Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9524
Peer-review started: April 15, 2022
First decision: June 7, 2022
Revised: June 11, 2022
Accepted: August 9, 2022
Article in press: August 9, 2022
Published online: September 16, 2022
Processing time: 140 Days and 0.3 Hours
Sulfonylurea (SU) is a commonly used antidiabetic drugs effective for type 2 diabetes mellitus. Previous studies have reported that the SU treatment could alter the serum free fatty acid (FFA) concentration in diabetic patients; however, their exact effects remain unknown.
To assess the impact of SU on the FFA level in diabetic patients.
A systematic literature search was conducted by consulting the PubMed, EMBASE, Cochrane Library, Reference Citation Analysis (https://www.referen
A total of 13 studies with 2273 individuals were selected. Results indicated that FFA concentration increased slightly after treatment with SU (MD = 0.08, 95%CI: 0.03-0.12, P < 0.01). In addition, we found that SU treatment combined with other antidiabetics could also increase the concentration of serum FFA (MD = 0.14, 95%CI: 0.01-0.28, P < 0.01). Regarding the type of SU, there was no significant difference in FFA concentration with glimepiride or glibenclamide. FFA concentration was higher at ≥ 12 wk (MD = 0.09, 95%CI: 0.04-0.13) but not at < 12 wk (MD = 0.01, 95%CI: -0.07-0.09).
SU treatment could increase the serum FFA concentration in diabetic patients. The fundamental underlying mechanism still needs further investigation.
Core Tip: The effect of sulfonylurea (SU) therapy on free fatty acid (FFA) concentration in diabetic patients has not been determined. This is the first systematic review and meta-analysis to assess the impact of SU on FFA. The present study indicated that SU therapy could increase FFA concentration in diabetic patients. Further research is required to confirm the association between FFA concentration and SU treatment.
- Citation: Yu M, Feng XY, Yao S, Wang C, Yang P. Certain sulfonylurea drugs increase serum free fatty acid in diabetic patients: A systematic review and meta-analysis. World J Clin Cases 2022; 10(26): 9524-9535
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9524.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9524
The worldwide epidemic of type 2 diabetes mellitus (T2DM) is an important public health problem. It is predicted that the number of people with diabetes will increase to 642 million by 2040. In 2015, about 5 million people aged 20–79 years died from diabetes, accounting for 12.8% of all-cause mortality worldwide[1]. Sulfonylurea (SU) medicines are widely applied for T2DM management[2]. Their mechanism of action is based on enhanced insulin release from the pancreatic beta cells by binding to ATP-sensitive K+ channels[3]. There is also some evidence that SU can limit hepatic glucose production by sensitizing beta cells to glucose[4]. However, SU drugs have limitations, such as being useless against type 1 diabetes or post-pancreatectomy. Several studies have demonstrated that treatment with SU might increase cardiovascular disease-related death risk and stroke in T2DM patients[5,6]. SU medications include certain generations of drugs. The first-generation drugs consist of acetohexamide, glycyclamide, carbutamide, etc. The second-generation drugs comprise gliclazide, glibenclamide, glipizide, glibornuride, etc. The third-generation drugs include glimepiride, which is occasionally considered a second-generation drug as well.
Circulating free fatty acids (FFAs), also known as non-esterified fatty acids, are released from phospholipids and adipocyte triglyceride stores after hydrolysis by phospholipases and by lipolysis, respectively[7]. FFA is a key causal factor implied in the association between obesity and T2DM[8]. FFAs play pivotal roles in multiple metabolic processes[9,10]. They can work by promoting the creation and triglyceride release inducing the enhanced production of very-low-density lipoproteins, thereby leading to the development of atherogenic dyslipidemia[11,12]. Furthermore, higher very-low-density lipoproteins levels may enhance serum FFA flow to the liver, causing inflammation and hepatic insulin resistance[13,14]. In addition, they inhibit the production and release of insulin, which alongside insulin resistance is a cornerstone of T2DM etiology[15]. In recent years, an increasing number of studies has confirmed the association between FFA and heart disease, and serum FFA has been concomitant to an augmented risk of coronary heart disease. High FFA levels reflect the severity of myocardial ischemia and necrosis[16].
Fatty acid metabolism is considered an effective factor during the SU-mediated treatment of T2DM[17]. However, because of the presence of different SUs and their varied combination with other antidiabetic drugs, such as metformin, rosiglitazone, or pioglitazone, the exact effect of SU therapy on serum FFA concentration remains unclear. As a result, the current meta-analysis searched for the possible link between SU therapy and serum FFA concentration. The analysis could help gain a better understanding of SU-mediated treatment impact on FFA of T2DM patients.
This meta-analysis was conducted according to PRISMA statement guidelines. A systematic literature search was conducted by consulting the PubMed, EMBASE, Cochrane Library, Reference Citation Analysis (https://www.referencecitationanalysis.com/), and Web of Science databases to find articles dedicated to the study of the relationship between SU therapy and plasma FFA concentration. A literature search was performed independently by two of the authors (Yu M and Feng XY) using keywords: “Sulfonylurea,” “glyburide,” “glipizide,” “glibenclamide,” “gliclazide,” “glimepiride,” “free fatty acids,” “FFA,” and “non-esterified fatty acids” in different combinations. For any further possible eligible research, suitable references from all prospective papers were also retrieved and studied. In the literature review, no language restrictions were implemented. The last retrieval was made on July 30, 2021. The approval of the ethics committee was not needed because this meta-analysis does not contain patient personal information.
The titles and abstracts of the primary studies were screened independently by two authors. The original studies were added in our meta-analysis if the below-mentioned conditions were met: (1) Studies using SU treatment for diabetic patients; (2) Studies where SU drugs used alone or in combination were equated to placebo or other active medications; (3) Studies examining the effect of SU treatment on serum concentration of FFA; and (4) Studies with sufficient data on FFA concentration at baseline and endpoint. Studies were omitted if they were: (1) Duplicate studies; (2) Non-human research; (3) Studies without sufficient data to extract the detailed information; (4) Pooled studies, comments, and review articles; and (5) Irrelevant studies.
The studies were thoroughly examined, and data were extracted using a predefined criterion. Data extraction was performed by two investigators independently, and disagreements were resolved by consensus. The following data were extracted: First author’s name, publication year, study location, ethnicity, number of participants in the SU and control groups, age, disease, median body mass index (BMI), and concentrations of serum FFA within the treatment group and control group.
The Cochrane criteria were used to undertake a systematic assessment of methodological quality in the selected studies. The items used for evaluating studies were as follows: Randomization method, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete/missing outcome data, selective reporting, and other potential bias. The quality was checked by two authors independently, and the disagreement was resolved by mutual discussion.
The FFA value change was measured according to the Cochrane handbook recommended formula if the study provided only endpoint and baseline FFA values, and the correlation coefficient was 0.5. There was a uniform unit for FF; thus, mean difference (MD) with a 95% confidence interval (CI) was used to assess the effect sizes by evaluating the association between SU therapy and FFA concentration. I2 index was used to assess the heterogeneity, and a random-effect model or fixed-effect model was used according to the test of heterogeneity. I2 of ≥ 50% and a P of < 0.1 indicating statistical heterogeneity were present between studies, and a random-effect model was implemented. Otherwise, the fixed-effect model was applied. The values were merged into a single group by the inverse variance method when performing the overall subgroup analysis if more than one SU group was provided. R3.5.1 software was utilized for conducting all the statistical analyses.
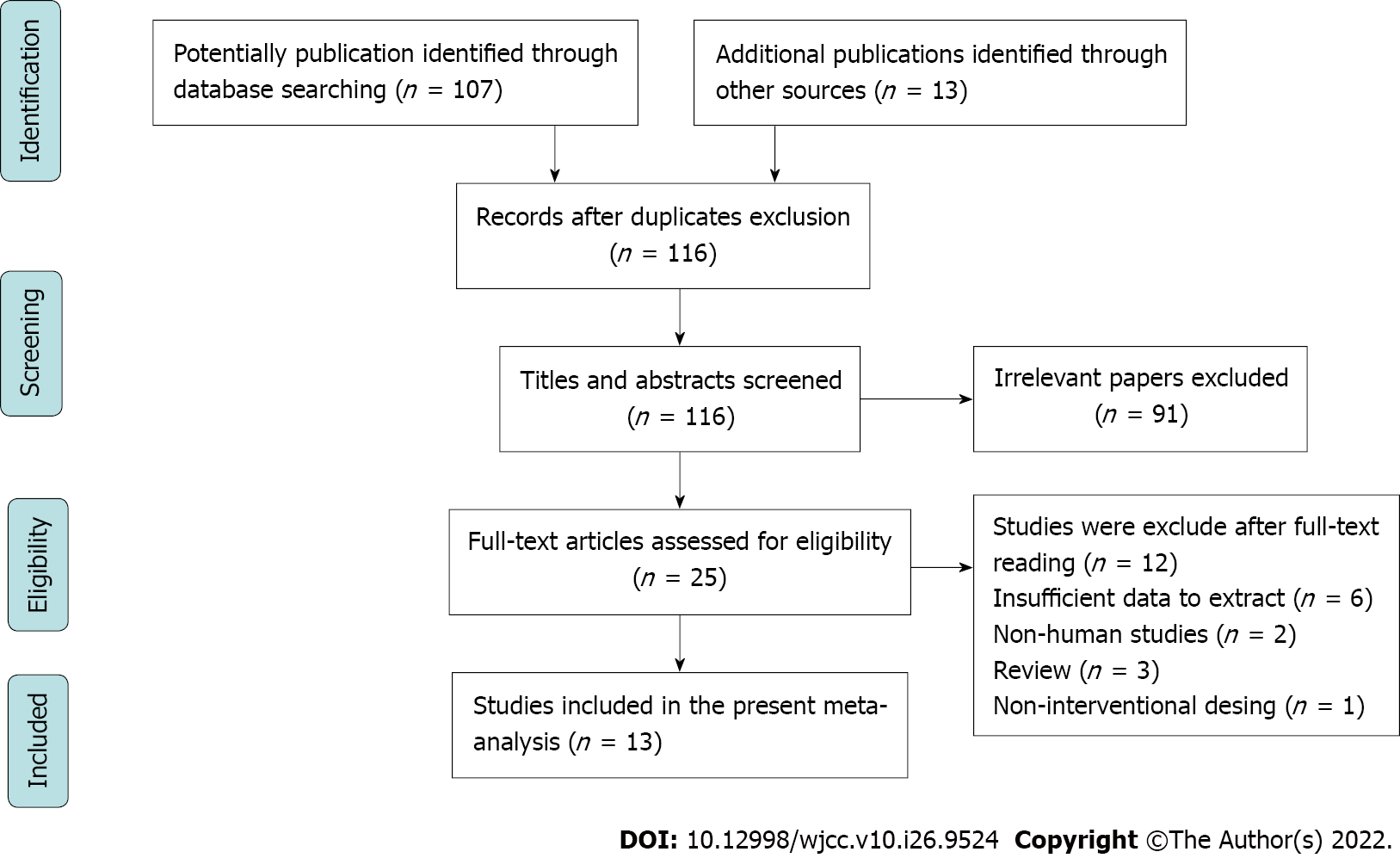
The procedure for the selection of studies is shown in Figure 1. Initially, 116 studies were selected by comprehensively searching the electronic database. Following an assessment of the titles and abstracts, 91 studies were excluded as irrelevant. Then, the remaining 25 studies were carefully assessed for eligibility through full-text reading. Among them, six studies were excluded without sufficient data to extract. Three studies were excluded as they were reviews, while two studies were non-human research, and one study was conducted without interventional design. Eventually, 13 studies comprising 16 treatment arms were used in our meta-analysis.
Data of 2273 individuals were included in the present study, comprising 995 subjects in the control group and 1278 subjects in the SU treatment group. Thirteen studies included in our meta-analysis were published from 1991 to 2010. Among them, four were conducted in China, three were conducted in Germany, and two were conducted in Japan. The remaining four studies were conducted in Italy, Finland, Greece, and the United States. SU treatment time ranged from 2 wk to 52 wk. Two studies focused on non-insulin-dependent diabetes, and the rest focused on T2DM. Two studies were published in the Chinese language, while the rest were in the English language. Glibenclamide was used as a therapeutic drug in five randomized controlled trials (RCTs). Gliclazide was used as a therapeutic drug in two RCTs, and glimepiride was used as a therapeutic drug in seven RCTs. Four studies used SU combined with rosiglitazone, pioglitazone, or metformin. All detailed information is listed in Table 1.
| Ref. | Publication yr | Country | Ethnicity | Disease | Duration time | Intervention | N | FFA in mmol/L | SD | Age | Sex, M/F | BMI | |
| 1 | Hasegawa et al[24] | 2008 | Japan | Asian | T2DM | 12 wk | Control | 11 | 0.50 | 0.19 | 56.1 ± 6.6 | 5/6 | 23.5 ± 3.3 |
| Glimepiride | 13 | 0.48 | 0.20 | 55.6 ± 7.0 | 6/7 | 23.5 ± 3.3 | |||||||
| 2 | Ebeling et al[25] | 2001 | Finland | Caucasian | T2DM | 24 wk | Control | 10 | 0.72 | 0.07 | 55.2 ± 1.8 | NM | 31.9 ± 1.5 |
| Glibenclamide | 10 | 0.73 | 0.06 | 55.2 ± 1.8 | NM | 30.2 ± 1.7 | |||||||
| 3 | Hanefeld et al[26] | 2007 | Germany | Caucasian | T2DM | 52 wk | Control | 195 | 0.70 | 0.30 | 60.4 ± 8.2 | 133/62 | 28.7 ± 3.7 |
| Glibenclamide | 203 | 0.80 | 0.30 | 60.1 ± 8.3 | 143/60 | 28.7 ± 3.9 | |||||||
| 4 | Li et al[27] | 2010 | China | Asian | T2DM | 4 wk | Control | 20 | 0.43 | 0.18 | 52.05 ± 8.06 | 9/11 | 24.91 ± 2.99 |
| Glimepiride | 16 | 0.61 | 0.44 | 51.93 ± 11.07 | 9/7 | 25.76 ± 3.37 | |||||||
| Gliclazide | 16 | 0.39 | 0.16 | 50.06 ± 8.68 | 7/9 | 25.17 ± 3.24 | |||||||
| 5 | Pfützner et al[28] | 2005 | Germany | Caucasian | T2DM | 26 wk | Control | 89 | 0.50 | 0.20 | 62.2 ± 8.4 | 55/34 | 31.7 ± 5.0 |
| Glimepiride | 84 | 0.56 | 0.19 | 63.0 ± 7.4 | 52/32 | 31.8 ± 4.3 | |||||||
| 6 | Tang et al[29] | 2004 | China | Asian | T2DM | 24 wk | Control | 29 | 0.60 | 0.20 | 53.8 ± 9.7 | 18/11 | 24.6 ± 2.2 |
| Glimepiride | 33 | 0.70 | 0.20 | 56.4 ± 8.8 | 21/12 | 23.3 ± 1.7 | |||||||
| Glimepiride + metformin | 32 | 0.60 | 0.20 | 56.8 ± 7.3 | 17/15 | 24.0 ± 2.8 | |||||||
| 7 | Hamann et al[30] | 2008 | Germany | Caucasian | T2DM | 52 wk | Control | 294 | 0.45 | 0.20 | 58.5 ± 9.6 | 155/139 | 33.0 ± 5.9 |
| Glienclamide + metformin | 301 | 0.58 | 0.27 | 59.3 ± 9.2 | 158/143 | 32.2 ± 4.9 | |||||||
| 8 | Yu et al[31] | 2003 | China | Asian | T2DM | 12 wk | Control | 48 | 0.52 | 0.19 | 54.9 ± 8.1 | NM | 25.9 ± 3.0 |
| Glimepiride + pioglitazone | 56 | 0.57 | 0.21 | 55.9 ± 8.5 | NM | 25.5 ± 3.5 | |||||||
| 9 | Riccio et al[32] | 1996 | Italy | Caucasian | NIDDM | 4 wk | Control | 17 | 0.26 | 0.06 | 52 ± 5 | 8/9 | 27.7 ± 0.5 |
| Gliclazide | 17 | 0.23 | 0.04 | 52 ± 5 | 8/9 | 27.7 ± 0.5 | |||||||
| 10 | Shen et al[33] | 1991 | China | Asian | NIDDM | 16 wk | Control | 6 | 0.11 | 0.63 | 60 ± 2 | NM | 26.1 ± 0.92 |
| Glibenclamide | 6 | 0.47 | 0.06 | 58 ± 5 | NM | 27.6 ± 1.35 | |||||||
| 11 | Chou et al[34] | 2008 | United States | Caucasian | T2DM | 28 wk | Control | 230 | 0.40 | 0.21 | 53.6 ± 10.7 | 138/92 | 31.3 ± 5.8 |
| Glimepiride | 222 | 0.47 | 0.22 | 53.0 ± 11 | 128/94 | 31.8 ± 7.2 | |||||||
| Glimepiride ± rosiglitazone | 224 | 0.41 | 0.20 | 54.5 ± 10.6 | 132/92 | 31.8 ± 6.4 | |||||||
| 12 | Skrapari et al[35] | 2001 | Greece | Caucasian | T2DM | 2 wk | Control | 8 | 0.80 | 0.30 | 54 ± 11 | NM | 26.9 ± 0.6 |
| Glibenclamide | 8 | 1.0 | 0.7 | 54 ± 11 | NM | 26.9 ± 0.6 | |||||||
| 13 | Yamanouchi et al[36] | 2005 | Japan | Asian | T2DM | 48 wk | Control | 38 | 0.24 | 0.14 | 55.2 ± 9.2 | 18/20 | 25.8 ± 4.2 |
| Glimepiride | 37 | 0.48 | 0.20 | 55.6 ± 9.3 | 19/18 | 25.6 ± 3.5 |
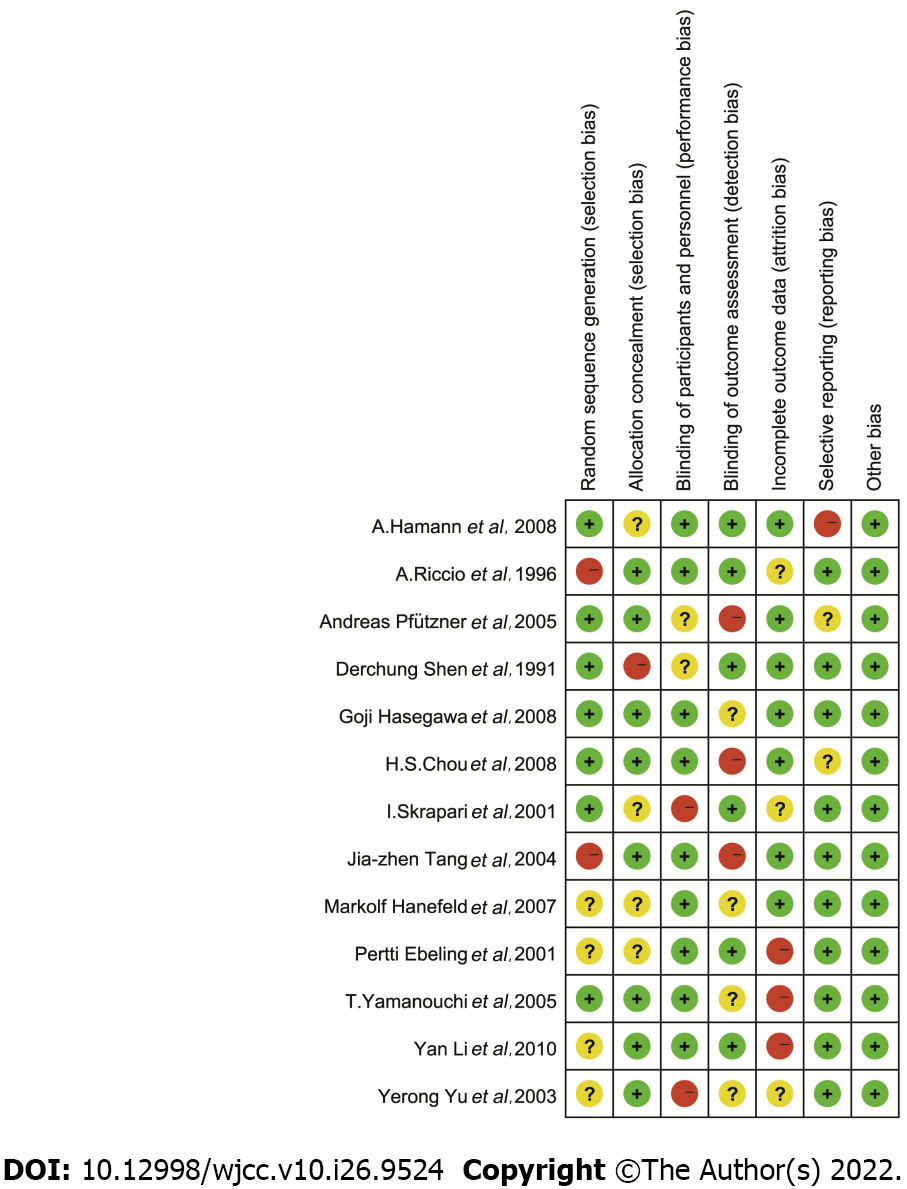
The detailed information on the risk of bias assessment is shown in Figure 2. Seven (53.8%) of the included studies generated random sequences adequately. Eight (61.5%) studies concealed allocation of treatment sufficiently, while two (15.4%) studies had a high risk of blinded participants and personnel. Six (46.2%) studies reported a low risk of outcome assessors. Three (23.1%) studies had an elevated risk of incomplete outcome data, and one (7.7%) study had an increased risk of selective reporting. Finally, all included studies had a low risk of other bias. Overall, the included studies were of high quality.
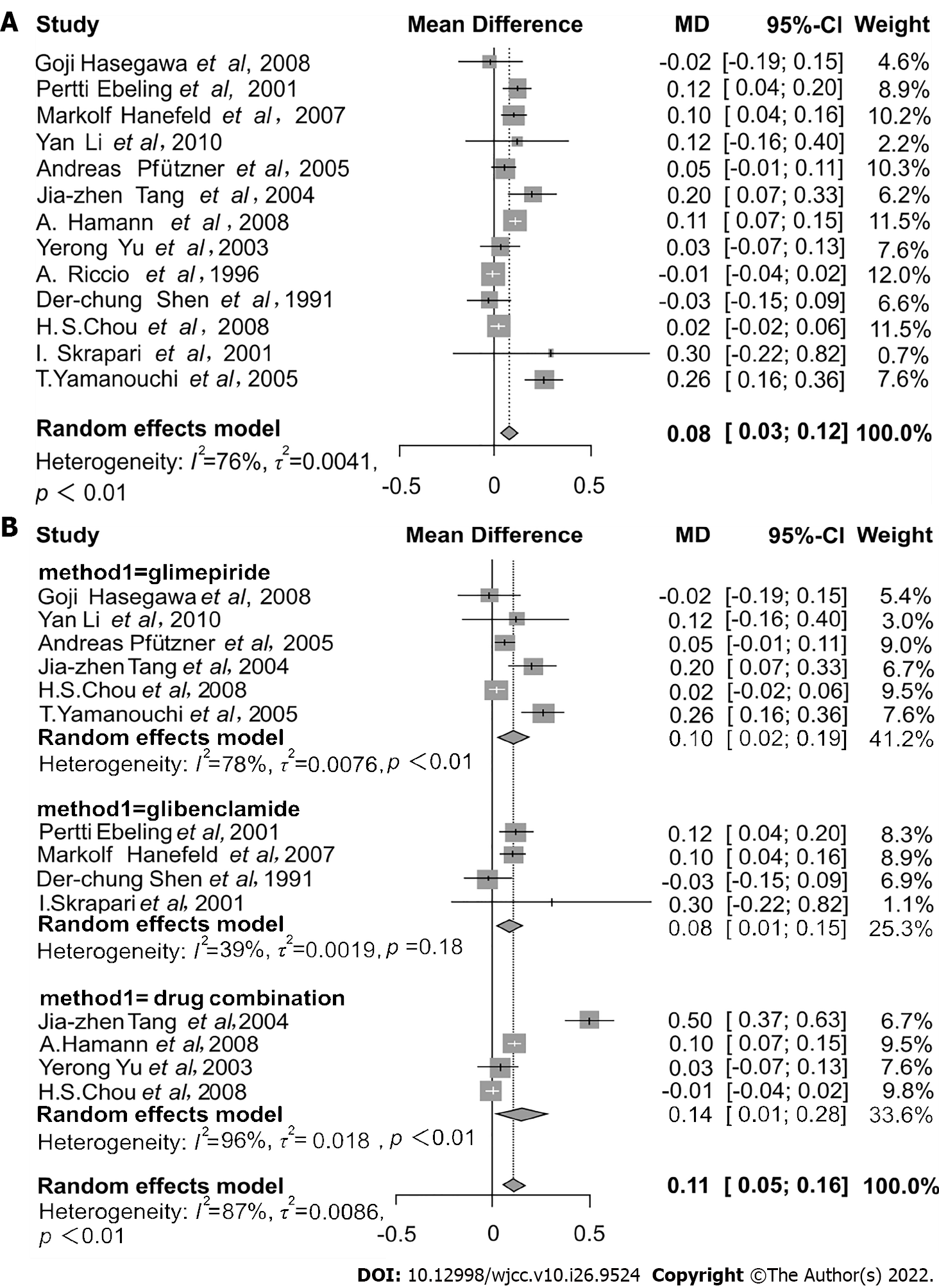
A total of 13 RCTs described the outcome of SU on the concentration of FFA. According to the heterogeneity test (I2 = 76%, P < 0.01), a random-effect model was utilized to evaluate the effect of SU treatment on the serum FFA concentration. The results indicated that FFA concentration was slightly increased after the treatment with SU (MD = 0.08, 95%CI: 0.03–0.12, P < 0.01) (Figure 3A). In addition, four studies reported the treatment with SU combined with rosiglitazone, pioglitazone, or metformin. The results indicated that treatment with SU combined with other antidiabetics could effectively raise the serum FFA concentration (MD = 0.14, 95%CI: 0.01–0.28, P < 0.01) (Figure 3B).
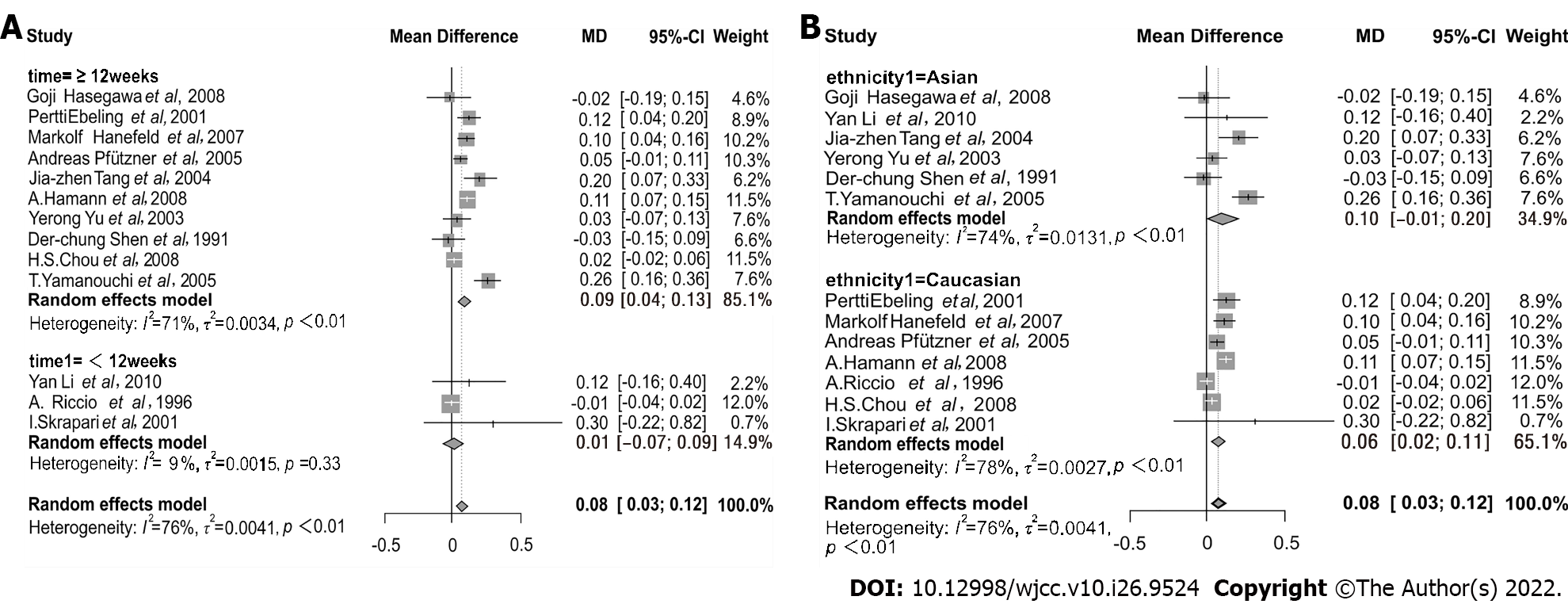
Regarding SU types, there was no significant difference in effect on FFA concentration using treatment with glimepiride or glibenclamide (interaction P = 0.72). Summaries of subgroup analyses are provided in Table 2. When the data were stratified by treatment length, serum FFA concentration was higher in subsets of time spanning several weeks, i.e. ≥ 12 wk (MD = 0.09, 95%CI: 0.04–0.13), and not in time durations of < 12 wk (MD = 0.01, 95%CI: -0.07–0.09) (Figure 4A). Thus, 12 wk was identified as a critical assessment point.
| Subtype | Categories | RCTs | No. of patients | MD | 95%CI | I2 (%) | P value |
| Total effect analysis | 13 | 2273 | 0.08 | 0.03-0.12 | 76 | < 0.01 | |
| Combination drug therapy | 4 | 613 | 0.14 | 0.01-0.28 | 96 | < 0.01 | |
| Duration time (interaction P < 0.01) | ≥ 12 wk | 10 | 2171 | 0.09 | 0.04-0.13 | 71 | < 0.01 |
| < 12 wk | 3 | 102 | 0.01 | -0.07-0.09 | 9 | 0.33 | |
| Ethnicity (interaction P = 0.59) | Asian | 6 | 361 | 0.10 | -0.01-0.20 | 74 | < 0.01 |
| Caucasian | 7 | 1912 | 0.06 | 0.02-0.11 | 78 | < 0.01 | |
| Different SU (interaction P = 0.72) | Glimepiride | 6 | 941 | 0.10 | 0.02-0.19 | 78 | < 0.01 |
| Glibenclamide | 4 | 528 | 0.08 | 0.01-0.15 | 39 | 0.18 | |
| Age (interaction P = 0.34) | ≥ 55 | 8 | 1395 | 0.09 | 0.04-0.14 | 65 | < 0.01 |
| < 55 | 5 | 878 | 0.05 | -0.02-0.11 | 65 | 0.02 | |
| BMI (interaction P = 0.57) | ≥ 28 | 5 | 1895 | 0.07 | 0.03-0.11 | 96.2 | 0.03 |
| < 28 | 8 | 379 | 0.10 | -0.01-0.21 | 88.1 | < 0.01 |
Furthermore, elevation of FFA concentration was seen in both Asians (MD = 0.10, 95%CI: -0.01–0.20) and Caucasians (MD = 0.06, 95%CI: 0.02–0.11) (Figure 4B). The effect of SU medications on FFA concentration was also proved in the age group of ≥ 55 years (MD = 0.09, 95%CI: 0.4–0.14) and in the age group of < 55 (MD = 0.05, 95%CI: -0.02–0.11) (Supplementary Figure 1A). We also assessed the effect of SU drugs on FFA concentration in the group with a BMI of ≥ 28 (MD = 0.08, 95%CI: 0.03–0.12) and in the group with a BMI of < 28 (MD = 0.08, 95%CI: -0.01–0.17) (Supplementary Figure 1B). The results showed that there was no significant difference between the two groups.
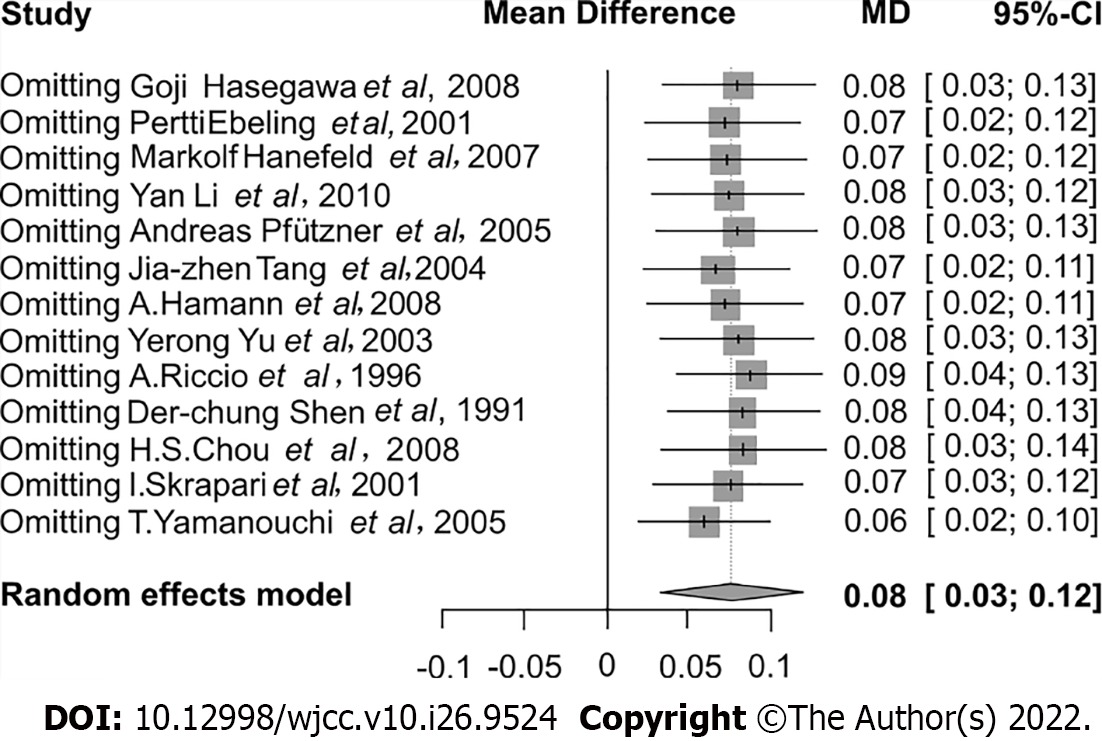
By removing one study at a time, the sensitivity examination was done to explore the robustness of our results. The data indicated that the significance of the comprehensive effect size did not significantly change throughout the analysis, suggesting that our results were relatively steady (Figure 5).
Different FFA concentrations have been shown to alter various biological processes in metabolism[18]. High expression of FFA has been reported to induce oxidative stress insulin resistance and facilitate inflammation by modulating the nuclear factor-kappa B pathway[14]. As a result, the altered FFA concentration might have important clinical roles in regulating the risk of cardiovascular disease and atherogenesis progression. FFA can be produced from adipose tissue lipolysis via lipase[19]. FFA interacts with peroxisome proliferator-activated receptors after being derived from lipolysis. Peroxisome proliferator-activated receptor activation boosts FFA oxidation, decreases triglyceride levels, raises plasma high-density lipoprotein, and lowers very-low-density lipoprotein production and secretion[20]. The current study attempted to examine the effects of SU therapy on FFA in-depth and produce a reliable conclusion by merging as many valuable studies as feasible. This could be the first meta-analysis to investigate the relationship between SU and FFA.
In the present study, the results indicated that SU treatment could increase the concentration of FFA (MD = 0.08, 95%CI: 0.03–0.12). Importantly, when combined with other antidiabetics, such as rosiglitazone, pioglitazone, or metformin, the effects of SU treatment on FFA concentration were more pronounced (MD = 0.14, 95%CI: 0.01–0.28). To further elaborate our findings, we performed a subgroup analysis, which assisted us in reaching a firmer conclusion. We found that there was no significant difference in the efficacy of different SU types. In addition, no apparent alteration of the effects was found between the Asian and Caucasian populations, indicating that ethnicity did not affect the impact of SU treatment on FFA concentration. Furthermore, we discovered that serum FFA had no discernible effect on SU therapy in highly obese (BMI of ≥ 28) individuals compared to moderately underweight people (BMI of < 28). Additionally, the elevation of FFA was only observed when T2DM patients were treated with SU for a duration time ≥ 12 wk.
Interestingly, another meta-analysis provided a slightly different conclusion compared to our findings. Chen et al[21] focused on the lipid alteration after administration of SU in T2DM treatment. Our study specifically focused on the FFA concentration after SU treatment in diabetic patients. Moreover, Chen et al[21] only included eight RCTs, which reported the effect of SU on FFA concentration in T2DM (based on their criteria for inclusion). They excluded all other studies focusing on the FFA concentration instead of lipids. We also investigated the impact of FFA changes when paired with other antidiabetics. Following treatment with SU, both studies found an increase in FFA concentration. However, Chen et al[21] enrolled three RCTs that reported that FFA elevation was only observed after glibenclamide treatment. Our results were more robust compared to the analysis of Chen et al[21].
The underlying mechanism of this FFA concentration alteration after SU treatment is still unknown. It has been reported that SU could open Ca2+ channels in pancreatic beta cells; thus, the influx of calcium ions increases. This ultimately leads to enhanced insulin secretion. The potassium channel, which regulates lipid metabolism, is another essential membrane protein[22]. SU could form proteins, anchored to membranes, and interact with yeast glycosyl-phosphatidyl-inositol[23]. Thus, SU could affect the FFA concentration in patients with diabetes. However, the specific mechanism still needs further research.
Despite a thorough review, there are several limitations to our research. The most important is the existence of significant heterogeneity between the studies. Although we conducted the subgroup analysis to find the potential for bias, the heterogeneity still existed. The random-effect model was used to check the variation of treatment effect and limit heterogeneity, yet it still existed. It could be due to variations in patients’ age, a dose of a drug, etc. Secondly, the quality assessment indicated that not all studies generated random sequences adequately. Thus, they might have influenced the outcome of our analysis. Finally, the sample size in several included studies was comparatively small. Hence, more studies concentrating on the association between FFA concentration and SU treatment are encouraged.
SU treatment may raise the serum FFA concentration in T2DM patients. The elevated FFA concentration was also observed when SU was combined with other antidiabetics. The change in FFA is more sensitive than that of triglycerides and total cholesterol. Therefore, FFA levels can be tested regularly for diabetic patients as a reference indicator of whether their lipid metabolism is well controlled.
Previous studies suggested that free fatty acid (FFA) concentration was potentially associated with anti-diabetic drugs of sulfonylurea (SU). The results were inconsistent. We assessed the effects of SU on the level of FFA concentration in diabetic patients.
SU is one of the most commonly used anti-diabetic medications. Several studies reported that SU treatment increased the risk of cardiovascular death and stroke in diabetic patients. Despite the reason for this result is unclear but may be related to the effect of SU on FFA and blood lipids.
The primary objective was to perform a meta-analysis of diabetic patients treated with SU and analyze changes in FFA concentration.
We reviewed PubMed, EMBASE, Cochrane Library, Reference Citation Analysis (https://www.referencecitationanalysis.com/), and Web of Science databases to identify studies using SU for diabetic patients. The FFA value change was measured. A random-effect model or fixed-effect model was used according to the test of heterogeneity, and I2 index was used to assess the heterogeneity.
We included 13 observational studies comprising 16 treatment arms in the meta-analysis. FFA concentration was increased after the treatment of SU in diabetic patients. When combined with other antidiabetics, the effects of SU treatment on FFA concentration were more pronounced. There was no significant different effect of FFA concentration when treated with glimepiride or glibenclamide.
Some SU drugs increased serum FFA concentration in diabetic patients.
The association between FFA concentration and SU treatment requires more studies and longer follow-up.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Balbaa ME, Egypt S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2514] [Article Influence: 314.3] [Reference Citation Analysis (0)] |
| 2. | Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? J Clin Endocrinol Metab. 2012;97:4605-4612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Seino S. Cell signalling in insulin secretion: the molecular targets of ATP, cAMP and sulfonylurea. Diabetologia. 2012;55:2096-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Yang M, Chisholm JW, Soelaiman S, Shryock JC. Sulfonylureas uncouple glucose-dependence for GPR40-mediated enhancement of insulin secretion from INS-1E cells. Mol Cell Endocrinol. 2010;315:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:938-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Phung OJ, Schwartzman E, Allen RW, Engel SS, Rajpathak SN. Sulphonylureas and risk of cardiovascular disease: systematic review and meta-analysis. Diabet Med. 2013;30:1160-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Mozaffarian D. Free fatty acids, cardiovascular mortality, and cardiometabolic stress. Eur Heart J. 2007;28:2699-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Arner P, Rydén M. Fatty Acids, Obesity and Insulin Resistance. Obes Facts. 2015;8:147-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Hodson L, Bickerton AS, McQuaid SE, Roberts R, Karpe F, Frayn KN, Fielding BA. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes. 2007;56:2433-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Nestel PJ. Relationship between FFA flux and TGFA influx in plasma before and during the infusion of insulin. Metabolism. 1967;16:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Goh EH, Heimberg M. Stimulation of hepatic cholesterol biosynthesis by oleic acid. Biochem Biophys Res Commun. 1973;55:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 348] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Kwiterovich PO Jr. Clinical relevance of the biochemical, metabolic, and genetic factors that influence low-density lipoprotein heterogeneity. Am J Cardiol. 2002;90:30i-47i. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 463] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Arner P. Free fatty acids - do they play a central role in type 2 diabetes? Diabetes Obes Metab. 2001;3 Suppl 1:11-19. [PubMed] |
| 16. | Kan Y, Wang H, Lu J, Lin Z, Lin J, Gong P. Significance of plasma free fatty acid level for assessing and diagnosing acute myocardial infarction. Biomark Med. 2020;14:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441-2449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 647] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 18. | Jansson PA, Larsson A, Smith U, Lönnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992;89:1610-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Tripathy D, Aljada A, Dandona P. Free fatty acids (FFA) and endothelial dysfunction; role of increased oxidative stress and inflammation. --to: Steinberg et al (2002) Vascular function, insulin resistance and fatty acids. Diabetologia. 2003;46:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 1203] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 21. | Chen YH, Du L, Geng XY, Peng YL, Shen JN, Zhang YG, Liu GJ, Sun X. Effects of sulfonylureas on lipids in type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. J Evid Based Med. 2015;8:134-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Szewczyk A, Pikula S. Lipid metabolism as a target for potassium channel effectors. Biochem Pharmacol. 2000;60:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Müller G, Geisen K. Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at adipocytes. Horm Metab Res. 1996;28:469-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Hasegawa G, Kajiyama S, Tanaka T, Imai S, Kozai H, Fujinami A, Ohta M, Obayashi H, Park H, Nakano K, Tanaka M, Shiraishi E, Fukui M, Yoshikawa T, Nakamura N. The alpha-glucosidase inhibitor acarbose reduces the net electronegative charge of low-density lipoprotein in patients with newly diagnosed type 2 diabetes. Clin Chim Acta. 2008;390:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Ebeling P, Teppo AM, Koistinen HA, Koivisto VA. Concentration of the complement activation product, acylation-stimulating protein, is related to C-reactive protein in patients with type 2 diabetes. Metabolism. 2001;50:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Hanefeld M, Patwardhan R, Jones NP; Rosiglitazone Clinical Trials Study Group. A one-year study comparing the efficacy and safety of rosiglitazone and glibenclamide in the treatment of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2007;17:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Li Y, Xu L, Shen J, Ran J, Zhang Y, Wang M, Yan L, Cheng H, Fu Z. Effects of short-term therapy with different insulin secretagogues on glucose metabolism, lipid parameters and oxidative stress in newly diagnosed Type 2 Diabetes Mellitus. Diabetes Res Clin Pract. 2010;88:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Pfützner A, Marx N, Lübben G, Langenfeld M, Walcher D, Konrad T, Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005;45:1925-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Tang JZ, Mao JP, Yang ZF, Zhou ZG, Tang WL, Feng Q. [Effects of glimepiride and metformin on free fatty acid in patients with Type 2 diabetes mellitus]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29:631-634. [PubMed] |
| 30. | Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with Type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Yu Y, Li Y, Fan J, Wang Y, Yu H, Wang C, Liao Z, Gao H, Zhao W. [Pioglitazone hydrochloride monotherapy or in combination with sulfonylurea therapy improves glycemia control in patients with type 2 diabetes]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:117-120. [PubMed] |
| 32. | Riccio A, Lisato G, de Kreutzenberg SV, Marchetto S, Turrin M, Tiengo A, Del Prato S. Gliclazide potentiates suppression of hepatic glucose production in non-insulin-dependent diabetic patients. Metabolism. 1996;45:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Shen DC, Fuh MM, Shieh SM, Chen YD, Reaven GM. Effect of gemfibrozil treatment in sulfonylurea-treated patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;73:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Chou HS, Palmer JP, Jones AR, Waterhouse B, Ferreira-Cornwell C, Krebs J, Goldstein BJ. Initial treatment with fixed-dose combination rosiglitazone/glimepiride in patients with previously untreated type 2 diabetes. Diabetes Obes Metab. 2008;10:626-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Skrapari I, Perrea D, Ioannidis I, Karabina SA, Elisaf M, Tselepis AD, Karagiannacos P, Katsilambros N. Glibenclamide improves postprandial hypertriglyceridaemia in type 2 diabetic patients by reducing chylomicrons but not the very low-density lipoprotein subfraction levels. Diabet Med. 2001;18:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Yamanouchi T, Sakai T, Igarashi K, Ichiyanagi K, Watanabe H, Kawasaki T. Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed Type 2 diabetes. Diabet Med. 2005;22:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |