Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10085
Peer-review started: April 13, 2022
First decision: May 30, 2022
Revised: June 12, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 166 Days and 23.8 Hours
Entecavir (ETV) is a potent and selective nucleotide analog with significant activity against hepatitis B virus (HBV). ETV maleate is a derivative compound of ETV and was reported to have an efficacy and safety profile that is comparable to ETV (Baraclude) when used in Chinese patients with chronic hepatitis B (CHB) in phase III clinical trials (Clinical Trials.gov number, NCT
To investigate the antiviral potency and safety of ETV maleate at week 192 in Chinese CHB patients predominantly genotyped B or C.
In this double-blind study, we randomly assigned patients to receive 0.5 mg/d ETV (Group A) or ETV maleate (Group B) (ratio, 1:1), each with a placebo tablet for 48 wk. Then, all patients received open-label treatment with 0.5 mg/d ETV maleate starting at week 49. The primary efficacy endpoint was the reduction in HBV DNA levels from baseline. Secondary endpoints included the proportion of patients with undetectable HBV DNA (< 20 IU/mL), serologic response, serum alanine aminotransferase (ALT) normalization and development of resistance mutations.
Two hundred eighteen patients who were hepatitis B e antigen (HBeAg) positive and 57 who were HBeAg negative were analyzed and predominantly presented with genotype B (49.82%) or C (48.73%). For the HBeAg-positive CHB patients, the mean HBV DNA level decrease (6.61 Log10 IU/mL vs 6.69 Log10 IU/mL, P > 0.05), viral suppression with HBV DNA < 20 IU/mL (83.33% vs 79.17%, P > 0.05) and HBeAg seroconversion (28.77% vs 20.00%, P > 0.05) occurred similarly between Groups A and B at week 192. However, there was a significant difference in the pro
Long-term ETV maleate treatment for up to 192 wk is effective and safe in Chinese CHB patients predominantly genotyped as B or C.
Core Tip: This randomized, double-blind, double-dummy, controlled, multicenter trial showed that long-term treatment with entecavir (ETV) maleate provides safe, potent and reliable suppression of hepatitis B virus replication for 192 wk in Chinese chronic hepatitis B patients predominantly genotyped as B or C with little chance of developing ETV-resistant mutations.
- Citation: Xu JH, Wang S, Zhang DZ, Yu YY, Si CW, Zeng Z, Xu ZN, Li J, Mao Q, Tang H, Sheng JF, Chen XY, Ning Q, Shi GF, Xie Q, Zhang XQ, Dai J. One hundred and ninety-two weeks treatment of entecavir maleate for Chinese chronic hepatitis B predominantly genotyped B or C. World J Clin Cases 2022; 10(28): 10085-10096
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10085.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10085
Hepatitis B virus (HBV) infection remains a serious global public health concern due to its high prevalence, significant morbidity and mortality worldwide[1,2]. China is one of the Asia-Pacific countries with the highest prevalence of HBV infection. Of the 93 million chronic HBV carriers in China, more than 20 million develop chronic hepatitis B (CHB) and require effective and safe treatment[3,4], which places a heavy burden on the health care system for the Chinese government. It is critical to develop strong antiviral agents against CHB, especially those with high cost-effectiveness and easy availability.
Currently, entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) have been recommended as first-line antiviral drugs for the treatment of CHB[4-7] and showed similar antiviral effect for CHB[8,9]. Although TDF has demonstrated potent antiviral effectiveness in patients with CHB infection with no resistance throughout 8 years of use, its long-term use is associated with reductions in mineral bone density, increases in markers of bone turnover[10], and adverse renal toxic effects[11,12]. TAF is a novel prodrug of TDF with similar efficacy and less impact on the bones and kidney than TDF[13-16], but TAF is much more expensive and is not yet available in most countries. ETV showed significant histologic, virologic, and biochemical responses in CHB patients with a safety profile and minimal resistance[17-20] and by far is the most widely used and cost-effective antiviral drug in China due to its easy access[21].
Entecavir maleate is a derivative compound of ETV and was reported to have efficacy and safety comparable to those of ETV (Baraclude) in Chinese patients with CHB in phase III clinical trials at weeks 48, 96, and 144. The current article presents efficacy and safety reports for up to 192 wk of ETV maleate treatment in Chinese patients predominantly genotyped as B or C.
This multicenter phase III clinical trial (NCT01926288) was conducted at 10 sites throughout China and can be divided into two stages. The first stage was a randomized, double-blind, double-dummy, controlled study. All subjects were stratified by hepatitis B e antigen (HBeAg) status before being randomly assigned (1:1) within 14 d of screening to receive ETV maleate (Jiangsu Chia-tai Tianqing Pharmaceutical Co., Ltd, Jiangsu Province, China) or ETV (Bristol-Myers Squibb Co., Ltd, Shanghai, China) 0.5 mg once daily for up to 48 wk. All patients received placebo tablets matching the alternative treatment (Supplementary Figure 1). The second stage was an open-label study, where all the patients received ETVM 0.5 mg once daily for up to 240 wk. More detailed information about the study design is described in the Supplementary material.
The study was designed by the Jiangsu Chia-tai Tianqing Pharmaceutical Company in collaboration with the study’s primary researchers and approved by the Ethics Committee of Peking University First Hospital, China. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all study participants.
In brief, eligible patients were aged 18-65 years with detectable HBsAg for at least 24 wk and were either HBeAg-positive with HBV DNA ≥ 2 × 104 IU/mL (1 × 105 copies/mL) or HBeAg-negative with HBV DNA ≥ 2 × 103 IU/mL (1 × 104 copies/mL). We excluded patients who received any interferon, thymosin or antiviral agents with activity against hepatitis B within 6 mo before screening. We also excluded patients who received prior antiviral therapy lasting more than 12 wk and patients showing signs of decompensation (i.e., clinical ascites, encephalopathy, or variceal hemorrhage), hepatocellular carcinoma or coinfection or superinfection of other viruses, such as hepatitis A virus, hepatitis C virus, hepatitis delta virus, hepatitis E virus, Epstein-Barr virus or cytomegalovirus. Finally, a total of 279 patients were enrolled between December 2001 and September 2002. Full eligibility criteria are provided in the supplementary information.
The primary efficacy endpoint was the reduction of HBV DNA levels from baseline. Secondary efficacy points included the proportion of patients achieving the following: Undetectable HBV DNA (< 20 IU/mL), HBeAg loss, HBeAg seroconversion, and serum alanine aminotransferase (ALT) normalization.
Virological and serological tests were performed centrally in the viral laboratory of Peking University First Hospital. Serum HBV-DNA was assayed by a Roche Cobas Ampliprep/Cobas Taqman™ polymerase chain reaction (PCR) assay with a lower limit of detection (LLOD) of 20 IU/mL. Commercially available chemiluminescence immunoassays (CLIA) (Abbott Laboratories, North Chicago, IL, United States) were used for the detection of serum HBsAg, anti-HBs, anti-HBc, HBeAg, and anti-HBe. Genotyping and resistance analysis were carried out using a direct-sequencing PCR method[22]. Reverse transcription region sequences of HBV were amplified with the following primers: P-F1: 5′ACTCGTGGTGGACTTCTC3′ (254-271), P-R1: 5′AGGCAGGATADCCACATT3′ (D = T, A, G) (1053-1036), and P-R2: 5′TAAAAGGGGCRGCAAASC3′ (R = A, G) (S = C, G) (1032-1015). All of the above primers were designed according to the GenBank sequence AY206384. The sense primer P-F1 and antisense primer P-R1 were used for the first-round amplification of P gene. The second round PCR was carried out by sense primer P-F1 and antisense primer P-R2. The PCR product was purified and sequenced directly by forward primer P-F1.
All adverse events (AEs), including serious AEs (SAEs), laboratory abnormalities, discontinuation of the study drug due to adverse events, and deaths, were recorded and evaluated at every visit.
LMV-associated resistance was defined as rtM204 V with or without rtL180 M. ADV-associated resistance was defined as rtA181T and/or rtN236T. Resistance to ETV was defined as an additional substitution (rtI169 or rtT184, rtS202, or rtM250) predicated on preexisting LMV-associated resistance substitutions. HBV polymerase/reverse transcriptase substitutions were analyzed for all patients at baseline, and patients who had HBV-DNA levels greater than 52 IU/mL at weeks 48, 96, 144, and 192 or experienced virological breakthrough during treatment.
HBV DNA levels were logarithmically transformed for analysis. Continuous variables were expressed as the mean ± SD or median (M) with interquartile range (Q) and were compared by t tests or Wilcoxon rank sum test where appropriate. Binary variables were summarized in counts and percentages and were compared using the chi-squared method or Fisher’s exact test where applicable. The cumulative probability of achieving virological response was estimated by Kaplan-Meier analysis. All reported P values were 2-sided and were not adjusted for multiple testing, with P < 0.05 considered statistically significant. Statistical analysis was performed using SPSS ver. 17.0 (SPSS, Chicago, IL, United States). For further details regarding the statistical analysis, please refer to the supplementary information.
Of the 360 patients who were enrolled and screened, 58 didn’t meet the inclusion criteria, 22 refused to participate and 1 dropped out for personal reasons. A total of 279 patients were randomly assigned into two groups (138 in Group A and 141 in Group B) in a blinded way to receive 0.5 mg/d ETV (Group A) or ETV maleate (Group B). Four were excluded from the full analysis set (FAS) due to lower baseline HBV DNA levels than the inclusion criteria (Supplementary Figure 1). Accordingly, 275 patients were included in the FAS. In total, 67 patients discontinued therapy throughout the 192-wk study period for the following reasons: Lost-to-follow-up (40), withdrawal of consent (4), unwillingness to commit to long-term treatment (4), preparing for pregnancy (1), virological breakthrough (3), or overdose of study drug (1), baseline mutation and/or add-on ADV (14). No discontinuation due to AE or AR occurred. No deaths occurred. A total of 208 out of 275 patients remained in the cohort after 192 wk of treatment with ETV maleate monotherapy.
Baseline characteristics were comparable between the two groups (Supplementary Table 1). The majority of the patients were male. HBeAg-positive patients constituted 80.88% and 77.70% in Groups A and B, respectively. Three cases with LAM resistance and 4 with ADV resistance were identified at baseline.
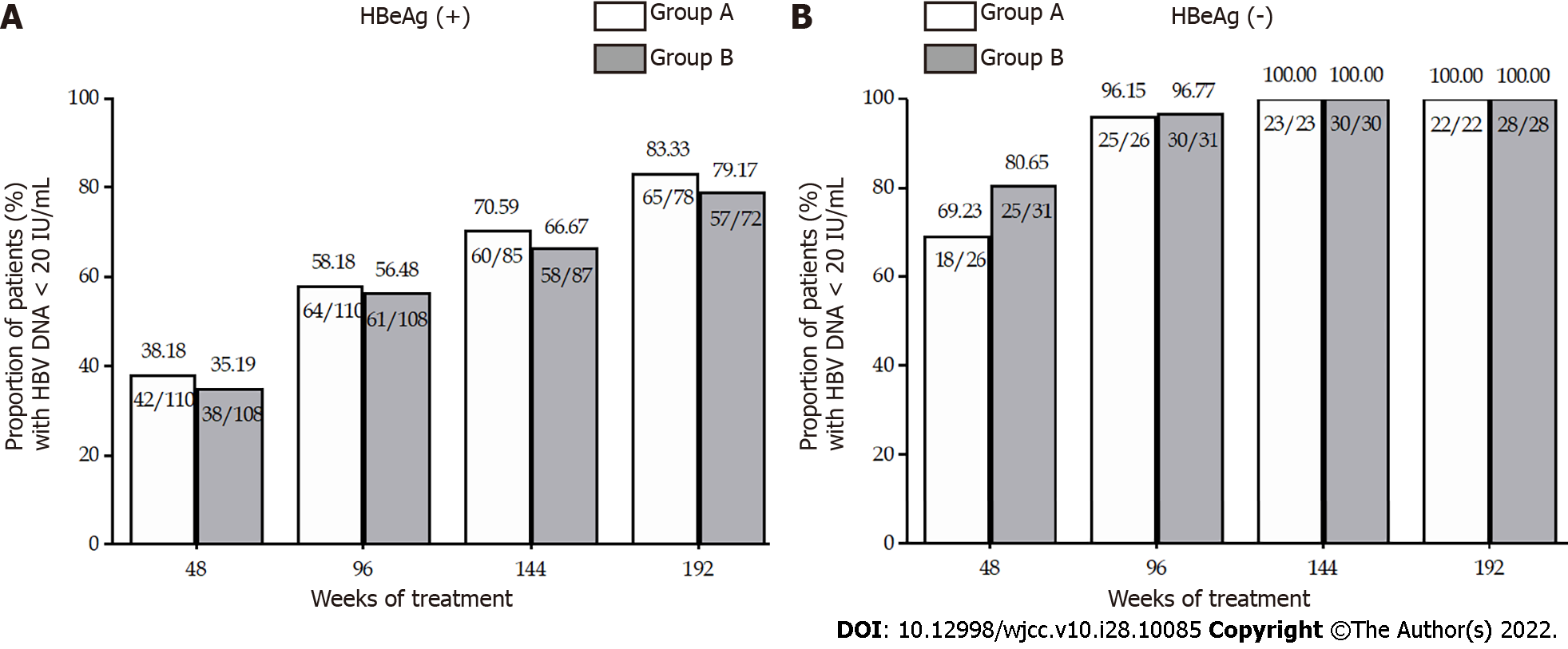
The mean HBV DNA levels at weeks 168 and 192 were similar between the two groups (Table 1). For HBeAg-positive patients, the mean reductions in serum HBV DNA levels from baseline to week 168 (6.44 vs 6.53 Log10 IU/mL, P > 0.05) and 192 (6.61 vs 6.69 Log10 IU/mL, P > 0.05) were similar in Groups A and B (Figure 1). For HBeAg-negative patients in Groups A and B, the mean decline in HBV DNA was comparable at week 168 (5.73 vs 5.99, log10 IU/mL, P > 0.05) and at week 192 (6.05 and 6.03 Log10 IU/mL, P > 0.05) (Table 2). The undetectable HBV DNA rates for HBeAg-positive patients were 83.33% in Group A and 79.17% (P > 0.05) in Group B at week 192 (Table 2). The HBV DNA level in all of the HBeAg-negative patients remained undetectable (< 20 IU/mL) at weeks 168 and 192 (Figure 1).
| Group | HBeAg-positive | HBeAg-negative | ||||
| Baseline | 168 wk | 192 wk | Baseline | 168 wk | 192 wk | |
| A | 7.64 ± 1.01 | 1.18 ± 0.86 | 0.9 ± 0.96 | 6.22 ± 1.25 | 0.62 ± 0.66 | 0.35 ± 0.59 |
| B | 7.65 ± 0.95 | 1.13 ± 0.82 | 1.02 ± 0.85 | 6.31 ± 1.37 | 0.36 ± 0.61 | 0.33 ± 0.57 |
| P | 0.937 | 0.728 | 0.560 | 0.798 | 0.150 | 0.867 |
| Group | HBeAg-positive | HBeAg-negative | ||
| 168 wk | 192 wk | 168 wk | 192 wk | |
| A | 6.44 ± 1.39 | 6.61 ± 1.28 | 5.73 ± 1.25 | 6.05 ± 1.20 |
| B | 6.53 ± 1.24 | 6.69 ± 1.12 | 5.99 ± 1.47 | 6.03 ± 1.49 |
| P | 0.654 | 0.696 | 0.533 | 0.952 |
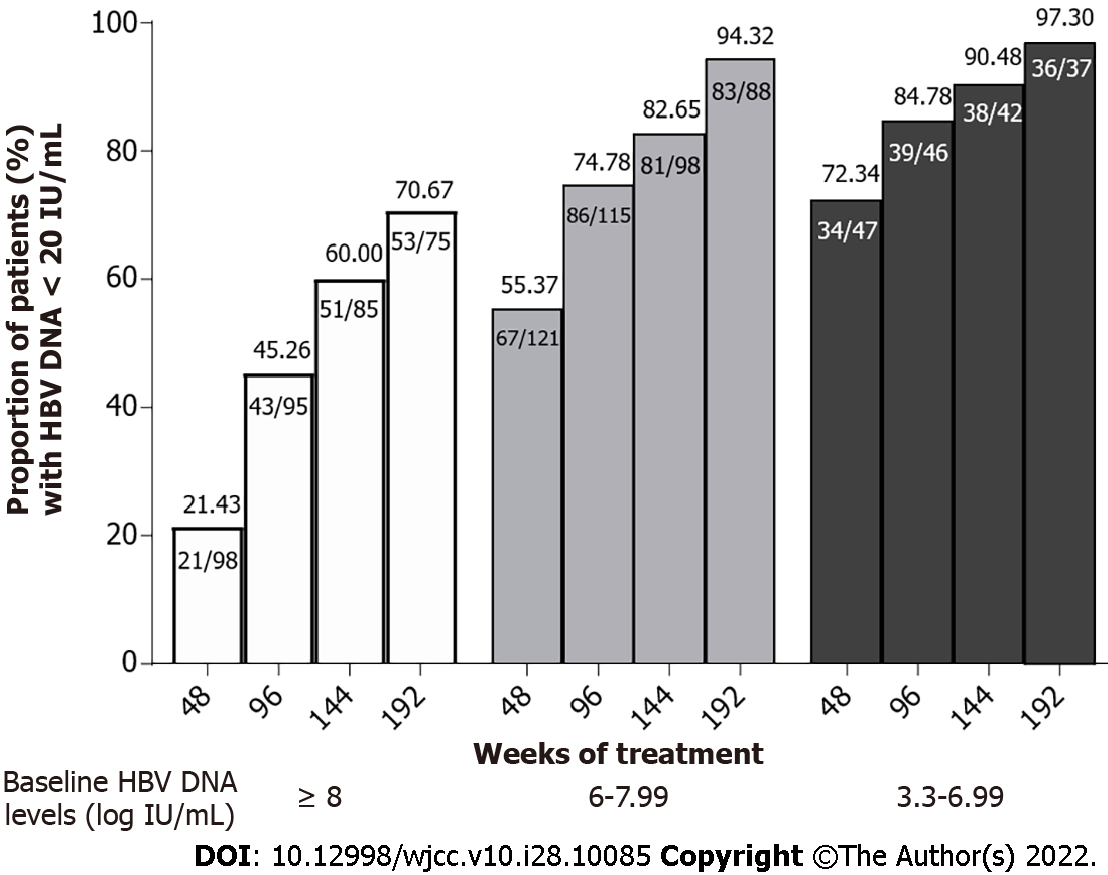
The undetectable HBV DNA rates in patients with different baseline HBV DNA levels (classified into 3 groups: 8 or more, 6-7.99, and 5.99 or less log10 IU/mL) at week 192 were 70.67%, 94.32%, and 97.30%, respectively (Figure 2). There was a significant difference in undetectable HBV DNA rates at week 192 between patients with baseline HBV DNA levels ≥ 8 Log10 IU/mL and < 8 Log10 IU/mL [P < 0.05, OR = 8.23, 95%CI (3.16, 21.48)], suggesting that patients with baseline HBV DNA levels < 8 Log10 IU/mL have an 8.23 times higher chance of achieving undetectable HBV DNA than patients with baseline HBV DNA levels ≥ 8 Log10 IU/mL after 4 years of treatment with entecavir or entecavir maleate. Twenty-four out of 28 (85.56%) patients who did not achieve undetectable HBV DNA levels at week 192 had HBV DNA levels lower than 3 Log10 IU/mL.
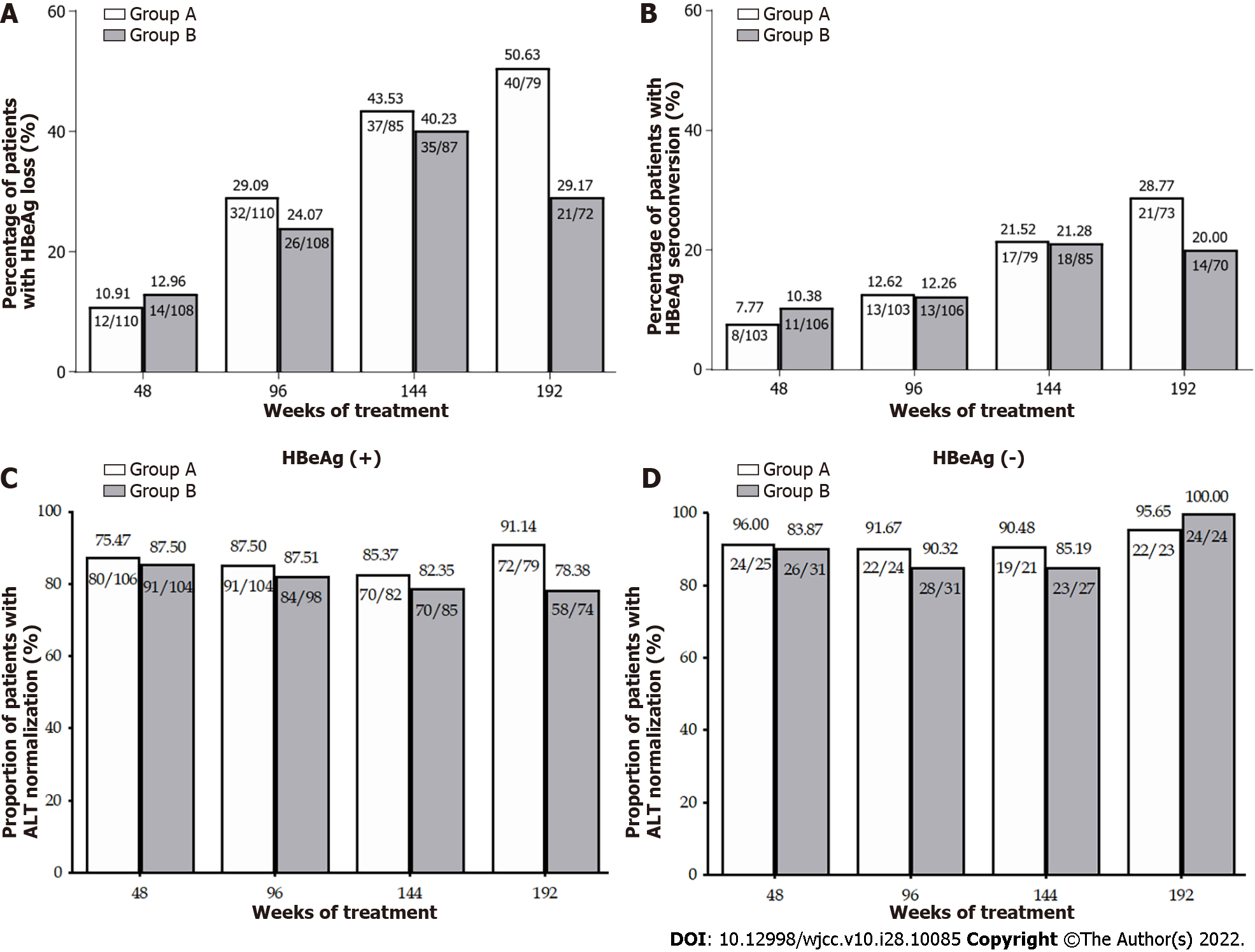
No significant difference in the HBeAg loss rate was observed in Groups A and B of HBeAg-positive patients from week 48 to week 144. However, owing to the instability of HBeAg status at the early stage after HBeAg loss, 14 patients who experienced HBeAg loss at week 144 regained HBeAg at week 192. As a consequence, there was a significant difference in the HBeAg loss rate at week 192 between Groups A and B (50.63% vs 29.17%, P < 0.05) (Figure 3A and B). HBeAg seroconversion occurred in 28.77% and 20.00% (P > 0.05), respectively, of the HBeAg-positive patients in Groups A and B (Figure 3A and B). No patient lost HBsAg after 4 years of treatment.
The rates of ALT normalization throughout the 192 wk of treatment are described in Figure 3C and D. Among HBeAg-positive patients, a significant difference was found in the ALT normalization rate at week 192 (91.14% vs 78.38%, P < 0.05). The ALT normalization rate was comparable (95.65% in Group A and 100% in Group B) in HBeAg-negative patients.
None of the patients in the HBeAg-negative group developed genotypic resistance to ETV throughout the 192 wk of this study. One patient in the HBeAg-positive group developed genotypic resistance (rtL180 M-rtM204 V/I-rtM250 M/V) to ETV at week 96, and then ADV was added at week 100 as a rescue therapy. Subsequently, this patient experienced a DNA reduction from 7.81 Log10 IU/mL at week 96.00 to 5.73 Log10 IU/mL at week 144 with a change of mutational pattern to rtV173 L-rtL180 M-rtM204 V. In the end, serum HBV-DNA became undetectable (< 20 IU/mL) at week 192. Seven cases in the HBeAg-positive group (2 in Group A and 5 in Group B) had confirmed ETV resistance at week 144, and no additional cases were found from week 144 to week 192. Three out of the 7 patients were treatment-naïve (all in Group B). The other four were nucleos(t)ide-experienced (one with LAM, three with both LAM and ADV) (Table 3). Among these four patients, baseline LMV resistance and ADV resistance were detected in one who was LAM-experienced and 3 who were LAM-and-ADV-experienced, respectively. ADV was added to 5 subjects, and a subsequent decrease in HBV DNA was achieved (Table 3).
| Group A (entecavir) | Group B (entecavir maleate) | ||||||
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
| Age, yr | 32 | 34 | 28 | 29 | 43 | 34 | 21 |
| Sex | Male | Male | Male | Male | Male | Male | Male |
| Prior LAM exposure | Yes | Yes | No | No | Yes | Yes | No |
| Prior ADV exposure | Yes | Yes | No | No | Yes | No | No |
| Baseline | |||||||
| HBeAg status | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| HBV DNA, log10 IU/mL | 6.69 | 7.99 | 8.06 | 5.32 | 7.77 | 7.47 | 7.81 |
| LAM resistance | No | No | No | No | No | Yes | No |
| ADV resistance | Yes | Yes | No | No | Yes | No | No |
| At maximum viral suppression | |||||||
| wk | 24 | 48 | 72 | 84 | 48 | 12 | 120 |
| HBV DNA, log10 IU/mL | 4.07 | 1.18 | 2.97 | 1.76 | 2.26 | 4.78 | TND |
| At time of resistance confirmed | |||||||
| wk | 144 | 144 | 144 | 144 | 144 | 144 | 144 |
| HBV DNA, log10 IU/mL | 6.91 | 6.04 | 4.41 | 1.73 | 6.73 | 4.96 | 1.79 |
| Mutational pattern | rtL180M | rtL180M | rtL180M | rtL180M | rtL180M | rtL180M | rtL180M |
| rtS202G | rtS202G | rtS202G | rtV173L | rtT184S | rtS202G | rtT184S | |
| rtM204V | rtM204V | rtM204V | rtM204V | rtM204V | rtM204V | rtM204V | |
| rtM250V | |||||||
| Addition of ADV | Yes | Yes | Yes | No | Yes | Yes | No |
| HBV DNA, log10 IU/mL | |||||||
| Week 168 | 5.14 | 5.08 | 3.18 | 1.84 | 3.43 | 4.86 | TND |
| Week 192 | 4.2 | 2.77 | 3.1 | Missing | 2.89 | 4.28 | Missing |
The safety analysis up to 144 wk showed no difference in the frequency of AEs in either group. From week 144 to week 192, the frequency of AEs was comparable between Groups A and B (9.3% vs 8.5%, P > 0.05). Two cases of SAE occurred, and both were judged to be unrelated to the study drug: one in Group A at week 169 caused by induced abortion and the other in Group B at week 168 due to the patient having to prepare for the pregnancy of his partner. Subject No. 358 in Group A had an adverse reaction (AR) of dull pain in the hepatic region, while No. 359 in Group B had both dull pain in the hepatic region and common cold, all of which were mild severity.
The results of this long-term, multicenter, prospective study show that ETV maleate treatment at the dosage of 0.5 mg/d for 192 wk is effective and safe in Chinese CHB patients predominantly genotyped as B and C.
The efficacy and safety of long-term ETV therapy has been clarified in many previous studies, but there are limitations in these reports, including the use of different dosages of ETV[23-25], high rates of loss to follow-up[26], low percentages of patients with genotype B or C, a small sample size[25] and the retrospective nature of the study[27]. To date, the longest prospective study of ETV treatment was 5 years and was derived from the follow-up study of two phase III, registration trials[17,18]. Patients were first given entecavir 0.5 mg/d or lamivudine 100 mg/d for 96 wk. In the rollover study ETV-901, most patients were first given ETV 1 mg plus LAM 100 mg once daily for half a year and then changed to ETV 1 mg once daily for up to 5 years[19,20]. In another follow-up study of ETV for 3 years in China, patients received entecavir 0.5 mg/d or lamivudine 100 mg/d for 96 wk and then ETV 1 mg since week 96[24]. In a large-scale study where CHB patients were treated with ETV 0.5 mg daily for only 3 years, more than half of the patients (101/222) discontinued treatment[26]. Of the 333 patients in a European cohort study of ETV for CHB patients, only 60 (22.6%) patients with genotype B or C were included in the study[28]. Another rollover study of ETV was conducted for 3 years on nucleoside-naïve Japanese patients with CHB and had a small sample size, as only 167 patients were enrolled in the study[25]. The ENUMERATE study was the largest and longest real-world study of ETV treatment, with 658 patients from 26 centers with a median duration of ETV of 4 (1.0-8.3) years; however, it was a retrospective study[27]. Therefore, long-term and large prospective studies on the efficacy and safety of ETV 0.5 mg daily in CHB patients with genotype B or C are still needed.
The primary goal of antiviral treatment in CHB patients is sustained HBV suppression, and ETV maleate demonstrated excellent and strong viral suppression. The results at weeks 144 showed that ETV maleate had similar efficacy and safety to ETV. In the current study, after 192 wk of ETV maleate treatment, 83.33% and 79.17% of HBeAg-positive patients in Groups A and B, respectively, and 100% of HBeAg-negative patients achieved undetectable HBV DNA levels (< 20 IU/mL). The percentage of patients with HBV DNA < 52 IU/mL at weeks 192 reached up to 89.74% in Group A and 90.28% in Groups B of HBeAg-positive patients (Supplementary Table 2). The mean decline in HBV DNA levels was 6.61 Log10 IU/mL and 6.69 Log10 IU/mL in Groups A and B of HBeAg-positive patients and 6.05 Log10 IU/mL and 6.03 Log10 IU/mL in Groups A and B of HBeAg-negative patients, respectively. Based on previous studies, with 3-5 years of entecavir administration, the rates of serum HBV DNA-negative conversion were 89%-96%[19,24,25,29]. According to a multinational rollover study ETV-901 from Study ETV-022 up to 5 years, the mean change from baseline in HBV DNA at year 5 was-7.2 Log10 copies/mL[19]. The difference in the results among these studies may be mainly due to the following reasons. First, and most importantly, at each laboratory, researchers employed different test agents with the LLOD of HBV-DNA varying from < 300 copies/mL to < 2000 copies/mL (approximately 400 IU/mL), while in our study, the PCR assay limit of detection of HBV DNA level was less than 20 IU/mL. Second, different sample sizes and study populations may also contribute to this difference in results to some extent. Finally, patient drop-out and missing data are common during long-term studies. In this study, 67 patients had discontinued treatment prior to week 192, and 8 patients who were still receiving treatment at week 192 had missing HBV DNA measurements. An LOCF analysis was conducted since week 96, potentially leading to biased outcomes.
The rollover study ETV-901 reported that 33 (23%, 33/141) patients achieved HBeAg seroconversion on-treatment at year 5. In a 3-year single-center study of ETV in Hong Kong, the rate of HBeAg seroconversion was as high as 43.9% (18/41) after 3 years of ETV treatment[26]. In our study, 21 (28.77%, 21/73) patients in Group A and 14 out of 70 (20.00%) in Group B of the HBeAg-positive patients achieved HBeAg seroconversion at week 192. The difference in the HBeAg seroconversion rate between different studies may be related to the proportion of HBV genotype C patients enrolled, which has previously been reported to be associated with delayed HBeAg seroconversion[30].
Other than durable and strong HBV suppression, ETV maleate showed minimal resistance and great safety. As reported, the resistance mutant emergence rate of ETV was reportedly 1.2% for 3-5 years for nucleoside treatment-naïve patients[19,20]. Consistent with previous studies, in our study, 1.44% (3/208) of NA-naïve CHB patients developed ETV resistance. In addition, no clinically serious AR related to the drug was found after 4 years of treatment.
This study is the largest prospective study focusing on a continuous daily dose of 0.5 mg ETV treatment, where patients from different geographical regions of China were followed closely for 5 years. Another strength is that we employed highly sensitive tests with a LLOD of 20 IU/mL to measure the serum HBV levels and to detect potential entecavir resistance. Last but not the least, the follow-up rate was up to 75.64%, which guaranteed the quality of this study. However, our study has some limitations. First, the study population was predominant with genotype B and C. Second, only 57 HBeAg-negative patients were included in the study. With a dropout rate for follow-up of 20%, the estimated sample size should be 60 in the HBeAg-negative group according to the previous literature[18].Third, subjects received open-label entecavir maleate treatment after weeks 49, which made the comparative result questionable to a certain extent.
Long-term treatment with entecavir maleate provides safe, potent and reliable suppression of HBV replication for 4 years in Chinese CHB patients predominantly genotyped as B or C. Among those patients treated with entecavir maleate, the proportion with undetectable HBV DNA levels increased to 83.33% of HBeAg-positive patients and 100% of HBeAg-negative patients with little chance of developing entecavir-resistant mutations. The safety profile of ETV maleate at week 192 was also promising.
Entecavir maleate is a domestically developed antiviral drug against HBV derived from entecavir and it has efficacy and safety profiles similar to those of ETV (Baraclude) in Chinese patients with CHB at weeks 48, 96, and 144.
In China, there are more than 20 million patients with chronic hepatitis B (CHB) requiring effective and safe treatment, which places a heavy burden on the health care system for the Chinese government. Thus, it is necessary to develop strong antiviral agents against CHB with high cost-effectiveness and easy availability.
To report the antiviral potency and safety of ETV maleate at week 192 in Chinese CHB patients.
In the first stage of this study, treatment assignments were allocated centrally on the basis of a permuted block. Patients, investigators, and all study personnel, including those assessing outcomes, were masked to treatment assignment throughout the 48 wk of the double-blind phase. During this stage, patients were randomly assigned to receive 0.5 mg/d ETV or ETV maleate (ratio, 1:1), each with a placebo tablet for 48 wk. Then, all patients received open-label treatment with 0.5 mg/d ETV maleate since week 49.
The present study demonstrated that ETV maleate has comparable antiviral activity and safety profiles in Chinese CHB patients with ETV.
Long-term entecavir maleate treatment was effective and safe in Chinese CHB patients predominantly genotyped as B or C.
The findings of this study provide an alternative effective drug with a low price and easy accessibility for Chinese CHB patients that lowers the financial burden on the Chinese medical system.
We gratefully thank all the investigators and patients involved in the study. We thank Dr. Min Yu and Ms. Dan Liu for the centrally detection of HBV markers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghoneim S, United States; Morozov S, Russia; Morozov S, Russia; Volynets GV, Russia S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 539] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 2. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (2)] |
| 3. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-6557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 713] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 4. | Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2844] [Article Influence: 406.3] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 7. | World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva, 2015. Available from: https://www.who.int/publications/i/item/9789241549059. |
| 8. | Mak LY, Wong DK, Cheung KS, Seto WK, Fung J, Yuen MF. First-line oral antiviral therapies showed similar efficacies in suppression of serum HBcrAg in chronic hepatitis B patients. BMC Gastroenterol. 2021;21:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Chon HY, Ahn SH, Kim YJ, Yoon JH, Lee JH, Sinn DH, Kim SU. Efficacy of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide in treatment-naive hepatitis B patients. Hepatol Int. 2021;15:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Marcellin P GE, Flisiak R. Long-term treatment with tenofovir disoproxil fumarate for chronic hepatitis B infection is safe and well tolerated and associated with durable virologic response with no detectable resistance: 8 year results from two phase 3 trials. Hepatology. 2014;60:313A-314A. |
| 11. | Jafari A, Khalili H, Dashti-Khavidaki S. Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol. 2014;70:1029-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Jung CY, Kim HW, Ahn SH, Kim SU, Kim BS. Tenofovir is Associated With Higher Risk of Kidney Function Decline Than Entecavir in Patients With Chronic Hepatitis B. Clin Gastroenterol Hepatol. 2022;20:956-958.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. |
Chuang WL AK, Hwang JS, Caruntu F, Wong F, Hann HW, et al Continued improvement in renal laboratory parameters in CHB patients treated with tenofovir alfenamide (TAF) compared with tenofovir disoproxil fumarate (TDF) over 96 wk.
|
| 14. | Agarwal K FS, Seto WK, Lim YS, Gane E, Janssen HL. A phase 3 study comparing tenofovir alafenamide (TAF) to tenofovir disoproxil fumarate (TDF) in patients with HBeAg-positive, chronic hepatitis B (CHB): efficacy and safety results at week 96. J Hepatol. 2017;66:S478. |
| 15. | Brunetto M LY, Gane E, Seto WK, Osipenko M, Ahn SH. A phase 3 study comparing tenofovir alafenamide (TAF) to tenofovir disoproxil fumarate (TDF) in patients with HBeAg-negative, chronic hepatitis B (CHB): efficacy and safety results at week 96. J Hepatol. 2017;66:S25-S26. |
| 16. | Gupta SK, Post FA, Arribas JR, Eron JJ Jr, Wohl DA, Clarke AE, Sax PE, Stellbrink HJ, Esser S, Pozniak AL, Podzamczer D, Waters L, Orkin C, Rockstroh JK, Mudrikova T, Negredo E, Elion RA, Guo S, Zhong L, Carter C, Martin H, Brainard D, SenGupta D, Das M. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, Cross A, DeHertogh D, Wilber R, Colonno R, Apelian D; BEHoLD AI463022 Study Group. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1090] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 18. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, Colonno R, Fernandes L; BEHoLD AI463027 Study Group. Entecavir vs lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 910] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 19. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, Zhang H, Tenney DJ, Tamez R, Iloeje U. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 20. | Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, Colonno RJ. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 633] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 21. | Lai K, Zhang C, Ke W, Gao Y, Zhou S, Liu L, Yang Y. Cost-Effectiveness Comparison Between the Response-Guided Therapies and Monotherapies of Nucleos(t)ide Analogues for Chronic Hepatitis B Patients in China. Clin Drug Investig. 2017;37:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Zheng J, Zeng Z, Zhang D, Yu Y, Wang F, Pan CQ. Prevalence and significance of Hepatitis B reverse transcriptase mutants in different disease stages of untreated patients. Liver Int. 2012;32:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 24. | Yao GB, Ren H, Xu DZ, Zhou XQ, Jia JD, Wang YM, Chen CW. Virological, serological and biochemical outcomes through 3 years of entecavir treatment in nucleoside-naive Chinese chronic hepatitis B patients. J Viral Hepat. 2010;17 Suppl 1:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Yokosuka O, Takaguchi K, Fujioka S, Shindo M, Chayama K, Kobashi H, Hayashi N, Sato C, Kiyosawa K, Tanikawa K, Ishikawa H, Masaki N, Seriu T, Omata M. Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J Hepatol. 2010;52:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106:1264-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Ahn J, Lee HM, Lim JK, Pan CQ, Nguyen MH, Ray Kim W, Mannalithara A, Trinh H, Chu D, Tran T, Min A, Do S, Te H, Reddy KR, Lok AS. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Zoutendijk R, Reijnders JG, Brown A, Zoulim F, Mutimer D, Deterding K, Petersen J, Hofmann WP, Buti M, Santantonio T, van Bömmel F, Pradat P, Oo Y, Luetgehetmann M, Berg T, Hansen BE, Wedemeyer H, Janssen HL; VIRGIL Surveillance Study Group. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naïve patients with a partial virological response. Hepatology. 2011;54:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Ono A, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitou S, Arase Y, Ikeda K, Watahiki S, Mineta R, Kumada H. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol. 2012;57:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Nakashima H, Furusyo N, Kubo N, Kashiwagi K, Etoh Y, Kashiwagi S, Hayashi J. Double point mutation in the core promoter region of hepatitis B virus (HBV) genotype C may be related to liver deterioration in patients with chronic HBV infection. J Gastroenterol Hepatol. 2004;19:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |