Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10146
Peer-review started: February 19, 2022
First decision: April 17, 2022
Revised: April 29, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: October 6, 2022
Processing time: 219 Days and 20.4 Hours
Small intestinal cavernous hemangioma is a rare disease, especially in the ileum. It is difficult to accurately diagnose due to its hidden location and nonspecific clinical symptoms. Here, we reported a case of ileal cavernous hemangioma with chronic hemorrhage in a 20-year-old man and review the literature to gain a better understanding of this disease.
The patient complained of intermittent melena and hematochezia for > 3 mo. The lowest hemoglobin level revealed by laboratory testing was 3.4 g/dL (normal range: 12-16 g/dL). However, the gastroscopy, colonoscopy and peroral double-balloon enteroscopy (DBE) showed no signs of bleeding. The transanal DBE detected a lesion at about 340 cm proximal to the ileocecal valve. Thus, we per
This report might improve the diagnosis and treatment of ileal cavernous hemangioma.
Core Tip: Small intestinal hemangiomas are rare benign tumors, and ileal cavernous hemangiomas are even rarer. Here, we reported a 20-year-old man with intermittent melena and hematochezia for > 3 mo. We successively performed colonoscopy, gastroscopy, contrast-enhanced computed tomography, transoral double-balloon endoscopy, and transanal double-balloon endoscopy, and finally found a lesion in the ileum. The lesion was resected by laparoscopy. Thus, to further improve the diagnosis and treatment of this disease, we collected cases of ileal cavernous hemangioma in the past 20 years and summarized its manifestations, complications, imaging features, endoscopic features, and therapeutic strategies.
- Citation: Yao L, Li LW, Yu B, Meng XD, Liu SQ, Xie LH, Wei RF, Liang J, Ruan HQ, Zou J, Huang JA. Cavernous hemangioma of the ileum in a young man: A case report and review of literature. World J Clin Cases 2022; 10(28): 10146-10154
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10146.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10146
Small intestinal hemangiomas are rare vascular malformations and account for 7%-10% of all benign tumors in the small intestine. Among them, 60% occur in the jejunum, and it is rare in the ileum[1,2]. Histologically, small intestinal hemangioma is a congenital vascular malformation that can be classified as capillary, cavernous or mixed-type, with cavernous hemangioma being the most prevalent[3]. It can be manifested as abdominal pain, gastrointestinal bleeding, intestinal obstruction, intussusception, and intestinal perforation in some patients[4-6]. Due to the lack of specific clinical symptoms and distinctive auxiliary examination characteristics, omission or misdiagnosis might occur. Recently, with the advancements in enteroscopy, the detection rates of small bowel diseases have increased, but rare cases of small intestinal cavernous hemangioma, especially cavernous hemangioma of the ileum, are still misdiagnosed. Here, we report a case of gastrointestinal bleeding caused by ileal cavernous he
On June 11, 2021, a 20-year-old man was hospitalized for intermittent melena and hematochezia that had persisted for > 3 mo.
For three months before hospitalization, the patient experienced intermittent melena between 50 and 200 g, up to twice daily, accompanied by dizziness and fatigue. He was admitted to the department of hematology of our hospital, and the laboratory testing revealed severe anemia (hemoglobin, 5.7 g/dL), with microcytic and hypochromic anemia. Thus, iron deficiency anemia was diagnosed. The treatment with blood transfusion and ferrous gluconate syrup improved the anemia-related symptoms and the hemoglobin levels increased to 6.0 g/dL. For diagnosis and treatment, he was admitted to our department 2 mo ago. His hemoglobin level was 6.2 g/dL, and the fecal occult blood test was positive. The gastroscopy, colonoscopy and contrast-enhanced computed tomography (CECT) examination suggested no specific abnormalities. Hence, small bowel endoscopy was recommended to determine the cause of bleeding, but the patient refused and was discharged. Nonetheless, the melena relapsed 9 d ago, averaging about 300 g each time, accompanied by dizziness and aggravated fatigue. He was sent to a nearby hospital and had a hemoglobin level of only 3.4 g/dL. After a blood transfusion, the symptoms of anemia were improved. Four days ago, the melena relapsed again, and the situation appeared to have significantly deteriorated and he returned to the department of gastroenterology of our hospital.
The patient had no past medical history.
The patient did not present previous personal and family medical history.
At admission, his heart rate was 92 beats/min and his blood pressure was 12.1/6.9 kPa. He was pale, and the other physical examinations were normal.
The laboratory testing revealed moderated microcytic and hypochromic anemia (hemoglobin: 6.95 g/dL). The fecal occult blood test was positive. The liver and kidney function, coagulation function, carcinoembryonic antigen, carbohydrate antigen 19-9, and cancer antigen 125 were normal.
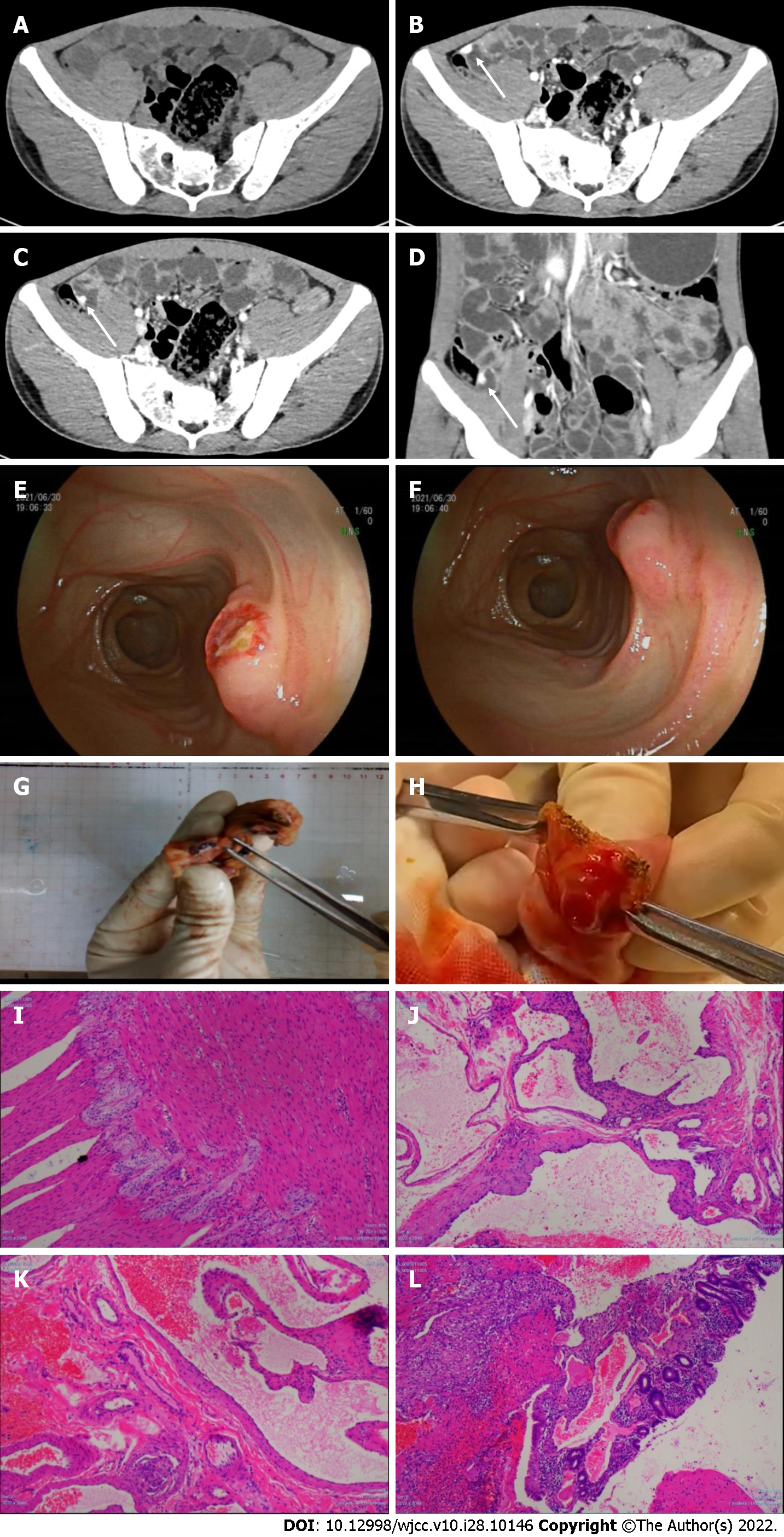
Endoscopy re-examination: Gastroscopy showed that the corpus had a spot of flushing and erosion, but there was no bleeding, and the stomach cavity was clean. The colonoscopy revealed dark red blood at the end of the ileum. After flushing with normal saline, there were no abnormalities in the mucosa, but dark red blood continued to gradually drip down from the ileum, indicating small intestinal bleeding. CECT re-examination (SOMATOM Definition Flash, Siemens, Germany) showed a lesion in the ileum that was enhanced in the arterial and venous phases (Figure 1A-D).
Peroral double-balloon enteroscopy (DBE): Mild inflammation of the upper jejunum was detected. Transanal DBE reached the upper segment of the ileum. In the middle and lower segments, the mucosa was rough and eroded, and the villi were absent. A submucosal lesion approximately 1.2 cm × 1.0 cm was found at about 340 cm proximal to the ileocecal valve with ulcer formation (Figure 1E and F). The surrounding mucosa was flushed, presented with edema, was hard in texture, easy to bleed, and had numerous thickened blood vessels supplying the tumor. The endoscopic diagnosis was: (1) Ileal submucosal bulge (stromal tumor? vascular malformation?); and (2) Ileal inflammation.
Our clinical diagnosis was small intestinal vascular malformation. The postoperative pathological diagnosis revealed ileal cavernous hemangioma.
First, laparoscopic exploration was performed and a tortuous collection of irregular vessels was found at about 300 cm proximal to the ileocecal valve. Then, the intestine was longitudinally opened near the irregular vessels. A 1.3 cm × 1.2 cm submucosal lesion with an ulcer (0.3 cm × 0.5 cm) and pulsatile bleeding on the surface was found in the ileum. The lesion was completely resected (Figure 1G-L), and we have uploaded a video that helps in understanding the morphology of the lesion (Video 1). The optical microscopy showed twisted and dilated blood vessels in the submucosa, surrounding the bleeding onset. Finally, the pathological diagnosis was cavernous hemangioma of the ileum.
The patient quickly recovered and had no further episodes of bleeding after the operation. He returned to our hospital for a follow-up visit 1 mo after the operation and his hemoglobin levels increased to 10.7 g/dL (normal range: 12-16 g/dL).
Small intestinal bleeding accounts for 5%-10% of all gastrointestinal bleeding[7]. Hence, it should be considered for persistent or recurrent bleedings that are not detected by routine endoscopy, including upper gastrointestinal endoscopy and colonoscopy. The common etiologies of small intestinal bleeding in individuals younger than 40 years include Crohn’s disease, tumor, Merkel’s diverticulum, Dieulafoy lesions, vasodilatory disorder, and polyp syndrome[7].
Small intestinal hemangiomas are rare vascular malformations that can cause small intestinal hemorrhages. They can occur in various segments of the small intestine, but are more frequent in the jejunum and rare in the ileum[8-10]. Pathologically, hemangiomas are classified into capillary, cavernous and mixed types, with capillary types being the most common[3]. Here, we presented a case of cavernous hemangioma in the upper ileum causing recurrent gastrointestinal bleeding.
Furthermore, a small intestinal hemangioma can originate from the mucosal, submucosa, muscularis propria, or even serosal layer[11]. Its most common manifestations include recurrent gastrointestinal bleeding and iron deficiency anemia, which might be accompanied by nonspecific symptoms such as dizziness and fatigue[12]. Generally, no specific findings are observed in the laboratory testing, making it difficult to diagnose. Thus, to analyze the clinicopathological features of ileal cavernous hemangiomas, we searched PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Google Scholar (https://scholar.google.com/) databases between 2001 and 2021 using “hemangioma”, “ileum”, “intestinal”, and “cavernous” as keywords. The language was limited to English. Case reports were included if the final diagnosis was ileal cavernous hemangioma. The exclusion criteria were: Non-English literature; location of the hemangioma was not mentioned; and nonhuman ileal cavernous hemangioma.
A total of 15 cases (9 women and 6 men; mean age: 30.3 years) were retrieved and reviewed (Table 1)[1,13-26]. The most common symptoms (73.3%) were related to anemia, including fatigue, weakness, pallor, dizziness, loss of consciousness, and even syncope. Melena was the second most common symptom (40.0%). Nonspecific symptoms of the digestive system (33.3%) included abdominal pain, nausea, vomiting, bloating, abdominal mass and loss of appetite (Table 1). Among cases, a 6-year-old girl developed hemangioma hemorrhage after a mild abdomen blunt trauma, and a diagnostic cum therapeutic laparoscopy was performed[14]. Our current patient had melena as the main symptom, accompanied by anemia-related dizziness and fatigue, consistent with the literature.
| Ref. | Age (yr) | Sex | Manifestation | Preoperative diagnosis study | Imaging | Endoscopic feature | Preoperative diagnosis | Size (cm) | Complications |
| Pinho et al[13], 2008 | 9 | F | Fatigue, nausea, dizziness, growth retardation, melena | 99 m-Tc-RBC-scintigraphy, CE | NM | A bluish polypoid mass with a dark spot | Y | 2 × 2.5 | None |
| Abdul Aziz et al[14], 2011 | 6 | F | Abdominal pain, pallor, vomit, lethargy, bloating, abdominal mass | X-ray, US | NM | Submucosal hematoma | N | NA | None |
| Huang et al[15], 2013 | 64 | F | Abdominal pain, nausea, vomit, bloating, abdominal mass | MRI, colonoscopy | Wall thickening | Submucosal hematoma | N | NA | Intussusception, perforation |
| Fernandes et al[16], 2014 | 58 | F | Melena, syncope, melena | Colonoscopy, ileoscopy, CE, CT | Wall thickening, calcification | NM | Y | 14 | None |
| Purdy-Payne et al[17], 2015 | 20 | F | Abdominal pain, nausea, vomit, syncope | CT, colonoscopy | Calcification | Annular growth nodular irregular mass | Y | 4 × 3.5 × 3 | None |
| Ocampo Toro et al[18], 2018 | 29 | M | Chronic anemia | Small bowel study (SBS) with barium, CECT, X-ray | Wall thickening, calcification | NM | Y | 10 | None |
| Kano et al[19], 2021 | 29 | M | None | CECT, DBE | Wall thickening | Multiple reddish-purple submucosal masses | Y | 15 × 6 | None |
| Magnano et al[20], 2005 | 13 | M | Fatigue, malaise, weakness, pallor, anemia | Gastroscopy, colonoscopy, technetium 99m-Tc-RBC-scintigraphy, mesenteric arteriography, CE | NM | A large polypoid erythematous lesion | N | 2 | None |
| Chan et al[21], 2006 | 36 | F | Dizziness, loss of consciousness, pallor | Gastroendoscopy, X-ray, colonoscopy, 99m-Tc-RBC-scintigraphy, angiogram, Proctoscopy, DBE | NM | Two large hemangiomas appeared to be vascular and thrombosed | Y | 1 & 3, respectively | None |
| Quentin et al[22], 2007 | 32 | F | Fresh red blood stool, anemia | Gastroscopy, colonoscopy, CE | NM | A submucosal and circumferential lesion, blue-colored, with superficial red spots | Y | 2 | None |
| Chen et al[23], 2009 | 23 | M | Fatigue, dizziness, chest pain, dyspnea on exertion, anemia | CE | NM | A purple-blue subepithelial mass | Y | 3 × 2.5 | None |
| Guardiola et al[24], 2012 | 19 | M | Anemia, melena | Gastroscopy, colonoscopy, small bowel barium, CT, CE | Wall thickening | A large violet-colored polypoid submucosal lesion | N | 8 | None |
| Peng et al[25], 2016 | 47 | M | Melena, anemia, weakness, dizziness | CE, CECT | NM | A huge heterogeneous mixed mass | N | 50 × 15 | None |
| Hu et al[1], 2018 | 24 | F | Melena, fatigue, anemia | Gastroscopy, colonoscopy, CE, DBE | NM | A reddish-purple lesion | Y | 5 × 3 × 3 | None |
| Majethia et al[26], 2021 | 45 | F | Vomiting, abdominal pain, loss of appetite | CECT | NM | NM | N | NM | Obstruction |
Nine of the 15 lesions (60%) were diagnosed by endoscopy before surgery. Among them, five were detected by capsule endoscopy, two by DBE, one by both capsule endoscopy (CE) and DBE, and one in the terminal ileum by colonoscopy. In our current case, colonoscopy revealed dark red blood near the end of the ileum and the fecal occult blood was positive. However, Fernandes et al[16] performed a colonoscopy and ileoscopy during asymptomatic disease and discovered no sign of blood or bleeding lesions. Therefore, we hypothesized that conducting an emergency endoscopy during the active stage of the disease might improve diagnostic performance. Regarding the endoscopic characteristics of ileal cavernous hemangioma in the literature reviewed, five cases were represented as submucosal lesions, four as polypoids, two as irregular nodules that can be easily misdiagnosed as cancers, and one as a large heterogeneous mixed lesion. Three case reports did not describe the morphology of the lesions in detail. The surface of the lesion can present as erythema, thrombosis or bleeding. The color of the lesion was mostly reddish-purple, blue, or purple–blue, indicating a vascular network. The patient reported here also had a submucosal lesion, but with ulcers on the surface and no blue–purple appearance, thrombosis, or bleeding, and could be easily misdiagnosed as a stromal tumor, especially a malignant stromal tumor. Therefore, we should strengthen our understanding of the characteristics of ileal cavernous hemangioma to prevent misdiagnoses. CT is a noninvasive examination but has limited significance in diagnosing small intestinal hemangioma. In contrast, CT can be helpful to initially determine the location and general shape of the lesion and guide the next step of endoscopy or surgery. Thus, we summarized the imaging features of these 15 patients: Five (33.3%) presented with intestinal wall thickening and three (20.0%) with calcifications. These results were different from those of Fu et al[12], in which 50% of patients had phleboliths. CT imaging of our patient revealed wall thickening and enhancement in the arterial and venous phases, consistent with previous reports[27]. Then, we investigated the hemangioma size of five patients with wall thickening of the ileum (one case did not mention the size[15]). The median hemangioma length of these patients was 4 cm, and the mean diameter was 9.5 cm. Besides, the lesions either originated from the submucosa or reached the submucosa. Thus, we suggest that the thickening of the intestinal wall might indicate the size and depth of the lesions. Furthermore, among the cases reviewed, one had secondary intussusception with perforation[15], consistent with previous reports[4].
In these 15 cases, various preoperative diagnostic methods were used, including CECT (46.7%), 99m-Tc-RBC-scintigraphy (20%), and magnetic resonance imaging (6.7%). CECT can clearly show the structures of the intestinal wall and extraintestinal lesions in multiple directions and angles, which can initially be used to determine the location and general shape of the lesions, contributing to more information for clinical diagnosis. Moreover, CE and DBE are used for diagnosing small bowel diseases and there is no significant difference in the overall diagnosis rate between these methods (50% and 53%, respectively)[28]. CE is noninvasive, painless, and tolerable[29]. However, disadvantages might also exist due to the inability of tissue biopsy or endoscopic treatment, inaccurate positioning, and being unsuitable for patients with massive bleeding or intestinal obstruction[30,31]. Meanwhile, DBE has intuitiveness, operability, biopsy feasibility, as well as the efficacy of endoscopic treatment, which significantly improves the diagnosis and treatment of small bowel diseases. However, the high cost, invasiveness, and risk of acute pancreatitis or intestinal perforation in DBE should not be ignored[32,33]. For better understanding, we summarize the advantages and disadvantages between CE and DBE in Table 2. Endoscopic treatments such as electrocoagulation and titanium clamp hemostasis are suitable for some small intestinal bleeding caused by vascular diseases. For hemangioma, endoscopic biopsy is not recommended, since it can easily lead to uncontrollable bleeding. In our current case, the patient presented with active bleeding, and the cause of gastrointestinal bleeding was highly suspected as vascular malformations. Thus, we selected DBE to diagnose and perform the endoscopic treatment.
| Capsule endoscopy | Double-balloon enteroscopy | |
| Diagnosis rate | 50% | 53% |
| Invasive | N | Y |
| Tissue biopsy | N | Y |
| Endoscopy treatment | N | Y |
| Cost | Low | High |
| Technical difficulty | Low | High |
| Complications | Obstruction | Perforation |
Polidocanol injection using DBE is a safe, feasible and effective treatment method for small intestinal hemangioma[34]. A retrospective cohort study showed that 66.3% of individuals improved after receiving DBE polidocanol injections. Additionally, in younger patients, the effectiveness rate was 100% during long-term follow-up[35]. However, for large or diffuse hemangiomas, or lesions originating from the submucosa or deeper layers, the endoscopic procedure might result in perforation and uncontrolled bleeding. In this case, surgical resection might be safer[1] and was selected to finally treat our patient.
Small intestinal cavernous hemangioma is a rare disease. Its most common symptoms are anemia and melena. CECT can initially be used to determine the location and general shape of the lesions. The wall thickening and high-density shadow in the CECT might have diagnostic value for small intestinal cavernous hemangiomas. CE and DBE can contribute to the identification of lesions, mostly represented as submucosal masses, polypoid or irregular nodules. A bluish-purple vascular network is generally visible on the surface of a typical lesion, and atypical lesions are easily misdiagnosed as stromal tumors and other cancers. Regarding the detection rate, there is no significant difference between CE and DBE, but, since CE is noninvasive, it is more recommended to detect the lesions. Both endoscopy and surgery are feasible for this disorder. However, for large or diffuse hemangiomas or lesions derived from the submucosa, surgical resection might be chosen. A detailed treatment plan for each case should be prepared to effectively manage patients with variable risks. Finally, more case reports or observational studies are needed to help in the diagnosis and treatment.
We would like to thank Zhao B for operating on the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kumar S, India; Shariati MBH, Iran; Singh Y, United States S-Editor: Yan JP L-Editor: Kerr C P-Editor: Yan JP
| 1. | Hu PF, Chen H, Wang XH, Wang WJ, Su N, Shi B. Small intestinal hemangioma: Endoscopic or surgical intervention? World J Gastrointest Oncol. 2018;10:516-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Varma JD, Hill MC, Harvey LA. Hemangioma of the small intestine manifesting as gastrointestinal bleeding. Radiographics. 1998;18:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Beraldo RF, Marcondes MB, da Silva DL, Grillo TG, Baima JP, de Barros JR, Quera R, Saad-Hossne R, Sassaki LY. Small Intestinal Hemangioma: A Case Report. Am J Case Rep. 2021;22:e929618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Hyun BH, Palumbo VN, Null RH. Hemangioma of the small intestine with gastrointestinal bleeding. JAMA. 1969;208:1903-1905. [PubMed] [DOI] [Full Text] |
| 5. | Gordon FH, Watkinson A, Hodgson H. Vascular malformations of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2001;15:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Rao AB, Pence J, Mirkin DL. Diffuse infantile hemangiomatosis of the ileum presenting with multiple perforations: a case report and review of the literature. J Pediatr Surg. 2010;45:1890-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Editorial Board of Chinese Journal of Digestion. Consensus on diagnosis and treatment of small bowel bleeding (2018, Nanjing). Zhonghua Xiaohua Zazhi. 2018;38:577-582. [DOI] [Full Text] |
| 8. | Fan GW, Chen TH, Lin WP, Su MY, Sung CM, Hsu CM, Chi CT. Angiodysplasia and bleeding in the small intestine treated by balloon-assisted enteroscopy. J Dig Dis. 2013;14:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ruiz AR Jr, Ginsberg AL. Giant mesenteric hemangioma with small intestinal involvement: an unusual cause of recurrent gastrointestinal bleed and review of gastrointestinal hemangiomas. Dig Dis Sci. 1999;44:2545-2551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Garvin PJ, Herrmann V, Kaminski DL, Willman VL. Benign and malignant tumors of the small intestine. Curr Probl Cancer. 1979;3:1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Handra-Luca A, Montgomery E. Vascular malformations and hemangiolymphangiomas of the gastrointestinal tract: morphological features and clinical impact. Int J Clin Exp Pathol. 2011;4:430-443. [PubMed] |
| 12. | Fu JX, Zou YN, Han ZH, Yu H, Wang XJ. Small bowel racemose hemangioma complicated with obstruction and chronic anemia: A case report and review of literature. World J Gastroenterol. 2020;26:1674-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Pinho R, Rodrigues A, Proença L, Silva AP, Fernandes S, Leite S, Amaral I, de Sousa P, Fraga J. Solitary hemangioma of the small bowel disclosed by wireless capsule endoscopy. Gastroenterol Clin Biol. 2008;32:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Abdul Aziz DA, Khandasamy Y, Tamba RP, Zaki FM. Bleeding small bowel cavernous haemangioma following blunt trauma to the abdomen presenting as subacute intestinal obstruction in a child. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Huang Y, Zhang Q, Feng JF, Liu H, Liu J. Adult intussusception with perforation caused by hemangioma in the distal ileum. Kaohsiung J Med Sci. 2013;29:582-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Fernandes D, Dionísio I, Neves S, Duarte P. Cavernous hemangioma of small bowel: a rare cause of digestive hemorrhage. Rev Esp Enferm Dig. 2014;106:214-215. [PubMed] |
| 17. | Purdy-Payne EK, Miner JF, Foles B, Tran TA. The "Endothelialized Muscularis Mucosae": A Case Report Describing a Large Cavernous Hemangioma at the Terminal Ileum and a New Histologic Clue for Preoperative Diagnosis from Endoscopic Biopsy. Case Rep Gastrointest Med. 2015;2015:454836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Ocampo Toro WA, Corral Ramos B, Concejo Iglesias P, Cubero Carralero J, Blanco García DF, Barón Ródiz P. Haemangiomas of the Small Intestine: Poorly Known Cause of Gastrointestinal Bleeding of Uncertain Origin. Cureus. 2018;10:e3155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kano T, Fukai S, Okamoto R, Motomura Y, Lefor AK, Mizokami K. An incidentally identified 15 cm cavernous hemangioma of the small intestine: Case report and literature review. Int J Surg Case Rep. 2021;84:106144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Magnano A, Privitera A, Calogero G, Nanfito' L, Basile G, Sanfilippo G. Solitary hemangioma of the small intestine: an unusual cause of bleeding diagnosed at capsule endoscopy. J Pediatr Surg. 2005;40:e25-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Chan AO, Lai KC. A patient with long-standing iron-deficient anemia. Nat Clin Pract Gastroenterol Hepatol. 2006;3:112-6; quiz 117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Quentin V, Lermite E, Lebigot J, Marinnes MZ, Arnaud JP, Boyer J. Small bowel cavernous hemangioma: wireless capsule endoscopy diagnosis of a surgical case. Gastrointest Endosc. 2007;65:550-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Chen CH, Jones J, McGowan P. Profound iron deficiency anemia caused by a small-intestinal cavernous hemangioma. Gastrointest Endosc. 2009;69:1392-3; discussion 1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Guardiola A, Navajas J, Valle J, López-Pardo R, Rodríguez-Merlo R, Lombera Mdel M, Alcántara M. Small bowel giant cavernous hemangioma diagnosed by capsule endoscopy. Rev Esp Enferm Dig. 2012;104:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Peng C, Chen H, Li W, Xu R, Zhuang W. A Rare Cause of Recurrent Gastrointestinal Bleeding: Giant Diffuse and Cavernous Intestinal Mesentery Hemangioma in an Adult. Dig Dis Sci. 2016;61:3363-3365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Majethia HV, Dhakre VW, Gheewala H, Bhuta P. Ileal cavernous haemangioma in an adult presenting as a rare cause of small bowel obstruction. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Kumar N, Adam SZ, Goodhartz LA, Hoff FL, Lo AA, Miller FH. Beyond hepatic hemangiomas: the diverse appearances of gastrointestinal and genitourinary hemangiomas. Abdom Imaging. 2015;40:3313-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol. 2015;110:1265-87; quiz 1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 30. | Ben Soussan E, Antonietti M, Hervé S, Savoye G, Ramirez S, Lecleire S, Ducrotté P, Lerebours E. Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol. 2004;28:1068-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Akahoshi K, Kubokawa M, Matsumoto M, Endo S, Motomura Y, Ouchi J, Kimura M, Murata A, Murayama M. Double-balloon endoscopy in the diagnosis and management of GI tract diseases: Methodology, indications, safety, and clinical impact. World J Gastroenterol. 2006;12:7654-7659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Mensink PB, Haringsma J, Kucharzik T, Cellier C, Pérez-Cuadrado E, Mönkemüller K, Gasbarrini A, Kaffes AJ, Nakamura K, Yen HH, Yamamoto H. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 33. | Möschler O, May A, Müller MK, Ell C; German DBE Study Group. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Aoyama T, Fukumoto A, Shigita K, Asayama N, Mukai S, Nagata S. Successful Endoscopic Sclerotherapy Using Polidocanol for Small Bowel Hemangioma. Intern Med. 2020;59:1727-1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Hindryckx P, Botelberge T, De Vos M, De Looze D. Clinical impact of capsule endoscopy on further strategy and long-term clinical outcome in patients with obscure bleeding. Gastrointest Endosc. 2008;68:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |