Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10120
Peer-review started: January 25, 2022
First decision: March 23, 2022
Revised: March 25, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: October 6, 2022
Processing time: 245 Days and 0.3 Hours
Nocardia paucivorans is an infrequently found bacterium with the potential to cause severe infection, with a predilection for the central nervous system, both in immunocompromised and immunocompetent individuals. Rapid etiological diagnosis of nocardiosis can facilitate timely and rational antimicrobial treatment. Metagenomic next-generation sequencing (mNGS) can improve the rate and reduce the turnaround time for the detection of Nocardia.
A 49-year-old man was admitted to hospital with cough and hemoptysis. Imaging revealed pulmonary consolidation as well as multiple brain lesions. Nocardia asiatica and Nocardia beijingensis were rapidly detected by mNGS of bronchoalveolar lavage fluid (BALF) while bacterial culture of BALF and pathological biopsy of lung tissue were negative. In early stages, he was treated with trimethoprim-sulfamethoxazole (TMP-SMZ) and linezolid by individual dose adjustment based on serum concentrations and the adverse effects of thrombocytopenia and leukopenia. The treatment was then replaced by TMP-SMZ and ceftriaxone or minocycline. He was treated with 8 mo of parenteral and/or oral antibiotics, and obvious clinical improvement was achieved with resolution of pulmonary and brain lesions on repeat imaging.
mNGS provided fast and precise pathogen detection of Nocardia. In disseminated nocardiosis, linezolid is an important alternative that can give a better outcome with the monitoring of linezolid serum concentrations and platelet count.
Core Tip: Early detection of Nocardia paucivorans can optimize antibiotic management, shorten hospital stays and improve survival rates. We report rapid detection of N. paucivorans by metagenomic next-generation sequencing (mNGS) of BALF. The patient was treated with trimethoprim-sulfamethoxazole (TMP-SMZ) and linezolid by individual dose adjustment based on serum concentration and thrombocytopenia and leukopenia, then replaced by TMP-SMZ and ceftriaxone or minocycline. This case suggests that mNGS is a convenient and efficient technique for detecting Nocardia, especially suitable for rare, novel and atypical etiologies of complicated infectious diseases. Linezolid may be an important alternative in disseminated nocardiosis.
- Citation: Li T, Chen YX, Lin JJ, Lin WX, Zhang WZ, Dong HM, Cai SX, Meng Y. Successful treatment of disseminated nocardiosis diagnosed by metagenomic next-generation sequencing: A case report and review of literature. World J Clin Cases 2022; 10(28): 10120-10129
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10120.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10120
Nocardia paucivorans is a Gram-positive aerobic bacterium of the order Actinomycetales and has become an increasingly important opportunistic pathogen, mostly in immunocompromised individuals[1]. It causes a wide spectrum of illness, including pneumonia, cutaneous or subcutaneous infections, brain or other solid organ abscesses, thus resulting in high mortality[2]. Early detection of N. paucivorans can optimize antibiotic management, shorten hospital stays, improve survival rates and reduce medical costs. Delaying diagnosis may impede targeted therapies and lead to poor prognosis. However, the definitive diagnosis of nocardiosis still depends on the isolation and identification of organisms from the infected site, which may take days to weeks and the positive rates by these methods are very low. As these methods cannot meet the current demand for rapid and accurate diagnosis, metagenome next- generation sequencing (mNGS) has become available in clinical practice[3]. mNGS also known as shotgun deep-sequencing, is a high-throughput sequencing approach with more rapid and accurate diagnostic advantages than traditional methods, especially in culture-negative samples[4,5]. mNGS can simultaneously detect and identify pathogens by extracting only a small amount of DNA or RNA from samples. mNGS can theoretically detect all pathogens in clinical samples, especially for rare, novel, and atypical etiologies of complex infectious diseases[6]. Here, we describe a case of disseminated nocar
A 49-year-old Chinese man had symptoms of cough and hemoptysis for 2 mo.
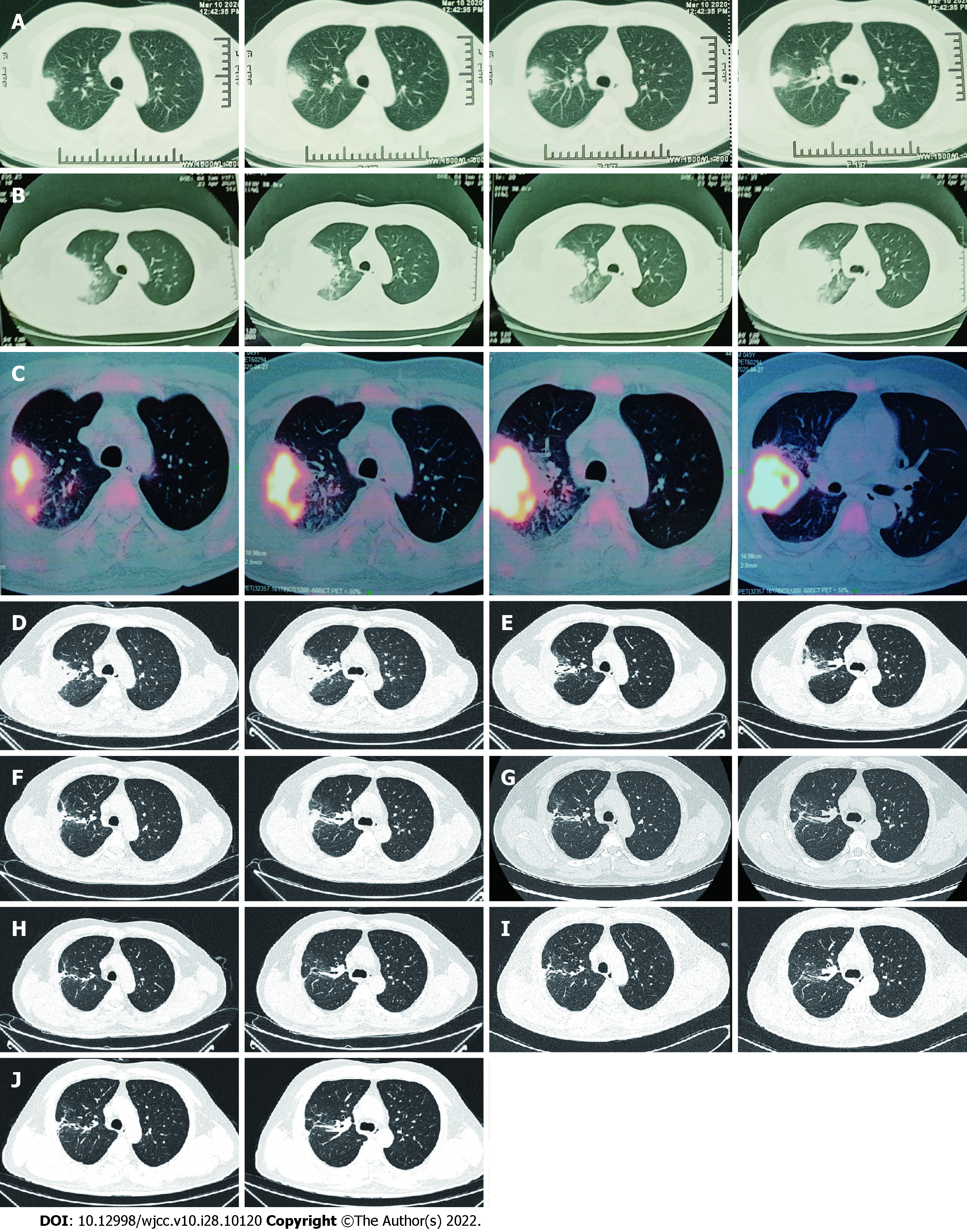
A 49-year-old Chinese man presented to the hospital with 2 mo of cough and hemoptysis, but without fever or chest pain. Daily blood loss was estimated to be 20-30mL. During a visit to a different hospital on March 10, 2020, chest computed tomography (CT) demonstrated a mass and atelectasis in the right upper lobe (Figure 1A). BALF was obtained by brochoscopy; however, the bacterial culture of BALF was negative. The patient was diagnosed with pulmonary infection and treated with oral cefixime capsules (0.2g every 12h) and azithromycin (500 mg/d) for 1 wk. However, there was no improvement and the patient was in a poor general status. He had dyspnea when walking hastily or going up stairs and had chest pain. Chest CT on April 23, 2020 showed aggravation of the original lesion, especially in the right upper lobe (Figure 1B). He was admitted to our respiratory department for further evaluation on April 25, 2020.
The patient had no previous medical history, and no history of drug allergy.
The patient worked as a welder and lived with his wife and children. He had no history of cigarette smoking or alcohol abuse. There was no family history to note.
On admission, the patient was afebrile and vital signs were normal. Breath sounds were decreased in the right lung, and neurological examination was unremarkable.
The oxygenation index in blood gas analysis was 265 mmHg (Table 1). Leukocytosis was 14.36 × 109/L, C-reactive protein was 117.50 mg/L, and procalcitonin was 0.126 ng/mL, and liver and kidney functions were normal. GeneXpert MTB/RIF, quantitative immunoglobulins, 1-3-β-D-glucan, galactomannan (GM), cryptococcal and deep sputum bacteriological culture antigen were all negative.
| April 25, 2020 | May 23, 2020 | June 16, 2020 | |
| Cough | 2 | 1 | 0 |
| Hemoptysis | 20-30 mL | 0 | 0 |
| Dyspnea index | 2 | 1 | 0 |
| Supplemental O2 in L/min | 4 L/min | 2 L/min | 0 |
| Oxygenation index | 265 mmHg | 388 mmHg | 412 mmHg |
Positron emission tomography (PET)-CT was ordered on the suspicion of lung cancer. PET-CT showed that most of the mass lesions in the posterior segment of the right upper lobe had significantly increased glucose metabolism inhomogeneously with peripheral exudative changes, which were suspicious for infection on April 27, 2020 (Figure 1C).
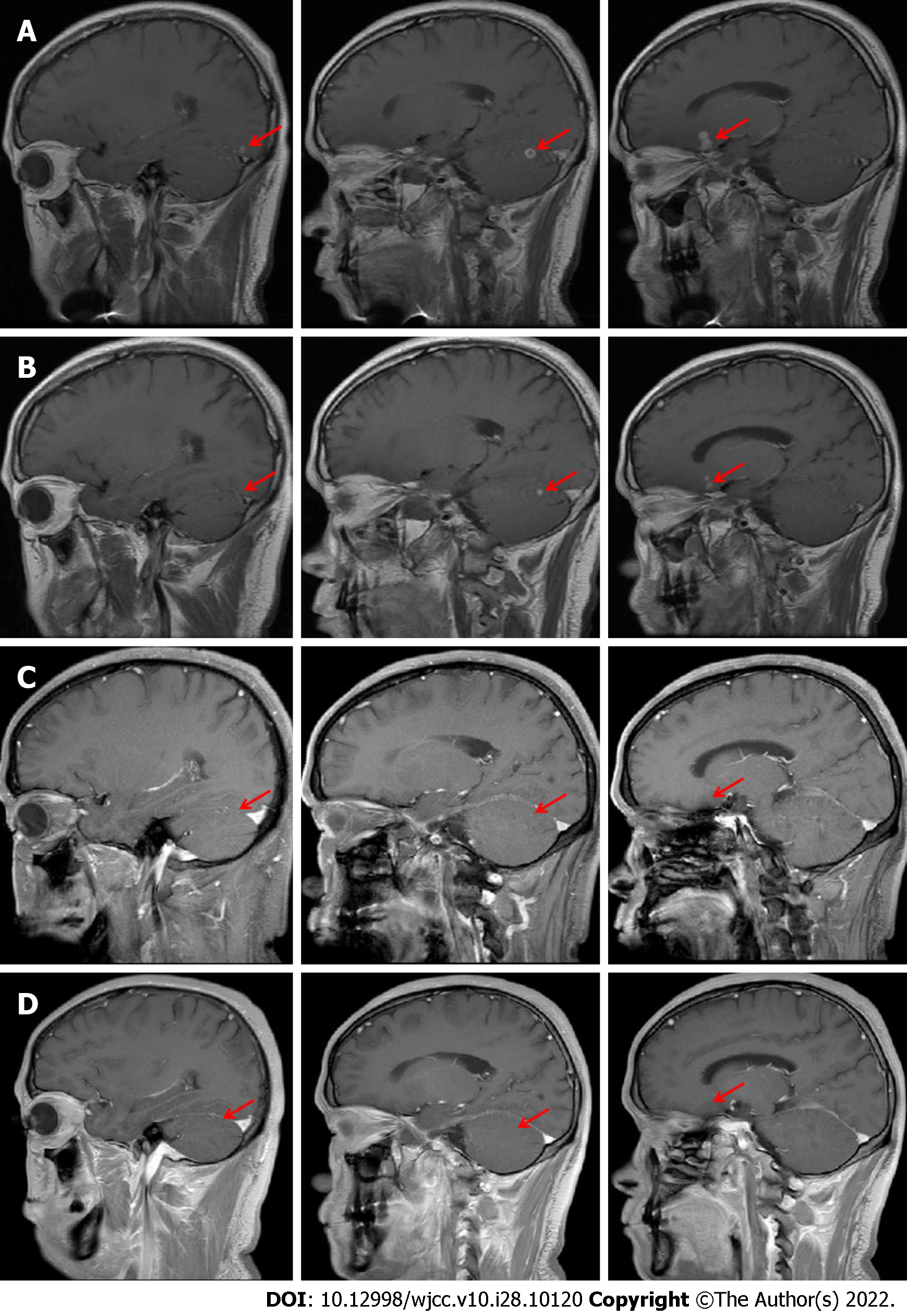
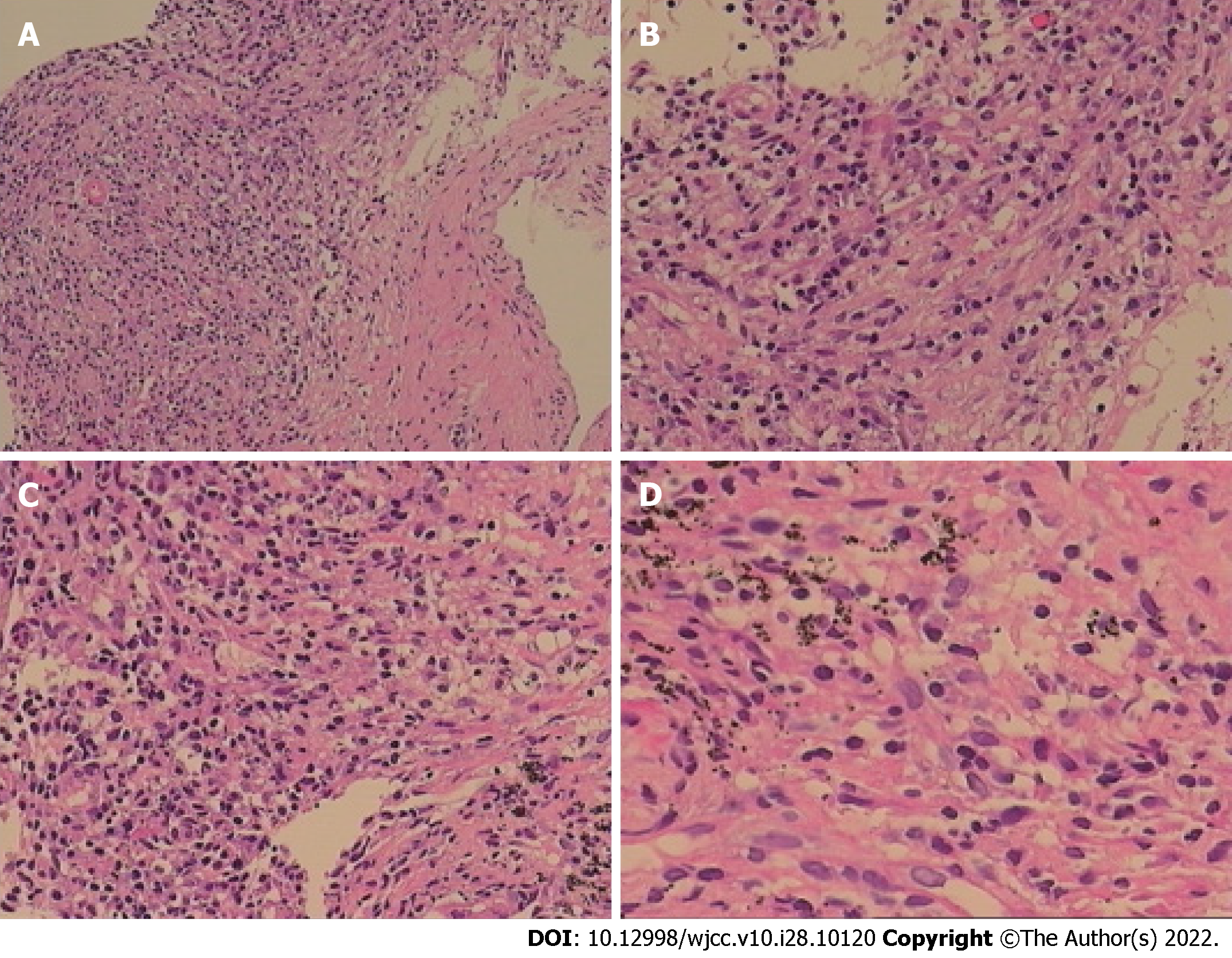
The patient was inhaled oxygen 4 L/min and treated empirically with simultaneous cefoperazone-tazobactam and teicoplanin for 7 d, but he continued to experience cough and hemoptysis with elevated C-reactive protein (109.92 mg/L) and procalcitonin (0.189 ng/mL). Meropenem and teicoplanin were then administered. In addition, bronchoscopy was performed to obtain specimens including BALF and lung tissue. Transbronchial biopsy of soft tissue of the right upper lung showed chronic inflammation with acute activity and fibrous tissue hyperplasia with focal necrosis. GeneXpert MTB/RIF, GM (1-3)-β-D-Glucan, fungi and acid-fast bacillus stains, and BALF culture for bacteria were negative. However, mNGS analysis of BALF detected N. asiatica and N. beijingensis. The patient developed a new symptom of headache at the same time. Cranial enhanced magnetic resonance imaging (MRI) was ordered on the suspicion of intracranial Nocardia infection and it showed multiple lesions in the left occipital lobe, right cerebellar hemisphere and left frontal lobe on May 13, 2020 (Figure 2A). Cerebrospinal fluid (CSF) obtained by lumbar puncture identified no white or red blood cells, glucose 3.42 mmol/L and protein 0.54 g/L. The bacterial culture, cryptococcal antigen, virus DNA and exfoliative cell examination of CSF were all negative, as was NGS analysis of CSF (Figure 3).
Disseminated nocardiosis affecting the CNS and lungs.
The patient was immediately treated with oral TMP-SMZ 0.96 g every 8 h and intravenous linezolid 600 mg every 12 h from May 9, 2020. After 2 wk of combined antibiotic therapy, his condition improved and pulmonary CT showed an obvious reduction in consolidation in both lung fields on May 23, 2020 (Figure 1D). The oxygenation index in blood gas analysis was 388mmHg (Table 1). Platelet count showed thrombocytopenia (171 × 109 /L 109 /L vs normal 233 × 109 /L). After 3 wk, because of the higher dose of linezolid in the plasma (9.36 mg/L) and reduction of hemamebas (3.34 × 109/L) and platelets (122 × 109/L), we adjusted the dose of intravenous linezolid (600 mg/d) and oral TMP-SMZ (0.96 g every 12 h) on May 31, 2020 (Table 2). The patient’s blood count recovered gradually, and at the same time his clinical conditions continued to improve. After 5 wk and improvement of his brain lesions on repeat MRI (Figure 2B), as the dose of linezolid in plasma was still high (6.38 mg/L), the treatment was replaced by ceftriaxone (2 g/d) on June 16, 2020 (Table 2). After 1 wk of treatment with ceftriaxone (2 g/d) and TMP-SMZ (0.96 g every 12 h), we shifted the antibiotic therapy to oral minocyline (100 mg every 12 h) and TMP-SMZ (0.96 g every 12 h) for 6.5 mo (Table 2). He completed nearly 8 mo of antibiotic therapy, with resolution of his pulmonary and brain lesions (Figure 1E-J), and complete cure was achieved (Figure 2C and D).
| April 9, 2020 | May 30, 2020 | May 31, 2020 | June 16, 2020 | June 23, 2020 | |
| Treatment | TMP-SMZ 0.96 g q8 h + linezolid 0.6 g q12 h | TMP-SMZ 0.96 g q12 h + linezolid 0.6 g qd | TMP-SMZ 0.96 g q12 h + ceftriaxone 2 g/d | TMP-SMZ 0.96 g q12 h and minocyline 100 mg q12 h | |
| WBC (109/L) | 4.03 | 3.34 | 4.07 | 3.91 | |
| PLT (109 /L) | 379 | 130 | 219 | 185 | |
| The dose of linezolid (mg/L) | No | 9.36 | 6.38 | No | |
He completed nearly 8 mo of antibiotic therapy, with resolution of his pulmonary and brain lesions (Figure 1E-J), and complete cure was achieved (Figure 2C and D).
Disseminated nocardiosis may affect many organs, especially the lungs, CNS, skin and even the pericardium. Among disseminated infections, 44% of patients had CNS involvement, highlighting the neurotrophic features of Nocardia spp. CNS manifestations of Nocardia infection can include isolated or multifocal brain abscesses and meningitis[7-9]. Consistent with the previous reports, our patient presented with disseminated disease spreading from a primary pulmonary infection to the central nervous system. He had a neurological symptom of headache and MRI revealed multiple lesions in the left frontal lobe, occipital lobe and right cerebellar hemisphere. Although bacterial culture and NGS analysis of CSF were both negative, his headache disappeared and MRI showed that the craniocerebral lesions were significantly reduced or even disappeared after antibiotic therapy, which confirmed that he had cerebral nocardiosis.
A definitive diagnosis of nocardiosis requires the isolation and identification of the organism from a clinical specimen. The traditional detection methods in specimens for microbial culture, histopathology, or smear microscopy, have low yield and are time-consuming; therefore, NGS has emerged[3]. NGS can detect unknown pathogens and a variety of mixed infectious pathogens by one-stop precision sequencing, therefore simplifying the detection process, improving pathogen detection sensitivity, and shortening detection time[4,5]. In this case, N. asiatica and N. beijingensis were rapidly detected by the mNGS of BALF, while the bacterial culture of BALF and the pathological biopsy of lung tissue were negative, contributing to prompt and accurate antibiotic treatment, and obvious clinical improvement was observed with resolution of pulmonary and brain lesions on repeat imaging. As reported, NGS can enable in-depth identification and classification of pathogens and their abilities to resist current treatment methods. In addition, the analysis of drug genes and virulence factors has an incomparable advantage over traditional detection methods[10,11]. However, as mNGS usually detects more than one pathogen in one test, clinicians need to comprehensively evaluate the clinical status and sequencing results of the patients to distinguish pathogens, symbiotic flora and pollutants. Although this method could detect the common resistant genes, it was unable to provide direct formation about antibiotic sensitivity. Furthermore, the high cost and low accessibility of NGS still limit its application in clinical practice. In the future, optimization of sample acquisition, sample preparation and bioinformatics analysis will be essential to the application of mNGS in clinical diagnosis. We hope that in the near future, this technology will benefit more patients.
Treatment of CNS nocardiosis is largely based upon expert opinion. Popular treatment strategies include empirical combination intravenous therapy with agents such as imipenem, TMP-SMZ, linezolid and amikacin for several weeks followed by antibiogram-guided oral therapy[12,13]. Consistent with these strategies, our patient initiated treatment with parenteral and oral antibiotics for 6 wk and had definite clinical improvement, followed by switching to oral minocyline and TMP-SMZ for 6.5 mo. He completed nearly 8 mo of antibiotic therapy, with resolution of his brain and pulmonary lesions and achieved complete cure. Furthermore, this successful case confirmed that in some cases of disseminated nocardiosis, linezolid may be an important alternative that can give a better outcome. However, it should be emphasized that the risk of adverse effects might be increased if linezolid was combined with other antibiotics. Postmarketing studies have reported higher rates of linezolid-associated thrombocytopenia, ranging from 15% to 50%[14,15]. The higher dose of linezolid in the plasma and prolonged duration of therapy have been suggested as more important risk factors[16-19]. Consistent with these findings, our study identified longer duration of therapy as a risk factor. The first thrombocytopenic platelet count occurred after 14 d of linezolid therapy. The severity of thrombocytopenia increased with the duration of treatment. Similarly, in this case, thrombocytopenia was closely related to the higher dose of linezolid in the plasma. The combined treatment of linezolid and TMP-SMZ may aggravate the myelosuppressive effects of reversible neutropenia and thrombocytopenia. Selection of appropriate antibiotics given at the optimal time is hence crucial for effective therapy. Although NGS can detect the presence of drug resistant genes in pathogens, it may not be consistent with clinical drug susceptibility results. Furthermore, there are still many drug resistant genes that have not yet been discovered and false negative results may exist.
A PubMed/MEDLINE search of literature found a total of 17 cases within a decade except for the references already cited in the article (Table 3)[20-36]. For patients with underlying conditions (87.5% in immunocompromised individuals), affected organs (lung involvement is most common, followed by the brain), and positive results (mostly from culture and histopathology, but not the NGS), TMP-SMZ is the most commonly used and effective drug.
| Ref. | Predisposing or underlying condition | Affected organ (s) | Positive specimen (s) | Anti-biotic therapy | Outcome |
| Komiya et al[20], 2013 | Hypersensitivity pneumonitis | Lung | sputum | Meropenem, TMP-SMZ | Improved |
| Yasar et al[21], 2014 | No | Lung | Lung tissue | TMP-SMZ | Improved |
| Cooper et al[22], 2014 | Smoking and alcohol consumption | Lung +Brain | Tissue of lung and brain | Vancomycin, Ceftriaxone, Amphotericin B, TMP-SMZ | Dead |
| Yagi et al[23], 2014 | Mycobacterium avium | Lung | Sputum | TMP-SMZ | Improved |
| Ibrahim et al[24], 2016 | Hematopoietic stem cell transplant | Lung +Brain | Lung tissue | TMP-SMZ | Improved |
| Mendonca et al[25], 2016 | Multiple Myeloma | Lung | BALF | TMP-SMZ, Meropenem, Tigecycline | Improved |
| Jayaschandran et al[26], 2016 | Idiopathic CD4 T-lymphocytopenia | Lung | BALF | Meropenemz, TMP-SMZ | Improved |
| Khadka et al[27], 2017 | Renal transplant, Pulmonary tuber-culosis | Lung+Brain+ Celiac lymph nodes | BALF | TMP-SMZ | Improved |
| Kobayashi et al[28], 2018 | Olfactory neuroblastoma, Ectopic adrenocorticotropic hormone | Lung | BALF | TMP-SMZ | Improved |
| Khaliq et al[29], 2019 | Cladophialophora bantiana Cerebral Phaeohyphomycosis | Lung | Lung tissue | TMP-SMZ, Meropenem, Voriconazole, Liposomal amphotericin | Died |
| Miyaoka et al[30], 2019 | Smoking | Lung | BALF | Meropenem, TMP-SMZ | Improved |
| Kato et al[31], 2019 | Radiation pneumonitis treated with prednisolone [PSL (50 mg/d)] | Lung | The PCR of 16S rRNA gene of the BALF | TMP-SMZ, Doripenem | Improved |
| Deterding et al[32], 2020 | heart transplant recipient requiring dialysis | lung | BALF | Amikacin, Imipenem | Improved |
| Campoli et al[33], 2020 | Liver transplant, Cutaneous infection by Alternaria alternata | Lung | BALF | TMP-SMZ, Linezolid, Ceftriaxone | Improved |
| Meena et al[34], 2020 | Pulmonary tuberculosis and COPD | Lung | BALF | TMP-SMZ | Improved |
| Wu et al[35], 2021 | Pulmonary alveolar proteinosis | Lung | Lung tissue | TMP-SMZ | Improved |
| Atemnkeng | COVID-19 Pneumonia | Lung +Brain | BALF | Meropenem, TMP-SMZ, Linezolid | Improved |
This case illustrates that Nocardia may cause severe disseminated infection in immunocompetent patients, which often involves the CNS and lungs. mNGS can establish a more rapid and accurate diagnosis than traditional methods. It is a convenient and efficient technique for the detection of Nocardia, and is especially suitable for rare, novel and atypical etiologies of complicated infectious diseases. This successfully treated case confirmed that, in some patients with disseminated nocardiosis, TMP-SMZ is still the first choice of treatment, but linezolid may be an important alternative that can give a better outcome with the monitoring of linezolid serum concentrations and platelet count. Notable risk factors for linezolid-induced thrombocytopenia included high daily weight-based dose and prolonged duration of therapy (some patients experience thrombocytopenia within 14 d of treatment). Clinicians should monitor patients for linezolid-induced thrombocytopenia throughout therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Roganovic J, Croatia; Watanabe T, Japan; Enomoto H, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 2. | Rouzaud C, Rodriguez NV, Catherinot E, Méchaï F, Bergeron E, Farfour E, Scemla A, Poirée S, Delavaud C, Mathieu D, Durupt S, Larosa F, Lengelé JP, Christophe JL, Suarez F, Lortholary O, Lebeaux D. Clinical Assessment of a Nocardia spp polymerase chain reaction PCR-Based Assay for Diagnosis of Nocardiosis. J Clin microbiol. 2018;56:e00002-18. |
| 3. | Bragg L, Tyson GW. Metagenomics using next-generation sequencing. Methods Mol Biol. 2014;1096:183-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Wang K, Li P, Lin Y, Chen H, Yang L, Li J, Zhang T, Chen Q, Li Z, Du X, Zhou Y, Wang H, Song H. Metagenomic Diagnosis for a Culture-Negative Sample From a Patient With Severe Pneumonia by Nanopore and Next-Generation Sequencing. Front Cell Infect Microbiol. 2020;10:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Huang J, Jiang E, Yang D, Wei J, Zhao M, Feng J, Cao J. Metagenomic Next-Generation Sequencing versus Traditional Pathogen Detection in the Diagnosis of Peripheral Pulmonary Infectious Lesions. Infect Drug Resist. 2020;13:567-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 6. | Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the Leap from Research Laboratory to Clinic: Challenges and Opportunities for Next-Generation Sequencing in Infectious Disease Diagnostics. mBio. 2015;6:e01888-e01815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine (Baltimore). 2004;83:300-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Mohanty A, Meena S, Kumar SP, Gupta PK, Kaistha N, Gupta P, Jha MK, Rekha S. Incidental Finding of Nocardia: A Case Series from a Tertiary Care Centre in Uttarakhand. Case Rep Infect Dis. 2020;2020:6874625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Anagnostou T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis E. Nocardiosis of the central nervous system: experience from a general hospital and review of 84 cases from the literature. Medicine (Baltimore). 2014;93:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Didelot X, Bowden R, Wilson DJ, Peto TEA, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13:601-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 543] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 11. | Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903; quiz 904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 421] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Shen Q, Zhou H, Li H, Zhou J. Linezolid combined with trimethoprim-sulfamethoxazole therapy for the treatment of disseminated nocardiosis. J Med Microbiol. 2011;60:1043-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Wang T, Jia Y, Chu B, Liu H, Dong X, Zhang Y. Nocardiosis in Kidney Disease Patients under Immunosuppressive Therapy: Case Report and Literature Review. Int J Med Sci. 2019;16:838-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Hirano R, Sakamoto Y, Tachibana N, Ohnishi M. Retrospective analysis of the risk factors for linezolid-induced thrombocytopenia in adult Japanese patients. Int J Clin Pharm. 2014;36:795-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Natsumoto B, Yokota K, Omata F, Furukawa K. Risk factors for linezolid-associated thrombocytopenia in adult patients. Infection. 2014;42:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Hanai Y, Matsuo K, Ogawa M, Higashi A, Kimura I, Hirayama S, Kosugi T, Nishizawa K, Yoshio T. A retrospective study of the risk factors for linezolid-induced thrombocytopenia and anemia. J Infect Chemother. 2016;22:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Ikuta S, Tanimura K, Yasui C, Aihara T, Yoshie H, Iida H, Beppu N, Kurimoto A, Yanagi H, Mitsunobu M, Yamanaka N. Chronic liver disease increases the risk of linezolid-related thrombocytopenia in methicillin-resistant Staphylococcus aureus-infected patients after digestive surgery. J Infect Chemother. 2011;17:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, Yata N, Yotsuyanagi H, Moriya K. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother. 2013;68:2128-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, Morikawa N, Takeda Y. Analysis of thrombocytopenic effects and population pharmacokinetics of linezolid: a dosage strategy according to the trough concentration target and renal function in adult patients. Int J Antimicrob Agents. 2014;44:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Komiya K, Ishii H, Tsubone T, Okabe E, Matsumoto B, Kadota J. Bird fancier's lung complicated by pulmonary nocardiosis. J Bras Pneumol. 2013;39:102-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Yaşar Z, Acat M, Onaran H, Ozgül MA, Fener N, Talay F, Cetinkaya E. An unusual case of pulmonary nocardiosis in immunocompetent patient. Case Rep Pulmonol. 2014;2014:963482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Cooper CJ, Said S, Popp M, Alkhateeb H, Rodriguez C, Porres Aguilar M, Alozie O. A complicated case of an immunocompetent patient with disseminated nocardiosis. Infect Dis Rep. 2014;6:5327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Yagi K, Ishii M, Namkoong H, Asami T, Fujiwara H, Nishimura T, Saito F, Kimizuka Y, Asakura T, Suzuki S, Kamo T, Tasaka S, Gonoi T, Kamei K, Betsuyaku T, Hasegawa N. Pulmonary nocardiosis caused by Nocardia cyriacigeorgica in patients with Mycobacterium avium complex lung disease: two case reports. BMC Infect Dis. 2014;14:684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ibrahim U, Saqib A, Mohammad F, Terjanian T. An Unusual Presentation of Nocardiosis in an Allogeneic Transplant Recipient. Cureus. 2016;8:e834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Mendonca NP, Kadayakkara DK, Forde IC, Rudkovskaia A, Saul ZK, Lobo DJ. Pulmonary Nocardiosis in a Multiple Myeloma Patient Treated with Proteasome Inhibitors. Am J Case Rep. 2016;17:76-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Jayaschandran V, Gjorgova-Gjeorgjievski S, Siddique H. Pulmonary nocardiosis in a patient with idiopathic CD4 T-lymphocytopenia. Respirol Case Rep. 2018;6:e00283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Khadka P, Basnet RB, Khadka P, Shah DS, Pokhrel BM, Rijal BP, Sherchand JB. Disseminated Nocardiosis in renal transplant recipient under therapy for pulmonary tuberculosis: a case report. BMC Res Notes. 2017;10:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Kobayashi K, Asakura T, Ishii M, Ueda S, Irie H, Ozawa H, Saitoh K, Kurihara I, Itoh H, Betsuyaku T. Pulmonary nocardiosis mimicking small cell lung cancer in ectopic ACTH syndrome associated with transformation of olfactory neuroblastoma: a case report. BMC Pulm Med. 2018;18:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Khaliq MF, Ihle RE, Schirtzinger CP. Cladophialophora bantiana Cerebral Phaeohyphomycosis Complicated by Pulmonary Nocardiosis: A Tale of Two Infections. Case Rep Infect Dis. 2019;2019:4352040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Miyaoka C, Nakamoto K, Shirai T, Miyamoto M, Sasaki Y, Ohta K. Pulmonary nocardiosis caused by Nocardia exalbida mimicking lung cancer. Respirol Case Rep. 2019;7:e00458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Kato K, Noguchi S, Naito K, Ikushima I, Hanaka T, Yamasaki K, Kawanami T, Yatera K. Pulmonary Nocardiosis Caused by Nocardia exalbida in a Patient with Lung Cancer and Radiation Pneumonitis: A Case Report and Literature Review. Intern Med. 2019;58:1605-1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Deterding L, Körner T, Borte G, Wirtz H, Seyfarth HJ. Nocardiosis mimicking lung cancer in a heart transplant patient with end-stage renal disease. Respir Med Case Rep. 2020;30:101101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Campoli C, Ferraro S, Salfi N, Coladonato S, Morelli MC, Giannella M, Ambretti S, Viale PL, Cricca M. Diffuse primary cutaneous infection by Alternaria alternata in a liver transplant recipient with pulmonary nocardiosis: Importance of prompt identification for clinical resolution. Med Mycol Case Rep. 2020;28:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Meena DS, Kumar D, Bohra GK, Garg MK, Yadav P, Sharma A, Abhishek KS, Garg P, Pamnani J. Pulmonary Nocardiosis with Aspergillosis in a patient with COPD: A rare co-infection. IDCases. 2020;20:e00766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Wu XK, Lin Q. Pulmonary alveolar proteinosis complicated with nocardiosis: A case report and review of the literature. World J Clin Cases. 2021;9:2874-2883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Atemnkeng F, Ducey J, Khalil A, Elemam A, Diaz K. Diagnosing Disseminated Nocardiosis in a Patient With COVID-19 Pneumonia. J Med Cases. 2021;12:319-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |