Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10162
Peer-review started: March 7, 2022
First decision: April 8, 2022
Revised: April 19, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: October 6, 2022
Processing time: 203 Days and 15.3 Hours
The endoscopic management of benign short post-anastomotic ileocolonic stricture (PAICS) that is refractory to primary and secondary treatment modalities remains challenging. The lumen-apposing metal stent (LAMS) is a novel device recently developed for therapeutic gastrointestinal endoscopy. LAMSs have demonstrated significantly better results with regard to stent migration than fully covered self-expandable metal stents (FCSEMSs).
This article presents six cases of symptomatic PAICS successfully treated with a LAMS and a review of the relevant literature. We report a life-saving technique not previously documented and the use of technology to improve patient outcomes. The six patients (median age, 75 years) suffered from vomiting, constipation and recurrent abdominal pain, with symptoms starting 23-25 wk post-surgery. The median stricture length was 1.83 cm. All six patients underwent successful and uneventful bi-flanged metal stent (BFMS)-LAMS placement for benign PAICS. All patients remained asymptomatic during the three months of stent indwelling and up to a median of 7 mo after stent removal. According to the literature, the application of LAMS for PAICS is associated with a < 10% risk of migration and a < 5% risk of bleeding. Conversely, FCSEMS has a high migration rate (15%-50%).
The evolving role of interventional endoscopy and the availability of LAMSs provide patients with minimally invasive treatment options, allowing them to avoid more invasive surgical inter
Core Tip: The lumen-apposing metal stent has demonstrated significantly better results with regard to stent migration than fully covered self-expandable metal stents. We present six cases of post-anastomotic ileocolonic strictures successfully treated with a bi-flanged metal stent (NAGI stent) and a review of the relevant literature. The long and broad flanges of the bi-flanged metal stent may reduce the migration rate and improve patient tolerance and thus may represent a suitable alternative to traditional endoscopic options, with better long-term results in the management of luminal gastrointestinal strictures longer than 10 mm and shorter than 30 mm.
- Citation: Kasapidis P, Mavrogenis G, Mandrekas D, Bazerbachi F. Short benign ileocolonic anastomotic strictures - management with bi-flanged metal stents: Six case reports and review of literature. World J Clin Cases 2022; 10(28): 10162-10171
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10162.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10162
Colonic anastomotic stricture occurs in 3%-28% of patients following colorectal surgery[1-3]. The mana
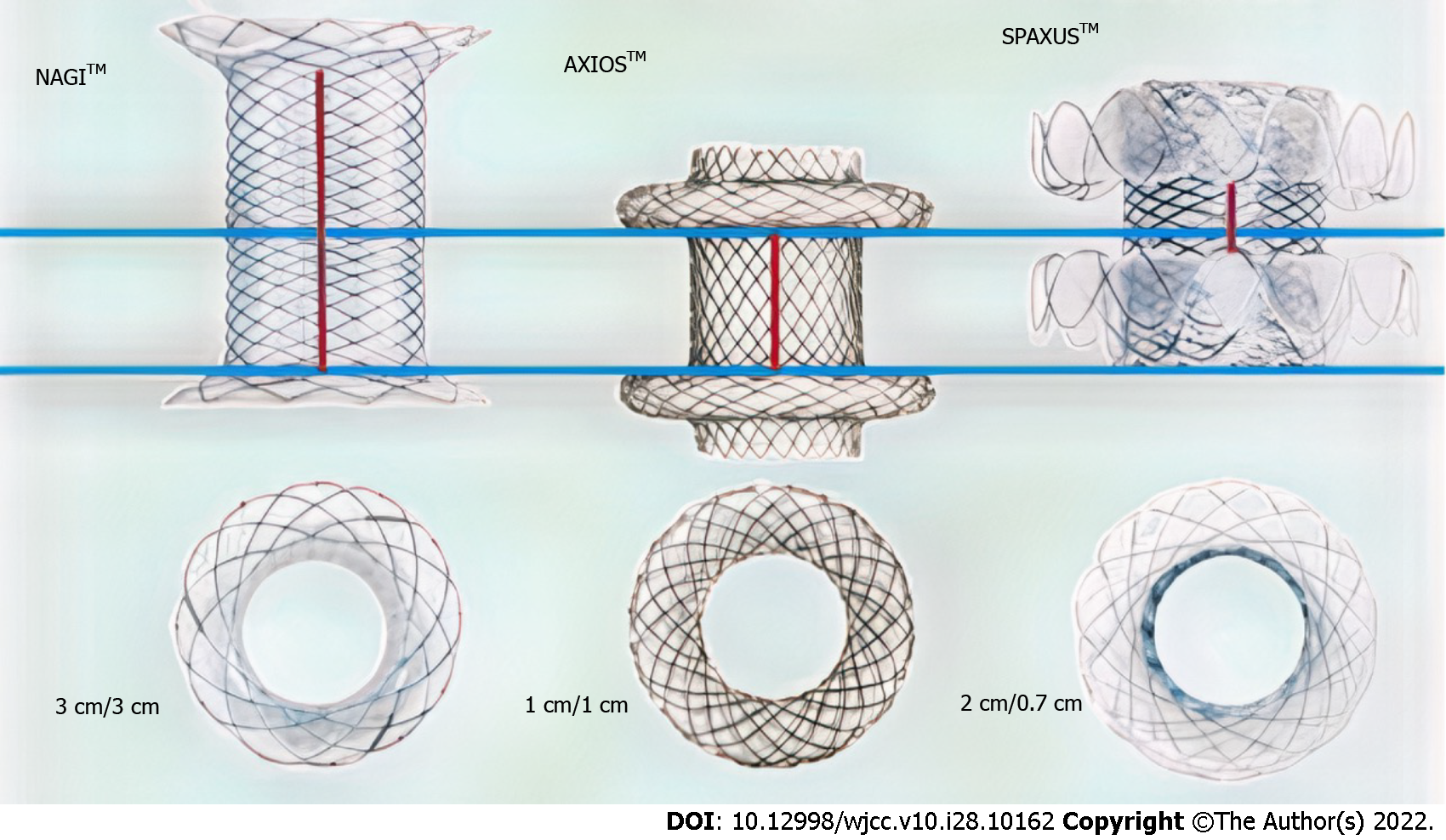
The lumen-apposing metal stent (LAMS) is a novel device recently developed for therapeutic GI endoscopy. The LAMS was originally designed for pancreatic fluid collection drainage but is currently being used for many off-label indications[2,5,7,8]. The bi-flanged metal stent (BFMS), another type of LAMS, is a saddle-shaped metal (nitinol) stent that achieves lumen apposition due to its bilateral anchoring flanges, thus decreasing the risk of stent migration[5-7,11]. Moreover, because of its larger intraluminal diameter, it can accomplish more efficient drainage, and its silicone coating prevents tissue ingrowth and thus facilitates easy removal[7,12]. The BFMS (Nagi-LAMS stent) has flared ends and a bi-flanged design, with a longer saddle (up to 3 cm) compared to the 1-cm saddle of the AXIOS-LAMS stent (Figure 1). Successful management of multiple types of GI stenoses (esophageal, gastric, and colonic) with the LAMS has been reported in several studies[2,3,5,13]. However, data on the role of the BFMS (Nagi-LAMS stent) in these scenarios are limited.
This study aims to evaluate the efficacy, feasibility, indications and safety of BFMS placement as a new endoscopic management approach for symptomatic short benign PAICS. This report describes a life-saving technique not previously documented and provides a review of the related literature.
These case reports adhere to the SCARE criteria[14]. The review follows the PRISMA guidelines[15]. All original studies and case reports concerning symptomatic benign GI strictures treated endoscopically with a LAMS, BFMS or FCSEMS were included. We performed an electronic literature search in PubMed, Cochrane Library, and Embase databases for articles published between March 2010 and November 2021.
Cases 1-5: These patients suffered from constipation and abdominal pain.
Case 6: This patient suffered from recurrent symptomatic small-bowel obstruction with nausea, vomiting, abdominal pain, and flatulence.
Cases 1-5: These patients developed symptoms starting at 23-25 wk post-surgery.
Case 6: This patient required hospitalization at the 28th week postoperatively.
Cases 1-6: All included patients underwent ileocecal resection and one-stage, end-to-end anastomosis without diverging ileostomy for adenocarcinoma in the right colon. The average age of the patients was 75 years (range: 68-82). None of the six patients received adjuvant chemotherapy as the tumor staging was < T3N0M0. The median time between surgery and the diagnosis of stenosis was 206.33 d (range: 167-257). The demographic characteristics, disease etiology, complaints, treatments administered and other characteristics of the luminal anastomotic benign strictures in patients who underwent BFMS placement are summarized in Table 1.
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Median |
| Sex | M | M | F | M | F | F | |
| Age (yr) | 82 | 79 | 70 | 76 | 68 | 75 | 75 |
| Surgical procedure (end-to-end anastomosis) | ICA | ICA | ICA | ICA | ICA | ICA | |
| Stricture length (cm) | 1.5 | 2.0 | 1.5 | 2.0 | 2.0 | 2.0 | 1.83 |
| Duration of procedure (min) | 19 | 23 | 26 | 12 | 14 | 15 | 18.16 |
| Technical success (%) | 100 | 100 | 100 | 100 | 100 | 100 | |
| Clinical success (short-term/long-term) | Yes/yes | Yes/yes | Yes/yes | Yes/yes | Yes/yes | Yes/yes | |
| BFMS details (mm) | L = 30, D = 16 | L = 30, D = 16 | L = 30, D = 16 | L = 30, D = 16 | L = 30, D = 16 | L = 30, D = 16 | |
| Stent indwelling (d) | 85 | 89 | 94 | 90 | 91 | 92 | 90.16 |
| Complications | None | Mild abdominal pain lasted 2 d | None | None | None | Mild discomfort lasted 2 d | |
| Follow-up (d) | 241 | 237 | 190 | 188 | 218 | 214 | 214.6 |
| Colonoscopy 4-5 wk after stent deployment | Yes | Yes | Yes | Yes | Yes | Yes | |
| Time span between surgery and the diagnosis of stenosis (d) | 167 | 193 | 204 | 257 | 222 | 195 | 206.33 |
Cases 1 and 3: These patients had a disease-free medical history.
Cases 2, 4 and 5: These patients had hypertension.
Case 6: This patient had obesity, osteoporosis, cataract, hypertension, and type 2 diabetes mellitus.
Cases 1-6: None of the patients had a family history of GI tumors.
Cases 1-5: These patients had tenderness in the right upper quadrant of the abdomen. Their temperature, blood pressure and pulse rate were normal.
Case 6: This patient had persistent periumbilical pain and was pale but alert at admission, with blood pressure of 90/60 mmHg and a heart rate of 98 bpm.
Cases 1-6: These patients had normal serum carcinoembryonic antigen (reference range: 0-5.0 ng/mL) and hemoglobin levels, with an average of 12.0 g/dL among men and 10.2 g/dL among women. Normal hemoglobin levels are 13.0-17.5 g/dL for men and 11.6-16 g/dL for women.
Case 6: This hospitalized patient had a white blood cell count of 12000 cells/μL, with the normal range being 4500-11000 cells/μL (4.5 × 109/L).
Cases 1-5: Computed tomography (CT) of the abdomen revealed anastomotic stricture.
Case 6: CT revealed partial small-bowel obstruction at the level of the ileocolonic anastomosis.
Cases 1-6: Colonoscopies demonstrated tight benign anastomotic stenoses that could not be transversed with a pediatric colonoscope. Preoperative evaluation by CT scan and intraprocedural assessment were performed to assess the length and degree of all strictures. Regarding the necessity of magnetic resonance imaging for the evaluation of stricture length, colonoscopy with injected contrast material and CT scan can accurately depict the anastomotic stricture[9,10].
All patients were found to have high-grade strictures (residual lumen, diameter < 7 mm) with a median length of 1.83 cm (range 1.5-2.0). Malignancy was ruled out in all patients via biopsies and histological examination.
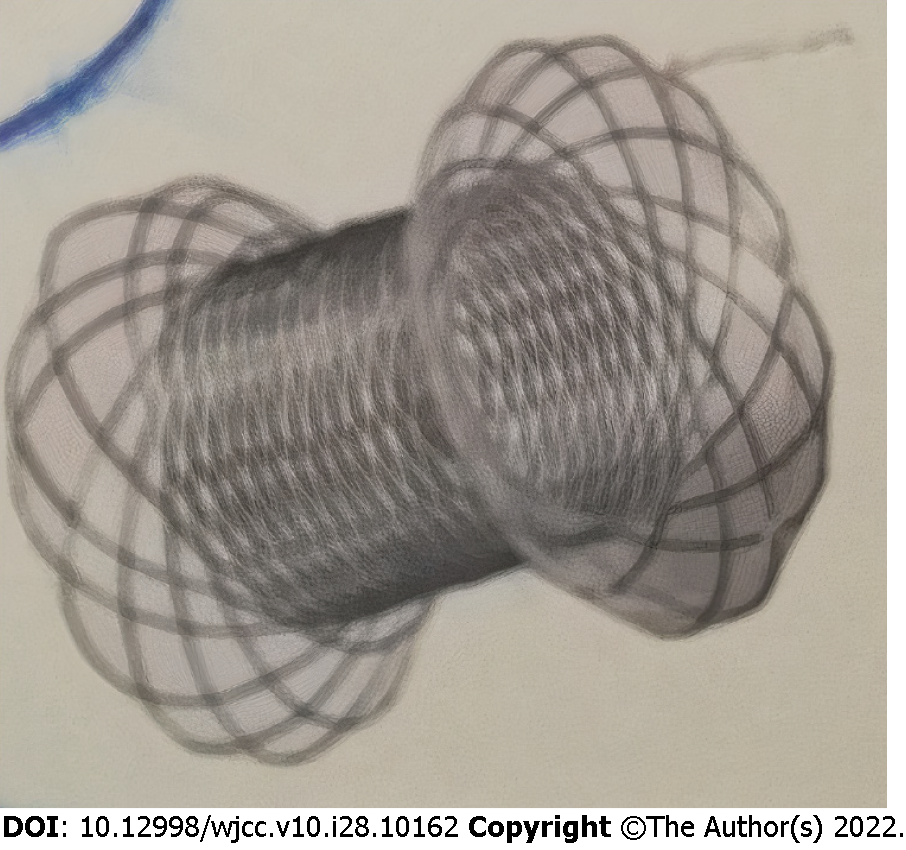
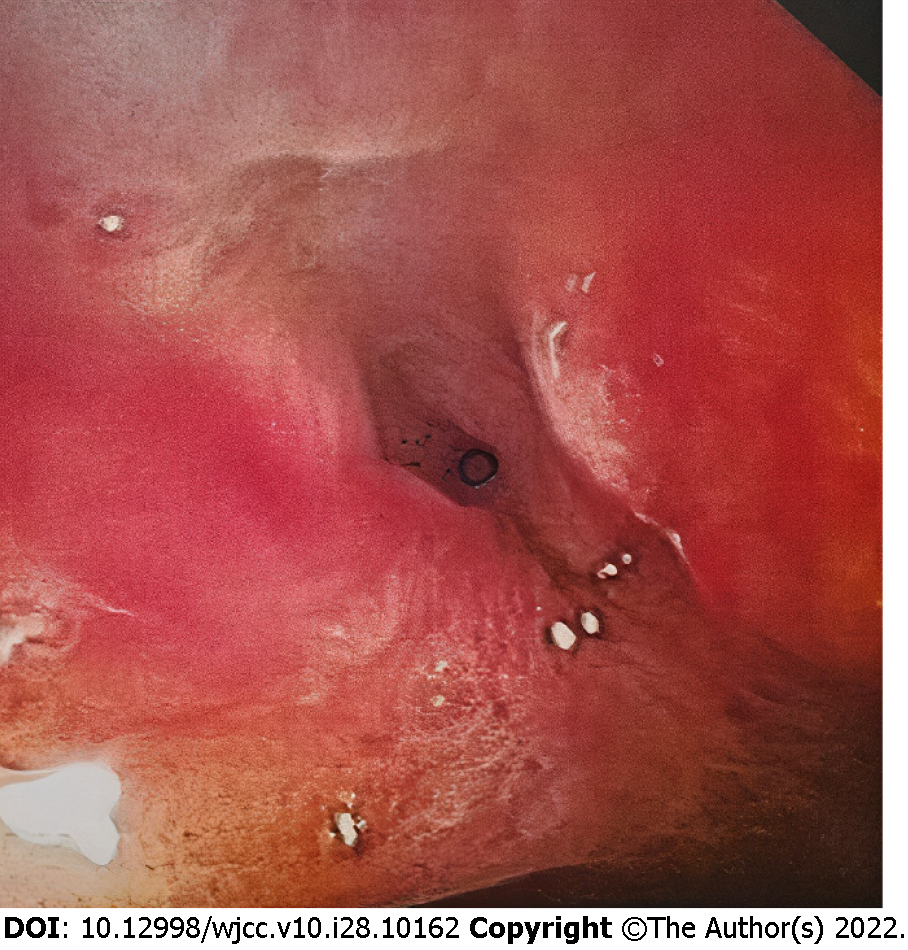
Midazolam was used for conscious sedation, or propofol was used if more profound sedation was needed. Fluoroscopic and direct endoscopic guidance, the latter with a flexible colonoscope, EC-590WM (Fujifilm, Tokyo, Japan), with a length of 1330 mm and a broad working channel 3.8 mm in diameter was employed to reach the stricture site in the transverse colon in all procedures. We delivered the BFMS via colonoscopy as the usable length of the NAGI-LAMS stent was longer than the working length of the colonoscope (1800 mm vs 1330 mm). The risk of intraprocedural perforation and stent migration was reduced by avoiding balloon dilatation either before or after BFMS deployment[8,9]. Access across the stricture was achieved using a 0.035 inch, 450-cm long guidewire (Jagwire, Boston Scientific, Natick, MA, United States). The deployed BFMSs were NAGI-LAMS stents (Taewoong Medical, Gyeonggido, South Korea) with a length of 30 mm, lumen diameter of 16 mm, 10 Fr delivery catheter, and flange diameter of 20 mm (Figure 2). Subsequently, over the guidewire and through-the-scope, a stent was inserted, and contrast injection revealed a stricture in the anastomotic area (Figure 3), which was stented with a BFMS under fluoroscopic guidance (Figure 4). The distal (downstream) flange of the BFMS was deployed under fluoroscopic guidance, and the proximal (upstream) end of the stent was deployed under direct endoscopic visualization. Clinical success was defined as the alleviation of GI obstructive symptoms, such as nausea, vomiting, constipation, abdominal distention and/or pain and occlusive ileus. Major adverse events were regarded as tissue perforation and stent migration. Minor adverse events were regarded as transient fever, vomiting, nausea, abdominal pain and self-limited hemorrhage. Any different endoscopic technique or urgent surgery was regarded as procedural failure.
Informed consent forms and authorization of the use of personal information forms were signed by all the patients before the procedure for the off-label use of BFMSs. Furthermore, the six patients provided written informed consent for publication. Ethics approval is not required for case reports at our institution.
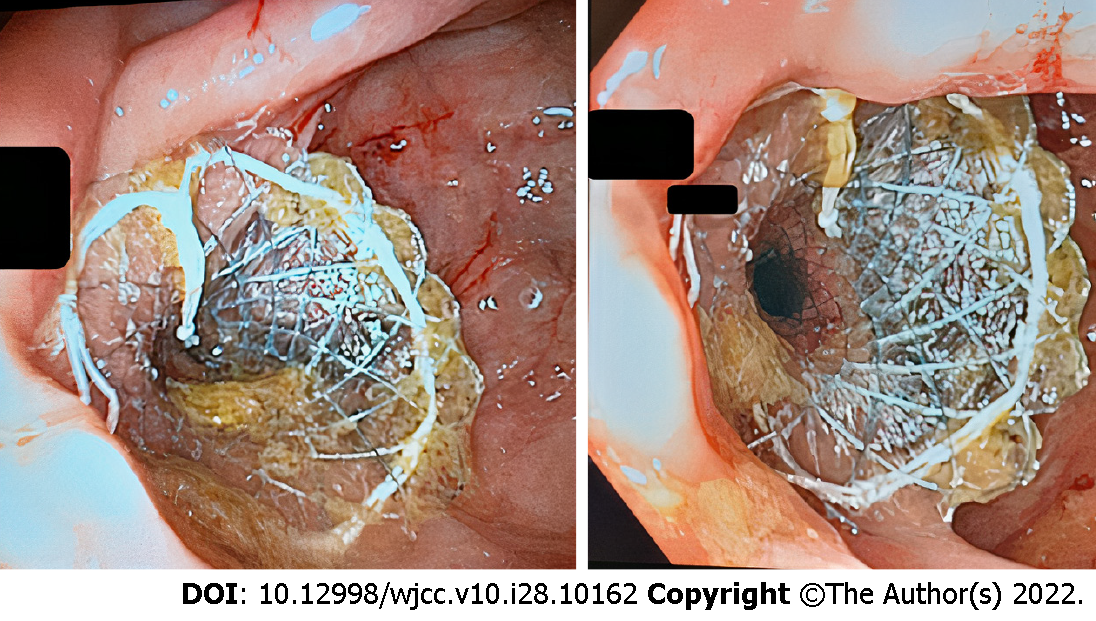
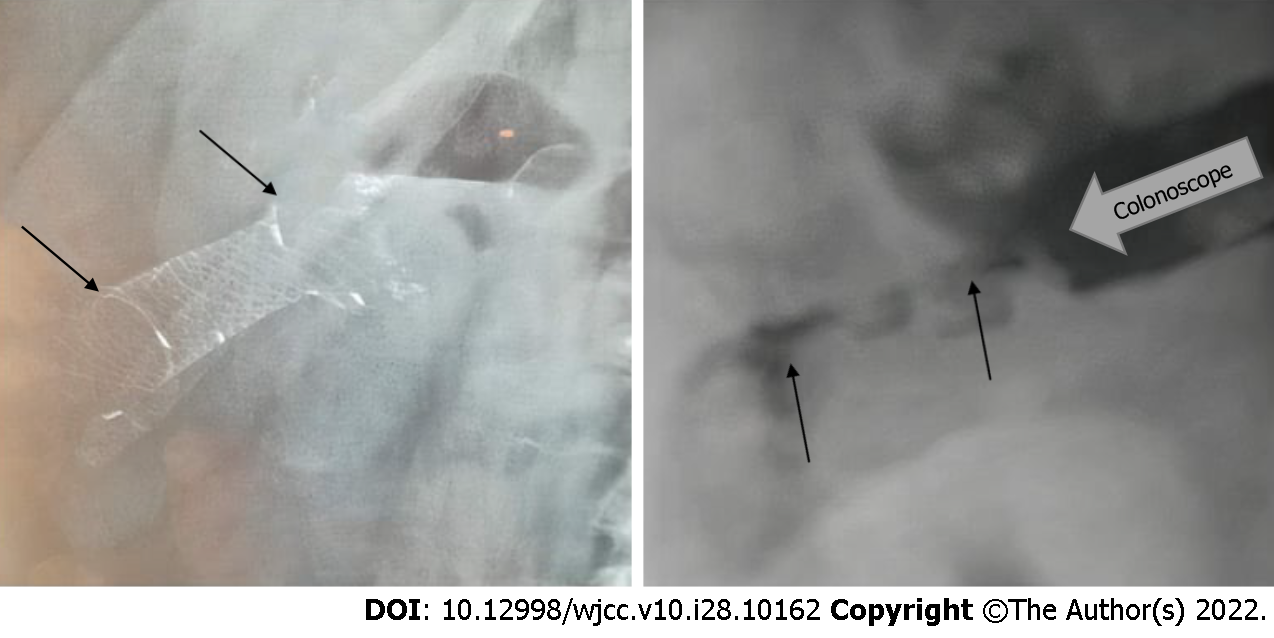
Cases 1-6: The endoscopic BFMS placement procedure, including scope insertion to reach the stricture site (Figure 5, right), required a median duration of 18.16 min (range: 12-26). There was constant stool and gas flow after BFMS deployment. All patients underwent colonoscopy 4-5 wk after stent deployment. Three months later, after a median of 90 d (range: 85-94), the stents (Figure 5, left) were removed without any adverse events. No patients required additional interventions at a median follow-up of 214.6 d (range: 188-241). The patients completed at least two follow-up visits during the subsequent seven months. The first and the second visits took place at the 3rd and 6th months, respectively.
Cases 1, and 3-5: No immediate adverse events occurred.
Case 2: The patient complained of mild post-procedural abdominal pain that lasted two days and he responded to analgesics (paracetamol, 1000 mg/24 h, for two days).
Case 6: The patient complained of mild post-procedural discomfort that lasted three days and she responded to analgesics (paracetamol, 1000 mg/24 h, for two days).
GI luminal stents are an appealing endoscopic option for managing selected colonic disorders, particularly post-anastomotic stricture. Strictures longer than 2 cm require multiple balloon dilatations, with limited long-term potency, perhaps due to a lack of stricture remodeling and scaffolding[8,15,16]. A solid proposition for managing benign anastomotic colon strictures are metal stents (SEMS, FCSEMS, LAMS-BFMS). This recommendation is based on the fact that constant radial force can be applied to the stricture for an extended time compared with balloon dilatation, thus inducing remodeling in these recalcitrant fibrotic strictures[10,13,17-19]. The major adverse event of SEMS and FCSEMS placement is stent migration, which has been reported to occur in 15%-50% of patients after a mean period of one month[4,8,14,20]. In addition, a high rate (40%-60%) of FCSEMS migration has been noted postoperatively in patients with colorectal diseases[6,8]. In a review of 8 studies involving 192 patients that evaluated LAMS for benign GI strictures, LAMS demonstrated significantly better results with regard to stent migration and post-procedural pain than FCSEMS and BDS stents[5,17].
The endoscopic placement of a LAMS and BFMS with fluoroscopic guidance is generally successful, with only a minority of cases requiring endoscopic ultrasound guidance because the lumen is fully obscured[7,12,17]. Early LAMS placement has been proposed as a viable option for long-term symptomatic relief in patients with short ileocolonic or colocolonic anastomotic strictures[3,6,18]. Patients with benign refractory stenosis who suffer from post-surgical ileocolonic anastomotic strictures may benefit from a LAMS if symptoms remain after two dilatations[2-4,18,21]. A LAMS has the potential to delay or ultimately prevent the need for consecutive dilations or surgical intervention[3,18]. Furthermore, the underlying risk of perforation (12%-20%) and migration (> 20%) increases with the deployment of pre- or post-balloon dilatations[8,9,18]. Due to the reasons mentioned above, a NAGI-LAMS stent was chosen, and non-pre-balloon dilatations were performed in these six patients.
In the literature, the rate of successful LAMS deployment for ileocolonic anastomosis is reported to be 89%-98%[3,7]. Overall stent migration has been reported in only 7%-9% of patients[3,18]. Proximal stent migration occurred during the first 3 wk in 6% of patients[1,3,4,7,13]. One patient (4.5%) had self-limiting bleeding, and this complication was associated with LAMS migration one week after placement[5,7]. No perforation or mortality has been attributed to LAMS placement[1,3,4,13]. Our results describing successful NAGI-LAMS placement for the management of PAICS are in line with the two most extensive studies of LAMS in the literature, showing its utility in the treatment of benign short PAICS[3,16]. Our clinical success rate in treatment-naïve patients was 100% (6/6). All patients in this study tolerated NAGI-LAMS deployment and indwelling for the entire therapy duration without severe post-procedural pain or stent migration. In the literature, LAMS (Nagi, Axios) intolerance is reported to be < 5% post-deployment[22-26]. In addition, LAMS has demonstrated less stent migration (9% vs 40%) and post-procedural pain than FCSEMS and BDS[24,27-29]. The overall rates of successful LAMS (Nagi, Axios) deployment (98%), migration (7%-9%), and bleeding (4.5%) without perforation in the treatment of luminal colonic strictures are all encouraging and indicate that LAMS should be considered in the treatment algorithm of benign, short ileocolonic post-surgical stenosis in a multidisciplinary setting[3,6,13,22,23]. Evidence of the clinical utility of NAGI-LAMS for ileocolonic or colocolonic anastomotic strictures has been presented in a few case reports[25,28]. Only 20 cases have been reported in the literature exploring the use of LAMS (Axios, Nagi) for the endoscopic management of colonic strictures[4].
The maximum efficacy and minimum adverse events regarding the optimal duration of LAMS placement remain undetermined. Many authors suggest limiting the indwelling period of LAMS (Nagi, Axios) to three months[5,6,12,18,22]. The novel design of these stents permits a longer indwelling time, which induces better clinical results and yields low recurrence rates[3,4,18,19]. Ιn the reviewed literature, the mean stent indwelling time was 3.56 mo for the NAGI-AXIOS-LAMS stent[4,5,11,13]. For these reasons, we decided to remove the NAGI-LAMS stents after a period of 3 mo, and our patients were followed clinically for a median of 7 mo without the need for additional interventions due to major or minor adverse events. The optimal period of stent indwelling remains to be evaluated in future studies.
One of the innovative aspects of our study is that we evaluated the efficacy, feasibility and safety of the NAGI-LAMS stent placement for the endoscopic management of symptomatic, short benign PAICSs. We report a life-saving technique that has not been previously documented, the off-label use of NAGI-LAMS stents and the application of technology to improve patient outcomes. The saddle length of this NAGI stent is longer than that of the AXIOS stent (30 mm vs 10 mm), and it may therefore be better for longer (> 1 cm) luminal GI strictures[5,10,12]. Another difference between the NAGI-LAMS and the AXIOS-LAMS is the stent diameter (diameter = 10, 12, 14, 16 mm vs 10, 15 mm)[10,12]. Stent diameter and length selection are crucial for the clinical success of the procedure (Figure 1). Given these parameters (length and diameter) of LAMS, the NAGI-LAMS stent would be effective for strictures < 30 mm in size, and the AXIOS-LAMS stent would be effective for strictures < 10 mm in size[5,11,13]. The anchoring effect of the NAGI stent stems from its bi-flanged design rather than lumen apposition[12]. Importantly, the NAGI stent delivery catheter can be introduced via colonoscopy, while the AXIOS stent delivery catheter, which is shorter, can only be delivered either via a therapeutic forward-viewing gastroscope or echoendoscope[4,5,12,17].
Our study focuses only on the endoscopic management of short (median length 1.8 cm) benign ileocolonic anastomotic strictures using a bi-flanged metal stent, not on the choice between stent placement and another treatment (e.g., re-surgery). In the literature, there is no concrete evidence of treatment preference based on long-term results. According to the literature, the average time of surgery was delayed by endoscopic management for 6.45 years. Therefore, endoscopic management (metal stent, balloon dilatation, etc.) prolongs the need for surgery for a significant period of time. Most of these benign anastomotic strictures are simple narrowings that are shorter than 2 cm, which can be successfully treated by endoscopic alternatives. Only 28% of these patients will require surgical correction, which could be technically difficult and carry the possibility of requiring colostomy. For these reasons, a part of the re-surgery strategy could include a bi-flanged metal stent, which could represent an alternative therapeutic option for this specific type of luminal stricture[30,31].
The small sample size in our study and the absence of comparative groups are significant limitations that should be acknowledged. Although our results are concordant with the current literature, there are some limitations related to the three-month indwelling time and the short duration (7 mo) of follow-up time. More prospective multicenter trials are required to develop guidelines for the utility of NAGI-LAMS in the endoscopic management of benign ileocolonic stricture. Further data are needed to validate the long-term safety and efficacy of BFMS (NAGI-LAMS) in treating luminal GI stenosis.
In conclusion, the long and broad flanges of the BFMS (NAGI-LAMS) may reduce the stent migration rate and improve patient tolerance. BFMS placement is a minimally invasive endoscopic procedure that may be beneficial as a bridge to surgery or definitive therapy when managing patients with refractory short benign post-anastomotic ileocolonic strictures. The BFMS could represent an important alternative to traditional endoscopic options and achieve better long-term results for the management of luminal GI strictures longer than 10 mm and shorter than 30 mm.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cao X, China; Virarkar M, United States S-Editor: Yan JP L-Editor: Webster JR P-Editor: Yan JP
| 1. | Majumder S, Buttar NS, Gostout C, Levy MJ, Martin J, Petersen B, Topazian M, Wong Kee Song LM, Abu Dayyeh BK. Lumen-apposing covered self-expanding metal stent for management of benign gastrointestinal strictures. Endosc Int Open. 2016;4:E96-E101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Tyberg A, Desai A, Zerbo S, Nieto J, Kahaleh M. Endoscopic management of an anastomotic stricture using a lumen-apposing metal stent. Gastrointest Endosc. 2016;83:464-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Irani S, Jalaj S, Ross A, Larsen M, Grimm IS, Baron TH. Use of a lumen-apposing metal stent to treat GI strictures (with videos). Gastrointest Endosc. 2017;85:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 4. | Larson B, Adler DG. Lumen-apposing metal stents for gastrointestinal luminal strictures: current use and future directions. Ann Gastroenterol. 2019;32:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Sharma P, McCarty TR, Chhoda A, Costantino A, Loeser C, Muniraj T, Ryou M, Thompson CC. Alternative uses of lumen apposing metal stents. World J Gastroenterol. 2020;26:2715-2728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (2)] |
| 6. | Hallac A, Srikureja W, Liu E, Dhumal P, Thatte A, Puri N. Economical effect of lumen apposing metal stents for treating benign foregut strictures. World J Gastrointest Endosc. 2018;10:294-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Jain D, Patel U, Ali S, Sharma A, Shah M, Singhal S. Efficacy and safety of lumen-apposing metal stent for benign gastrointestinal stricture. Ann Gastroenterol. 2018;31:425-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Cereatti F, Fiocca F, Dumont JL, Ceci V, Vergeau BM, Tuszynski T, Meduri B, Donatelli G. Fully covered self-expandable metal stent in the treatment of postsurgical colorectal diseases: outcome in 29 patients. Therap Adv Gastroenterol. 2016;9:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Tonolini M, Bareggi E, Salerno R. Endoscopic stenting of malignant, benign and iatrogenic colorectal disorders: a primer for radiologists. Insights Imaging. 2019;10:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Malgras B, Lo Dico R, Pautrat K, Dohan A, Boudiaf M, Pocard M, Soyer P. Gastrointestinal stenting: Current status and imaging features. Diagn Interv Imaging. 2015;96:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Bazerbachi F, Visrodia KH, Mavrogenis G, Wong Kee Song LM, Buttar NS. Extrabiliary applications of fully covered antimigration biliary metal stents. VideoGIE. 2020;5:437-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Saumoy M, Kahaleh M. Superiority of metal stents for pancreatic walled-off necrosis: bigger is better! Gastrointest Endosc. 2017;85:1253-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Santos-Fernandez J, Paiji C, Shakhatreh M, Becerro-Gonzalez I, Sanchez-Ocana R, Yeaton P, Samarasena J, Perez-Miranda M. Lumen-apposing metal stents for benign gastrointestinal tract strictures: An international multicenter experience. World J Gastrointest Endosc. 2017;9:571-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP; SCARE Group. The SCARE 2018 statement: Updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. 2018;60:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2070] [Article Influence: 295.7] [Reference Citation Analysis (0)] |
| 15. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13293] [Article Influence: 830.8] [Reference Citation Analysis (0)] |
| 16. | Caruso A, Conigliaro R, Manta R, Manno M, Bertani H, Barbera C, Mirante VG, Frazzoni M. Fully covered self-expanding metal stents for refractory anastomotic colorectal strictures. Surg Endosc. 2015;29:1175-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Bazerbachi F, Heffley JD, Abu Dayyeh BK, Nieto J, Vargas EJ, Sawas T, Zaghlol R, Buttar NS, Topazian MD, Wong Kee Song LM, Levy M, Keilin S, Cai Q, Willingham FF. Safety and efficacy of coaxial lumen-apposing metal stents in the management of refractory gastrointestinal luminal strictures: a multicenter study. Endosc Int Open. 2017;5:E861-E867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Yang D, Nieto JM, Siddiqui A, Riff BP, DiMaio CJ, Nagula S, Ismail AM, Ngamreungphong S, Khashab MA, Wagh MS, Tzimas D, Buscaglia JM, Strand DS, Wang AY, Chauhan SS, Forsmark CE, Draganov PV. Lumen-apposing covered self-expandable metal stents for short benign gastrointestinal strictures: a multicenter study. Endoscopy. 2017;49:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Jessamy K, Ozden N, Simon HM, Kobrossi S, Ubagharaji E. Self-Expanding Metal Stenting in the Management of a Benign Colonic Stricture. Case Rep Gastroenterol. 2016;10:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Ngamruengphong S, Sharaiha RZ, Sethi A, Siddiqui AA, DiMaio CJ, Gonzalez S, Im J, Rogart JN, Jagroop S, Widmer J, Hasan RA, Laique S, Gonda T, Poneros J, Desai A, Tyberg A, Kumbhari V, El Zein M, Abdelgelil A, Besharati S, Hernaez R, Okolo PI, Singh V, Kalloo AN, Kahaleh M, Khashab MA. Endoscopic suturing for the prevention of stent migration in benign upper gastrointestinal conditions: a comparative multicenter study. Endoscopy. 2016;48:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Martínez Alcalá F, Martínez-Alcalá García FR, Sánchez-Yague A, Martínez-Alcalá García A, Ciria Avila JA, Perez Pozo JM. Treatment of a benign, anastomotic refractory rectal stricture with an AXIOS stent. Endoscopy. 2015;47 Suppl 1 UCTN:E413-E414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Saumoy M, Yarber C, Kahaleh M. Novel Uses of Lumen-Apposing Metal Stents. Gastrointest Endosc Clin N Am. 2018;28:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Mussetto A, Fugazza A, Fuccio L, Triossi O, Repici A, Anderloni A. Current uses and outcomes of lumen-apposing metal stents. Ann Gastroenterol. 2018;31:535-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Mohan BP, Chandan S, Garg R, Mohamed S, Shakhatreh M, Dugyala S, Mashiana HS, Ponnada S, Asokkumar R, Adler DG. Lumen-apposing Metal Stents, Fully Covered Self-expanding Metal Stents, and Biodegradable Stents in the Management of Benign of GI Strictures: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2019;53:560-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Adler DG. Colonic strictures: dilation and stents. Gastrointest Endosc Clin N Am. 2015;25:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Tan S, Zhong C, Huang S, Luo X, Xu J, Fu X, Peng Y, Tang X. Clinical outcomes of lumen-apposing metal stent in the management of benign gastrointestinal strictures: a systematic review and meta-analysis. Scand J Gastroenterol. 2019;54:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Jain D, Chhoda A, Sharma A, Singhal S. De-novo Gastrointestinal Anastomosis with Lumen Apposing Metal Stent. Clin Endosc. 2018;51:439-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Reddy R, Patel U, Tarnasky P, Kedia P. Lumen-apposing stent placement for management of a short benign colonic anastomotic stricture. VideoGIE. 2018;3:99-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Nasser A, Cullen M, Barawi M. Lumen-apposing metal stent use to maintain a surgical anastomosis. VideoGIE. 2020;5:494-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Liu Z, Wang G, Yang M, Chen Y, Miao D, Muhammad S, Wang X. Ileocolonic anastomosis after right hemicolectomy for colon cancer: functional end-to-end or end-to-side? World J Surg Oncol. 2014;12:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 31. | Lian L, Stocchi L, Remzi FH, Shen B. Comparison of Endoscopic Dilation vs Surgery for Anastomotic Stricture in Patients With Crohn's Disease Following Ileocolonic Resection. Clin Gastroenterol Hepatol. 2017;15:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |