Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10180
Peer-review started: March 30, 2022
First decision: June 16, 2022
Revised: July 14, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 6, 2022
Processing time: 181 Days and 1.3 Hours
Renal cell carcinoma (RCC) with Xp11.2 translocation/TFE3 gene fusion is a rare and distinct subtype of RCC that is classified under tumors with translocation of the microphthalmia-associated transcriptional factor.
We report an adult case of Xp11.2 translocation advanced RCC with metastasis (T3aN1M1), after targeted treatment, alcohol ablation, and transarterial chemoembolization, who eventually underwent successful surgical excision. No recurrence or transfer was seen within one year, and the survival period was more than 3 years. A review of the relevant literature was conducted to improve our under
Transarterial chemoembolization and ablation did not achieve the desired tumor reduction in this patient, but had a significant effect on reducing intraoperative bleeding and inhibiting tumor activity.
Core Tip: Under the guidance of multimodality imaging, surgical resection, targeted chemotherapy, alcohol ablation, and transarterial chemoembolization were used to treat a case of Xp11.2/TFE3 (T3aN1M1) advanced renal cell carcinoma with good results. No recurrence or metastasis occurred within 1 year, with a partial response over 3 years. By reviewing this case and related literature, we can improve our understanding of the diagnosis, differential diagnosis and treatment of the disease.
- Citation: Wang P, Zhang X, Shao SH, Wu F, Du FZ, Zhang JF, Zuo ZW, Jiang R. Chemotherapy, transarterial chemoembolization, and nephrectomy combined treated one giant renal cell carcinoma (T3aN1M1) associated with Xp11.2/TFE3: A case report. World J Clin Cases 2022; 10(28): 10180-10185
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10180.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10180
Renal cell carcinoma (RCC) with Xp11.2 translocation/TFE3 fusion is rare but highly malignant, and is associated with poor prognosis due to the lack of early diagnosis[1,2] and the difficulty in resecting advanced stage giant tumors[3]. It was classified into the microphthalmia-associated transcriptional factor translocation family of tumors as per the 2016 WHO criteria[4]. A break in the short arm of the X chromosome gene TFE3 and the subsequent translocation generates a novel TFE3 fusion gene that is overexpressed under the new promoter, resulting in abnormal cell proliferation and malignant transformation[5,6]. We present a case of Xp11.2/TFE3 RCC stage T3aN1M1 with a tumor diameter > 30 cm that was successfully resected after chemotherapy and transarterial chemoembolization (TACE), and eventually achieved a favorable prognosis.
The 18-year-old female patient had been experiencing occasional abdominal distension without abdominal pain, vomiting, constipation and other discomfort for over one year. There was no gross hematuria but the patient lost more than 10 kg, which unfortunately was ignored by the patient and her family members.
No special medical history.
No special medical history.
No special medical history.
Physical examination indicated Zubrod-ECOG-WHO (ZPS) 2 points and Karnofsky (KPS) KPS 90 points. A tangible, immobile mass measuring approximately 30 cm in diameter was present in the middle and lower abdomen, and felt firm to the touch. There was no obvious tenderness, skin surface rupture or varicose vessels, and the skin temperature was normal.
Routine laboratory test results indicated hemoglobin of 56 g/L, albumin of 30 g/L and a platelet count of 604 × 109/L. Kidney and liver function indices were normal.
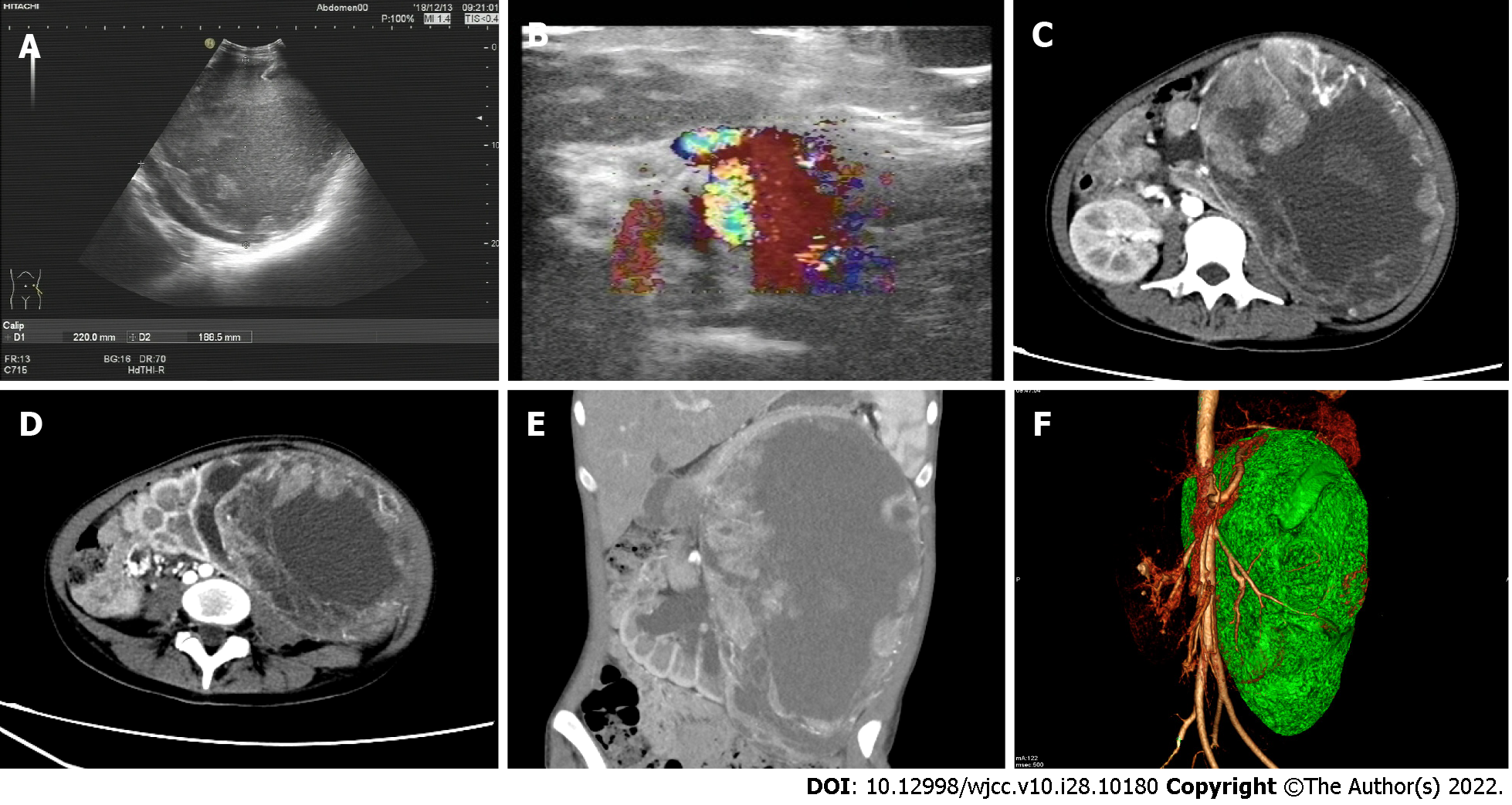
Multimodal imaging (Figure 1) revealed an elliptical mass measuring about 13.7 cm × 20 cm × 30.7 cm in the upper part of the left kidney with a distinct solid border, liquefaction in the center, and septa. Mural nodule blood flow was abundant with some spotty calcium. Computed tomography (CT) enhancement showed a solid margin with continuous uneven enhancement and high uptake of 18F-fluorodeoxyglucose (18F-FDG) (SUVmax - 6.1, SUVmean - 4.2). Computed tomography angiography showed that the mass was fed by the left renal artery and vein, and the left renal vein was tortuously dilated. The renal hilum of the left kidney was rotated due to hydronephrosis (Figure 2).
There was residual normal tissue in the lower left kidney. The pancreas, liver, stomach and spleen were displaced by compression, although no obvious ascites were observed. Magnetic resonance imaging (MRI) showed equal or high T1 signal in the liquefied center, suggesting necrosis with hemorrhage and no definite adipose composition. The left adrenal gland was indistinguishable. The mesentery around the mass was partially thickened and swollen, and retroperitoneal lymph nodes were enlarged. 18F-FDG metabolism increased in both cases (SUVmax - 10.5 and SUVmean - 7). Hematoxylin-eosin staining of the biopsied samples (10 × 30) showed extensive hemorrhage and necrosis involving the renal pelvis, along with lymph node metastasis (3/10). Immunohistochemical staining indicated that the mass was TFE3+, CD8/18+, CD10+, PAx-2+, CK+ and Ki-67+ (1%).
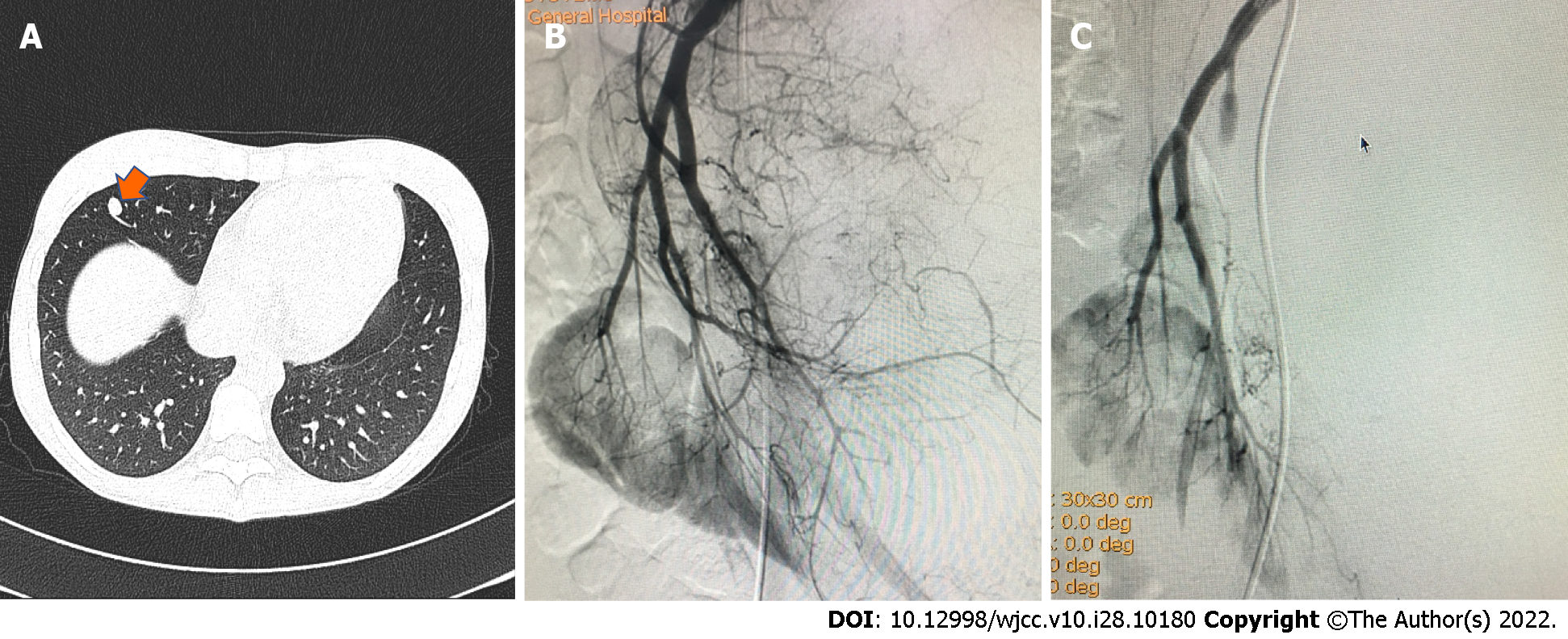
CT showed a single small nodule (Figure 3; orange arrow) in the middle lobe of the right lung and digital subtraction angiography showed obvious staining of the tumor (Figure 3; black arrow). The feeding artery of the tumor was almost completely embolized after TACE, while that of the normal renal tissue was preserved.
Xp11.2 translocation/TFE3 gene fusion associated renal cell carcinoma with metastasis (T3aN1M1).
The patient had a large tumor (T3aN1M1) along with distant metastases and severe anemia. In this setting, direct surgical resection can be extremely risky, and its long-term effect is unknown.
Nevertheless, the age of the patient, presence of a single metastatic focus in the right lung and the intact tumor capsule were consistent with the indication of abdominal tumor removal. Following a multidisciplinary consultation among the departments of urology, radiology, general surgery and oncology, we devised a therapeutic regimen consisting of targeted drugs, alcohol-mediated ablation and TACE, followed by surgical resection and post-surgery targeted drug therapy.
The chemotherapy regimen included oral axitinib 5 mg twice a day for 2 wk, and the treatment was repeated after 2 mo. In order to accelerate tumor shrinkage, we extracted liquid necrotic substances from the tumor and injected anhydrous alcohol for ablation. This was followed by infusion of red blood cells (RBCs), antibiotics and other symptomatic support treatment.
For TACE, 30 mg pirarubicin and 30 mg recombinant human endostatin were infused through the left renal artery trunk. The grade 2-3 branches of the tumor-feeding artery were preserved, and the remaining branches were embolized with polyvinyl alcohol foam embolic particles (PVA-100). There was 18% reduction in the tumor after TACE in conformity with the RECIST criteria.
Radical resection of left renal carcinoma, celiac lymph node dissection and intestinal adhesion lysis were performed under general anesthesia by the urological surgery team after two courses of axitinib treatment. The surgery was successful, and the intraoperative blood loss was only about 400 mL. Postoperative anti-infection, hemostasis and nutritional support were given as symptomatic treatment, along with 3U RBCs and 430 mL plasma. Oral axitinib therapy was continued after surgery.
Compensatory hyperplasia of the right kidney was detected 4 mo after surgery. SPECT and pelvic enhanced MRI scans revealed sacral bone metastases 14 mo after surgery, and zoledronic acid chemotherapy was initiated. Abdominal lymph node metastasis was detected 18 mo after surgery. Chemotherapy drugs included axitinib, karelizumab (Erica), and zoledronic acid (Elan). Radiotherapy was performed with linear accelerator 15 MV X-rays, and the enlarged lymph nodes received local and conventional irradiation with a total dose of 40–60 Gy. Partial response was achieved, and the patient survived with the tumor for more than 46 mo and her overall condition is good.
Xp11.2/TFE3 RCC is more common in children and adolescents, and accounts for roughly one-third of pediatric RCC and 0.5% of adult RCC cases[7]. Our patient was 18 years old at the time of diagnosis, and thus belonged to the age group that is predisposed to RCC.
Although surgical resection is an effective first-line treatment for RCC, it is not a feasible option for advanced or large tumors. TACE is often performed to reduce the size of the tumor before surgery. Some studies have shown that TACE can inhibit intra-tumor blood flow, accelerate tumor tissue necrosis, reduce tumor volume before surgery, and reduce intra-operative blood transfusion. The combination of embolic agents with high doses of chemotherapeutic drugs can improve the clinical outcomes of systemic chemotherapy and minimize side effects[8].
The effective clinical management of tumors is incumbent on imaging, regular re-examination and timely modification of the treatment plan[9]. Ultrasound and CT should be the primary screening methods for suspected renal neoplasms. The differential diagnosis of Xp11.2/TFE3 RCC should include MRI enhancement, fat suppression sequence, diffusion sequence and MRS to enhance understanding of tumor components. In addition, preoperative CT/MR enhancement can detect lymph node metastasis, distant metastasis or vascular invasion. PET/CT and SPECT scans are recommended for postoperative follow-up; while PET/CT can supplement focus metabolism, and SPECT can detect lesions related to bone metabolism.
In summary, the sequential application of multimodal imaging can be used for the early detection of Xp11.2/TFE3 RCC, and assist in surgical planning and postoperative follow-up. TACE and ablation did not achieve the desired tumor reduction in this patient, but had a significant effect on reducing intraoperative bleeding and inhibiting tumor activity.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boopathy Vijayaraghavan KM, India; Haddadi S S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1823] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 2. | Argani P. MiT family translocation renal cell carcinoma. Semin Diagn Pathol. 2015;32:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Xia QY, Wang Z, Chen N, Gan HL, Teng XD, Shi SS, Wang X, Wei X, Ye SB, Li R, Ma HH, Lu ZF, Zhou XJ, Rao Q. Xp11.2 translocation renal cell carcinoma with NONO-TFE3 gene fusion: morphology, prognosis, and potential pitfall in detecting TFE3 gene rearrangement. Mod Pathol. 2017;30:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Inamura K. Translocation Renal Cell Carcinoma: An Update on Clinicopathological and Molecular Features. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Wang Y, Feng M, Lian X, Lei Y, Zhou H. Renal cell carcinoma associated with Xp11.2 translocation/transcription factor E3 gene fusion: an adult case report and literature review. J Int Med Res. 2020;48:300060520942095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Liu N, Wang Z, Gan W, Xiong L, Miao B, Chen X, Guo H, Li D. Renal Cell Carcinoma Associated with Xp11.2 Translocation/TFE3 Gene Fusions: Clinical Features, Treatments and Prognosis. PLoS One. 2016;11:e0166897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2071] [Article Influence: 230.1] [Reference Citation Analysis (0)] |
| 8. | Seki A, Hori S, Sueyoshi S, Hori A, Kono M, Murata S, Maeda M. Transcatheter ar-terial embolization with spherical embolic agent for pulmonary metastases from renal cell carcinoma. Cardiovasc Intervent Radiol. 2013;36:1527-1535. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Liu C, Zhang W, Song H. Nephron-sparing surgery in the treatment of pediatric renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions. J Pediatr Surg. 2017;52:1492-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |