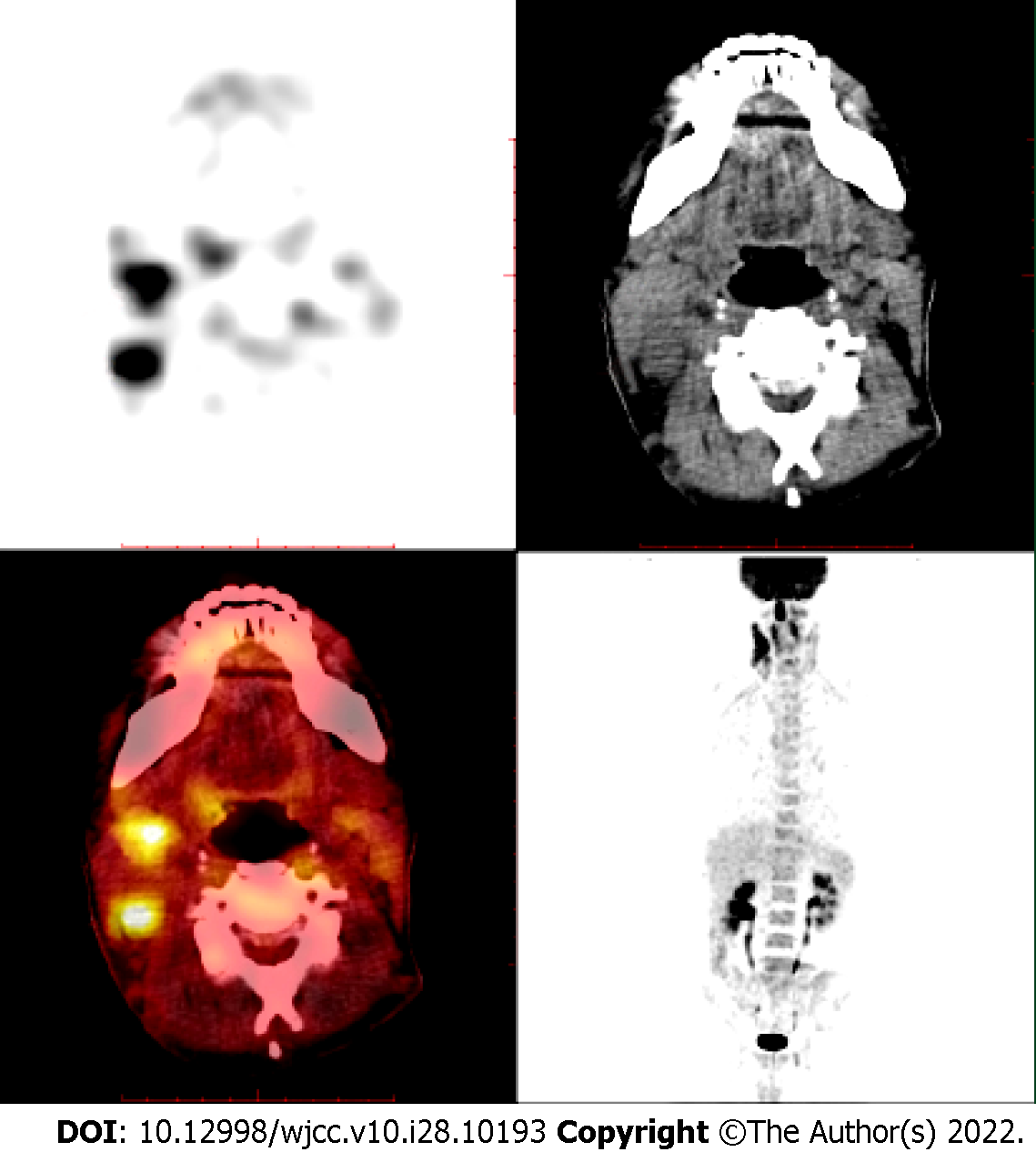
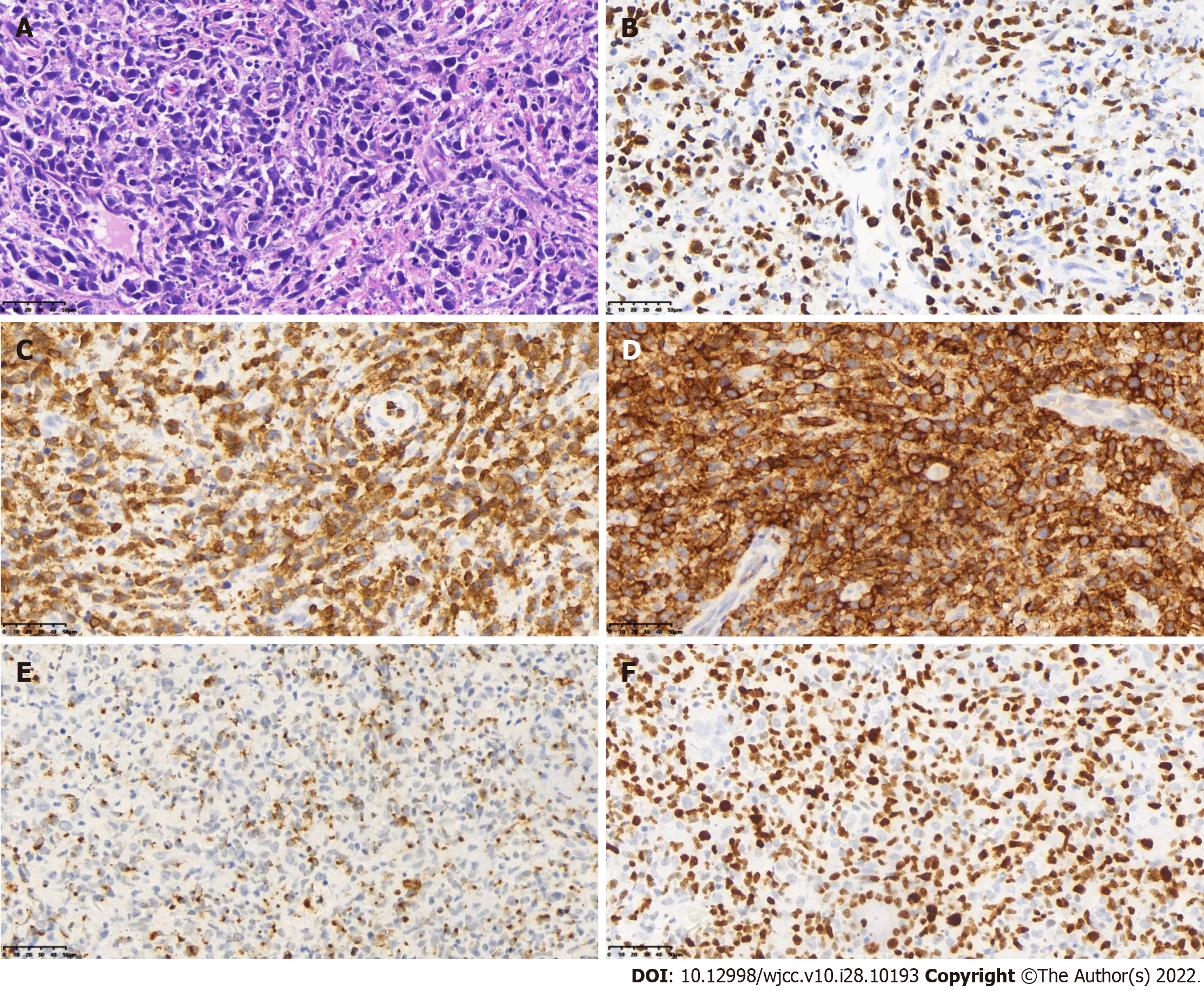
About 4 years ago, the patient visited our hospital for recurrent gingival bleeding accompanied by night sweats and a recent weight loss of > 10 kg. Positron emission tomography/computed tomography (PET/CT) showed multiple lymphadenopathies in the bilateral neck and supraclavicular area (especially in the right neck), strip-shaped soft tissue density shadow in the right nasal cavity, and significantly increased fluorodeoxyglucose (FDG) metabolism (Figure 1). Biopsy of the right nasal septum mucosa was performed postoperatively, and the pathology showed ENKL. Immunohistochemistry showed CD3 +, CD4 +, CD56 +, granzyme B +, CD8 -, CD20 -, Pax5 -, and Ki-67 + (about 80%). Molecular in situ hybridization demonstrated positivity for EBER (Figure 2).
The patient had no history of past illness.
No personal or family history was available.
There were multiple lymphadenectasis in the bilateral neck especially in the right neck. No palpable enlargement of the liver or spleen was noted.
The liver function, renal function, routine blood tests, myocardial enzymes, and coagulation function were normal. Bone marrow puncture was not performed.
On March 3, 2018, PET/CT showed multiple lymphadenopathies in the bilateral neck and supraclavicular area (especially in the right neck), strip-shaped soft tissue density shadow in the right nasal cavity, and significantly increased FDG metabolism and a high standard uptake value (SUV) with a Deauville score of 7.6 (Figure 1).
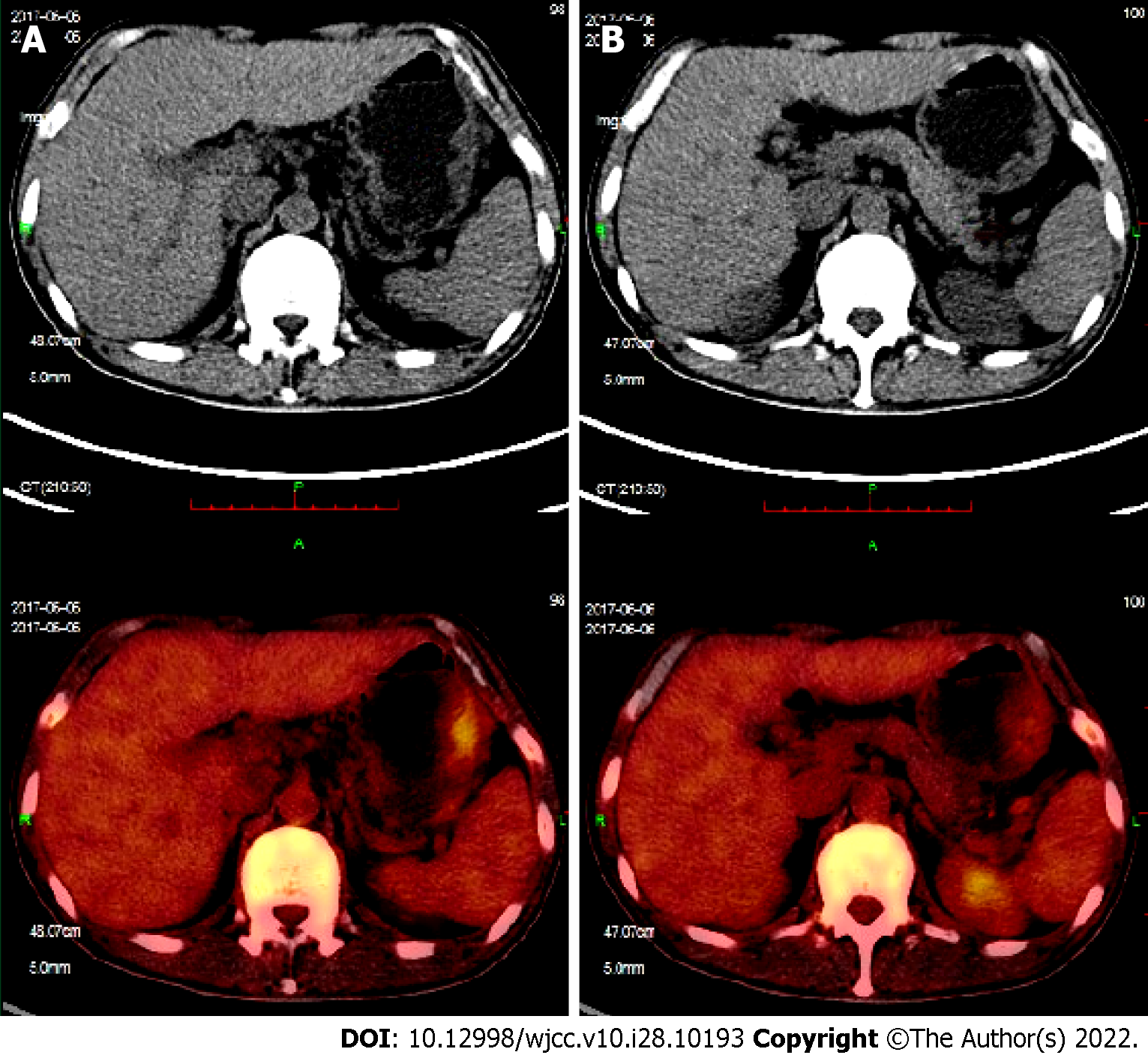
On June 6, 2017, PET/CT showed that the original lesions in the right nasal cavity, bilateral neck, and supraclavicular area had subsided and become inactive compared to the scan on March 3, 2017. However, there was new large, curved wall thickening of the gastric body, multiple nodules of the spleen, multiple lymphadenopathies around the stomach, mediastinum and left hilum, and increased FDG metabolism (Figure 3).
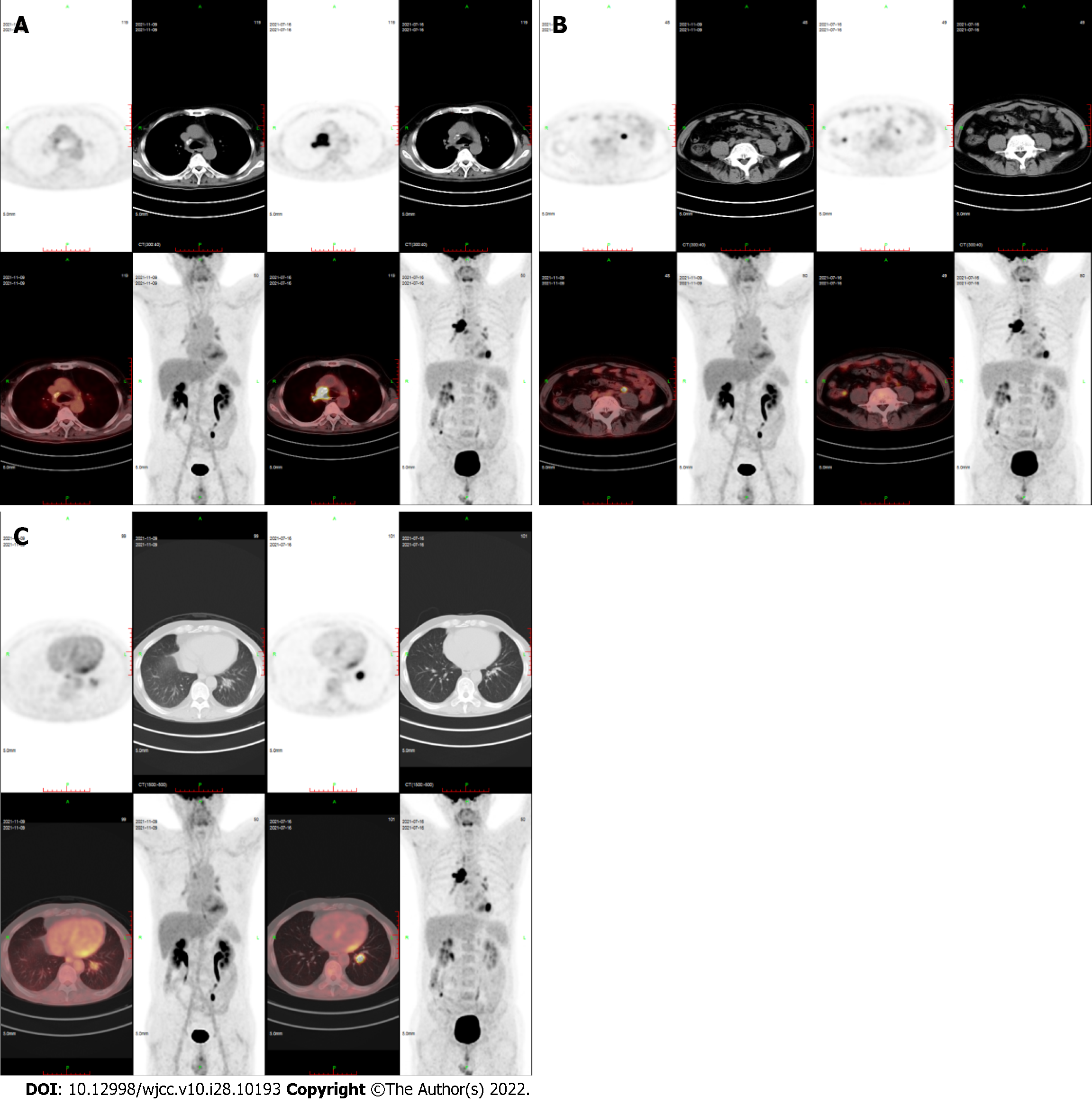
On November 9, 2021, PET/CT showed that right mediastinal paratracheal, right main bronchial, and subcarinal lymph nodes and ileocecal small nodules subsided and became inactive, and the metabolism of left lower lobe nodules was decreased significantly (SUVmax decreased from 10.2 to 3.8) (Figure 4).
According to the above medical history, the final diagnosis was ENKL.
After diagnosis, the patient was given SMILE regimen for two cycles (methotrexate 4.5 g d1, dexamethasone 40 mg d1–4, ifosfamide 2 g d2–4, etoposide 160 mg d2–4, L-asparaginase 3200 U d5) on March 10 and April 8, 2017, respectively. Since May 2, 95% planned target volume at 54Gy/28f was irradiated to the local lesion and irregular large-area fields on both sides of the neck. PET/CT on June 6, 2017 showed that the original lesions in the right nasal cavity, bilateral neck, and supraclavicular area have subsided and become inactive compared to the scan on March 3, 2017. However, there was new large, curved wall thickening of the gastric body, multiple nodules of the spleen, multiple lymphadenopathies around the stomach, mediastinum, and left hilum, and increased FDG metabolism (Figure 3). Gastroscopic pathological biopsy suggested ENKL. Then, DDGP regimen plus methotrexate (gemcitabine 1.63 g d1 and 8, methotrexate 3.20 g d1, dexamethasone 30 mg d1–4, L-asparaginase 3750 U d5) chemotherapy was administered for two cycles. On August 30, after SMILE regimen was repeated, stem cells were prepared for autologous stem cell transplantation, but the collection failed. On October 27, modified SMILE regimen (dexamethasone 32 mg d1–4, etoposide 100 mg d2–4, metho
The above scheme was repeated after 4 wk. PET/CT on July 16, 2021 showed that after 8 wk, the right mediastinal paratracheal, right main bronchial, subcarinal lymph nodes, and small ileocecal nodules subsided and were inactive, and the left lower lobe nodules, and FDG metabolism decreased significantly (Figure 4). This patient is still undergoing regular chemotherapy every 4 wk: Decitabine 25 mg d1–5, sintilimab 200 mg d6, and L-asparaginase 3750 U d10. To the best of our knowledge, this is the first case of decitabine combined with anti-PD-1 applied to R/R ENKL.
As an inhibitory receptor, PD-1, expressed on the surface of activated T cells, is involved in immune tolerance and the prevention of tissue damage associated with chronic inflammation. Inflammatory cytokines have been found to induce programmed cell death ligand 1 (PD-L1) expression in many studies. In particular, the involvement of IFN-γ in immune checkpoint induction has been reported. When the expression of proinflammatory cytokines was high, it suggests the possibility that the expression of PD-L1 Therefore, it suggests the possibility that the expression of PD-L1[6].
PD-1 interacts with its ligands PD-L1 and PD-L2 to inhibit T cell receptor signal, downregulate T cell activation and proliferation, and weaken the T cell-mediated anti-tumor immune response[7,8]. Therefore, the PD-1 pathway is an immune checkpoint that suppresses anti-tumor immunity. Other studies have reported the efficacy of anti-PD-1 in R/R ENKL[5,9].
Exhausted T cells, including memory-like exhausted T cells that respond to PD-1 inhibitors, ultimately differentiate into terminal exhausted T cells. These T cells are resistant to anti-PD-1-induced rejuvenation but can still exhibit distinct epigenetic profiles[10-12]. Another study identified low-dose DNA demethylating agents that alter T-cell’s epigenetic status and enhance the anti-tumor activity of PD-1/PD-L1 blockade therapy in mouse models[13,14]. Reportedly, T cell phenotype and function and the expression of PD-1 are strongly regulated by epigenetic changes[15,16]. Patients with hematological malignancies treated with hypomethylating agents have demonstrated increased expression of PD-1 transcripts and proteins on the cell surface[17,18]. With the expanding application of immune checkpoint inhibitors in hematological malignancies, immunotherapies targeting the PD-1/PD-L1 axis combined with hypomethylating agents may counteract the upregulation of PD-1/PD-L1 checkpoints and improve the clinical outcomes[18]. While anti-PD-1 alone had modest effects, hypomethylating agents followed by anti-PD-1 therapy inhibited tumor growth and prolonged the survival of the pancreatic cancer mouse model[19]. Wang et al[20] found that decitabine combined with anti-PD-1 achieves curative effects in patients with R/R Hodgkin’s lymphoma resistant to anti-PD-1 and is a safe and feasible approach. A randomized phase II study of R/R Hodgkin’s lymphoma demonstrated that anti-PD-1 plus decitabine was well-tolerated and improved the clinical outcomes compared to anti-PD-1 alone with regard to the duration of response (DOR) and progression-free survival (PFS)[21]. The common treatment-related adverse events, such as a benign and reversible skin condition, reactive capillary endothelial proliferation, and leukocytopenia, but no treatment-related infections, were identified[21]. Although this protocol has achieved good preliminary results in Hodgkin’s lymphoma and other tumors, this is the first attempt to use this combination therapy in R/R ENKL. Our patient was resistant to pembrolizumab after continual treatment for 2 years but still achieved near-complete remission by decitabine combined with sintilimab. Although the binding sites of the two antibodies are different, more over the Fc side of sinitinib was modified to avoid direct phagocytosis of antibody molecules. These mechanisms maybe result in the different response between these two antibodies. But as far as the current research is concerned, sintilimab has similar anti-tumor effects to other anti-PD-1[22]. No treatment-related adverse events were noted in our case.
L-asparaginase-based chemotherapy improves the response rate of R/R ENKL. However, no studies have yet reported that L-asparaginase has a synergistic effect with anti-PD-1 or demethylated drugs.
The experience of this case offers a new option for the treatment of R/R ENKL resistant to anti-PD-1 and other types of lymphomas. This case is only an empirical report of a single case, and the efficacy of decitabine combined with anti-PD-1 in R/R ENKL needs to be confirmed in more patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ekine-Afolabi B, United Kingdom; Hashimoto K, Japan S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121:4997-5005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 2. | Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, Lee DH, Huh J, Oh SY, Kwon HC, Kim HJ, Lee SI, Kim JH, Park J, Oh SJ, Kim K, Jung C, Park K, Kim WS. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 558] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Kwong YL, Anderson BO, Advani R, Kim WS, Levine AM, Lim ST; Asian Oncology Summit. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X, Wu J, Fu X, Ma W, Zhang X, Chang Y, Nan F, Li W, Su L, Wang J, Xue H, Zhang M. DDGP versus SMILE in Newly Diagnosed Advanced Natural Killer/T-Cell Lymphoma: A Randomized Controlled, Multicenter, Open-label Study in China. Clin Cancer Res. 2016;22:5223-5228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, Zhang X, Chang Y, Sun Z, Yu H, Zhang L, Wang X, Wu J, Li Z, Nan F, Tian L, Li W, Young KH. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 6. | Hashimoto K, Nishimura S, Ito T, Akagi M. Characterization of PD-1/PD-L1 immune checkpoint expression in soft tissue sarcomas. Eur J Histochem. 2021;65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2242] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 8. | Liu D, Wang S, Bindeman W. Clinical applications of PD-L1 bioassays for cancer immunotherapy. J Hematol Oncol. 2017;10:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 10. | Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, Hellmann MD, Wolchok JD, Leslie CS, Schietinger A. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 701] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 11. | Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R, Sowalsky AG, Valanparambil RM, Hudson WH, McGuire D, Melnick K, Khan AI, Kim K, Chang YM, Kim A, Filson CP, Alemozaffar M, Osunkoya AO, Mullane P, Ellis C, Akondy R, Im SJ, Kamphorst AO, Reyes A, Liu Y, Kissick H. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 569] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 12. | Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, Manos M, Gjini E, Kuchroo JR, Ishizuka JJ, Collier JL, Griffin GK, Maleri S, Comstock DE, Weiss SA, Brown FD, Panda A, Zimmer MD, Manguso RT, Hodi FS, Rodig SJ, Sharpe AH, Haining WN. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 1376] [Article Influence: 229.3] [Reference Citation Analysis (0)] |
| 13. | Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, Barnitz RA, Bartman C, Bengsch B, Huang AC, Schenkel JM, Vahedi G, Haining WN, Berger SL, Wherry EJ. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 979] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 14. | Weintraub K. Take two: Combining immunotherapy with epigenetic drugs to tackle cancer. Nat Med. 2016;22:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Suarez-Álvarez B, Rodríguez RM, Schlangen K, Raneros AB, Márquez-Kisinousky L, Fernández AF, Díaz-Corte C, Aransay AM, López-Larrea C. Phenotypic characteristics of aged CD4+ CD28null T lymphocytes are determined by changes in the whole-genome DNA methylation pattern. Aging Cell. 2017;16:293-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 4134] [Article Influence: 243.2] [Reference Citation Analysis (0)] |
| 17. | Kouba C, Liu D, Everett G, Kerber RE. Extreme intracardiac coiling of a transvenous pacemaker: a case report. Pacing Clin Electrophysiol. 1985;8:360-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, Nguyen M, Pierce S, Wei Y, Parmar S, Cortes J, Kantarjian H, Garcia-Manero G. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 572] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 19. | Gonda TA, Fang J, Salas M, Do C, Hsu E, Zhukovskaya A, Siegel A, Takahashi R, Lopez-Bujanda ZA, Drake CG, Manji GA, Wang TC, Olive KP, Tycko B. A DNA Hypomethylating Drug Alters the Tumor Microenvironment and Improves the Effectiveness of Immune Checkpoint Inhibitors in a Mouse Model of Pancreatic Cancer. Cancer Res. 2020;80:4754-4767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Wang C, Liu Y, Dong L, Li X, Yang Q, Brock MV, Mei Q, Liu J, Chen M, Shi F, Liu M, Nie J, Han W. Efficacy of Decitabine plus Anti-PD-1 Camrelizumab in Patients with Hodgkin Lymphoma Who Progressed or Relapsed after PD-1 Blockade Monotherapy. Clin Cancer Res. 2021;27:2782-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Wang C, Li X, Dong L, Yang Q, Chen M, Shi F, Brock M, Liu M, Mei Q, Liu J, Nie J, Han W. Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: A Promising Anti-Tumor PD-1 Antibody. Front Oncol. 2020;10:594558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |