Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9518
Peer-review started: May 20, 2022
First decision: June 8, 2022
Revised: June 25, 2022
Accepted: August 11, 2022
Article in press: August 11, 2022
Published online: September 16, 2022
Processing time: 104 Days and 21.8 Hours
Hemangioblastoma (HB) is a rare tumor, comprising about 2% of all intracranial tumors. Although it is a benign tumor, due to the abundant blood supply and its close relationship with adjacent cerebral blood vessels, surgical resection is difficult and may cause complications such as bleeding. If HB can be correctly diagnosed before surgery, complications can be avoided by methods such as vascular embolism before surgery.
A 51-year-old male patient was admitted to our hospital because of blurred vision in his left eye for 2 years. Ophthalmological examination revealed oculus dexter vision acuity of 1.0 and oculus sinister vision acuity of 0.6. His left vision had tubular visual field, while his right vision had a partial defect. Computed tomography and magnetic resonance imaging showed a mass lesion at the left anterior base of the skull, which could have been a meningioma. During the operation, the tumor was found to be located at the entrance of the left optic nerve tube, closely adhering to the left optic nerve and the blood supply was extremely abundant. The tumor was carefully separated and diagnosed as HB postoperatively after pathological examination.
A rare HB at the anterior skull base could be distinguished by its imaging features, which is essential to the surgical procedures.
Core Tip: Hemangioblastoma (HB) tends to occur in the infratentorial region, especially in the cerebellar hemisphere. Supratentorial HB incidence was only about 3.2%. HB at the anterior skull base is exceedingly rare and needs to be distinguished from other tumors that tend to occur in this region. Magnetic resonance imaging plays a key role in the differential diagnosis of supratentorial HB, allowing its correct diagnosis and guiding the choice of surgical procedure.
- Citation: Xu ST, Cao X, Yin XY, Zhang JY, Nan J, Zhang J. Supratentorial hemangioblastoma at the anterior skull base: A case report. World J Clin Cases 2022; 10(26): 9518-9523
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9518.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9518
Hemangioblastoma (HB) is a rare tumor, comprising about 2% of all intracranial tumors[1]. HB occurs most commonly in the cerebellar hemispheres, followed by the brainstem and spinal cord, and is least common in the supratentorial region[2]. In the supratentorial area, it mostly occurs in the frontal lobe and pituitary stalk[3], whereas in this case, as on rare occasions, HB can occur at the base of the anterior skull. This patient initially presented with a left visual field defect, with computed tomography (CT) and magnetic resonance imaging (MRI) suggesting a left anterior skull base mass. The pathological report revealed HB after surgery. Since the HB blood supply at the base of the anterior skull is abundant and located in regions of critical neural functions, correct preoperative diagnosis is very important to improve surgical safety.
Here, we provide characteristic imaging findings of anterior skull base HB and distinguish them from other anterior skull base mass holders.
A 51-year-old male patient had blurred vision in his left eye for 2 years, and was not receiving any medication at the time of admission.
The patient undergoes physical examination every year. Four months ago, during physical examination in the local hospital, a head CT revealed a mass-occupying space. The patient came to our hospital for further diagnosis and treatment.
The patient denied history of hepatitis, tuberculosis, hypertension, diabetes, heart disease, or other chronic diseases.
The patient’s personal and family history was unremarkable.
The ophthalmic examination showed that the patient’s consciousness was clear, both pupils were equal in size, and he was sensitive to light reflex. Cranial nerve examination showed a normal result except that the oculus sinister vision acuity was 0.6, his left eye had tubular constriction, and his right eye had a partial defect.
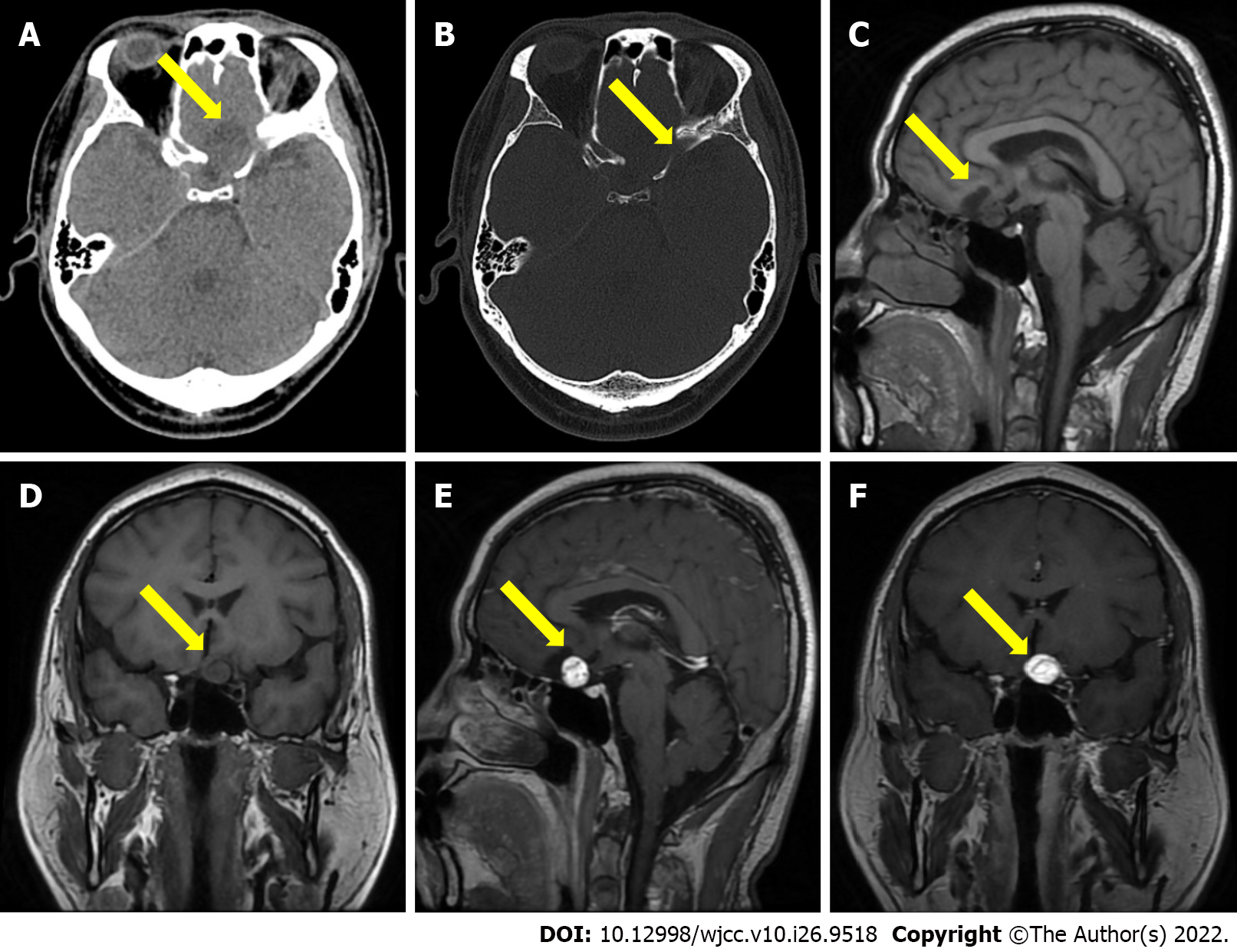
CT showed a quasi-circle-shaped lesion of 24.0 mm in diameter, with a hybrid density focus and a clear boundary, adjacent to some irregular bone, identified in the anterior skull base. MRI revealed a quasi-circular, cystic-solid mixed mass at the anterior skull base. T1-weighted imaging (T1WI) exhibited slightly low and low signals. Gadolinium-enhanced T1WI showed that the solid part was enhanced, and the inside of the mass showed flow void (Figure 1). Imaging diagnosis indicated that the mass in the anterior skull base was benign.
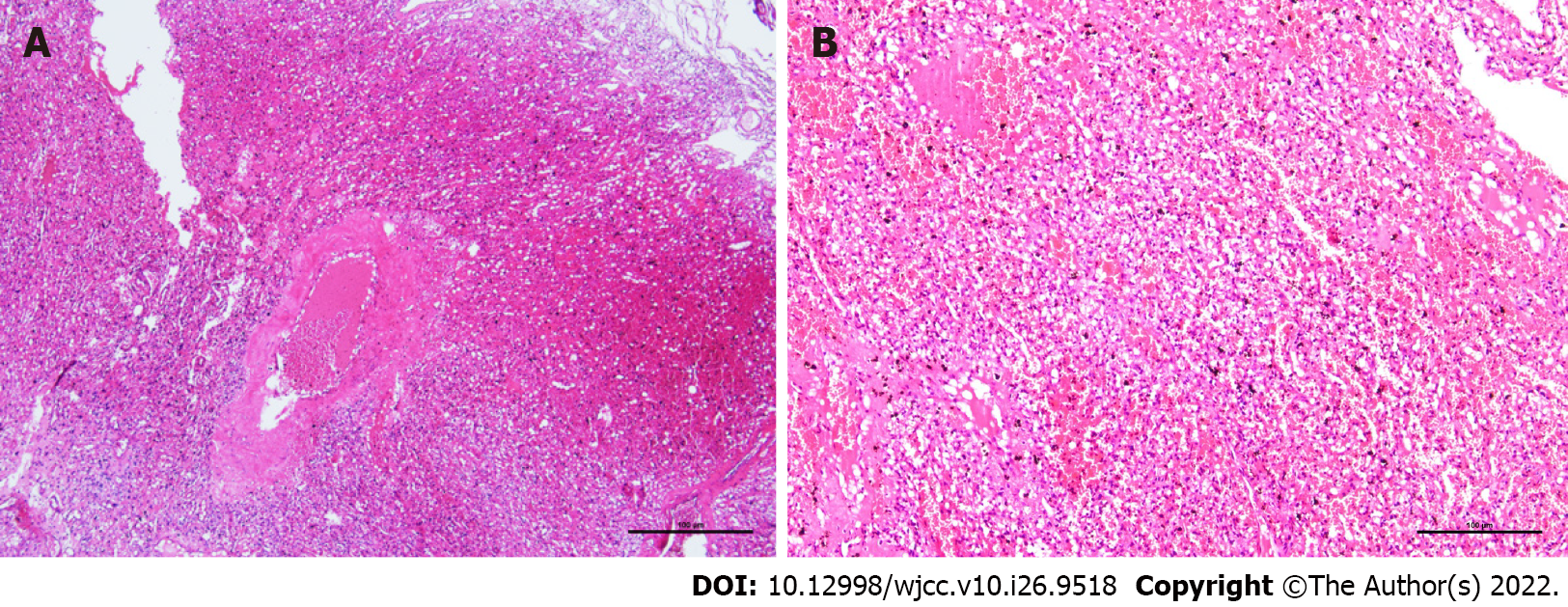
During the operation, the tumor was located at the entrance of the left optic canal, approximately 20 mm × 20 mm × 25 mm in size. The tumor was reddish-brown, and its texture was soft, with an abundant blood supply. Several thick and malformed blood vessels were observed on the surface of the tumor, which primarily supplied blood to the left ophthalmic artery. Gross pathology revealed a gray-yellow tumor with a diameter of 18 mm. Microscopic pathology demonstrated an abundant capillary network and foamy stromal cells, and the nucleus of these cells exhibited slight atypia (Figure 2). The immunohistochemistry results were as follows: cluster of differentiation 34 (CD34) (+), S-100 (10%-20%+ of tumor cells), inhibin A (-), D2-40 (-), glial fibrillary acidic protein (brain +), cytokeratin (-), CD10 (-), Ki67 (1%+ of tumor cells), epithelial membrane antigen (-), and progesterone receptor (10%-20%+ of tumor cells). The pathological diagnosis was of an HB at the anterior skull base [World Health Organization (WHO) grade I].
Supratentorial HB at the anterior skull base.
Left anterior skull base tumor resection was performed under general anesthesia. During the operation, the artery supplying the tumor was found to be an ophthalmic artery, and was cut and separated from the tumor. Although the tumor tightly adhered to the left optic nerve, the tumor was carefully isolated and completely removed under the microscope. The bilateral internal carotid arteries, optic crissus, right optic nerve, and pituitary stalk were well protected, and the left optic nerve was anatomically preserved.
After surgery, the patient was blind in the left eye, had no sense of light, and was slow to respond to light. One week after the operation, the patient suddenly had a generalized tonic-clonic seizure, which presented with generalized muscle rigidity and clonus, with loss of consciousness and autonomic dysfunction. After symptomatic treatment, there was no recurrence. At the most recent follow-up, the patient also had no further seizures. And, 3 mo after the operation, the patient underwent a head MRI at the local hospital, and the imaging results showed that HB had not recurred.
HB is also known as angioreticuloma. In 2021, the WHO categorized HB as grade I “mesenchymal, non-meningothelial tumor.” HB accounts for approximately 2% of all intracranial tumors and 7%-12% of all posterior fossa tumors. HB is divided into sporadic and familial hereditary types, with the latter accounting for approximately 20% of HBs[1]. HB is often diagnosed between 35 years and 45 years of age, and men are more likely to be diagnosed than women[2]. In accordance with the frequency of occurrences of HB at various locations, the descending order is as follows: Cerebellar hemisphere, brainstem and spinal cord, and supratentorial area[2]. The clinical symptoms of HB are primarily dependent on the location of the tumor, while the clinical symptoms of supratentorial HB are non-specific and include epilepsy, elevated intracranial pressure, and neurological dysfunction[4]. Histologically, HB comprises two components: the parenchymal vessels at various maturity stages and the interstitial cells in the vascular network[5]. Because HB has abundant blood supply and is located in essential neural functional areas, it is important to improve the safety of the operation to make the correct diagnosis before the operation.
The imaging of HB has shown the existence of solid and cystic types and a mixed type (cystic-solid mixed type). MRI plays a key role in the differential diagnosis of HB[6]. The typical feature of the solid-cystic mixed type is a large cystic part with small tubercles. In this type, the edge of the tumor is smooth, and the diameter of the mural tubercle is normally less than 2 cm, which is also often attached to the pia mater. CT has shown that the cystic part has a homogeneous low density, whereas the tubercle is isodense or of a slightly higher density. T1WI shows a hypointense signal of cystic fluid, which is slightly higher than the intensity of the cerebrospinal fluid, whereas the tubercle displays a slight hyperintense or isointense signal. T2WI shows a homogeneously hyperintense signal of the cystic fluid, while there is a slightly less intense signal of the mural tubercle than that of the cystic fluid. The signal of the mural tubercle is often covered by the hyperintense signal of the cystic fluid. Fluid attenuated inversion recovery shows a hypointense signal of the cystic fluid and a hyperintense signal of the mural tubercle. Enhanced T1WI signal indicates that the mural tubercle is obviously enhanced, but not in the cystic fluid and cyst walls. Flow voids are observed inside or in the vicinity of the tubercle. If there was fresh bleeding from the tumor, T1WI and T2WI would display a hyperintense signal. It is associated with no or mild peritumoral edema[1,7].
For solid-type HB, T1WI displays an isodense signal, and the tumor with its center necrotized could display an isodense and hypointense mixed signal. The enhanced T1WI shows that the solid part is obviously enhanced, and there are earthworm-shaped arteries with flow voids supplying blood to the tumor, around the tumor, and the majority of the peritumoral edema is obvious. A purely cystic tumor is the rarest circumstance, probably because the mural tubercles are too small to be displayed, and the overall tumor exhibits one cystic mass. Infratentorial and supratentorial HBs often differ from each other, as the former are primarily cystic, whereas the latter are cystic-solid mixed, with a few cystic components. The reason for this difference might be that the brain is believed to be less resistant to fluid diffusion than the cerebellum[1,7].
In this case, the HB was in the supratentorial region and at the anterior skull base, which is rather rare. T1WI displayed a slightly hypointense signal on the solid tubercle and a relatively low intensity signal of the cystic component. Enhanced T1WI indicated that the tubercle was enhanced, but not the cystic fluid and wall. The HB in this case was of the cystic-solid mixed type. A flow void was observed in the vicinity of the tumor, which was consistent with the typical imaging features of this type of HB. Because HB is abundant in blood supply, in anatomically conducive circumstances, vascular embolization could be performed to reduce bleeding during the perioperative period and associated complications[3,8]. If this case can be correctly diagnosed as HB before surgery, there may be a more suitable surgical plan, and the patient's prognosis will be better. Therefore, a correct diagnosis of HB before surgery plays a key role in surgery.
It is not difficult to discern the infratentorial HB in light of the typical location and its imaging features; however, it is the view of this article that the circumstance of the HB at the anterior skull base is to be distinguished from the following conditions: (1) Olfactory groove meningioma is a common space-occupying lesion at the anterior skull base and bears imaging features similar to those of meningiomas at other locations, including hyperosteogeny, less cystic degeneration, and the broad base of the tumor connected to the meninges. Most olfactory groove meningiomas exhibit signs of dural tail and cortical buckling[9]; (2) Olfactory neuroblastoma often appears as irregularly shaped lumps, with isodensity on CT and calcification in most cases. This type of tumor is usually located in the front two-thirds of the top of the nasal cavity, with longitudinal growth. It is also dumbbell-shaped with the anterior skull base as its center, and develops in both the nasal and intracranial directions, coupled with bone destruction of the ethmoid plate of the anterior skull base. Bone destruction often occurs in the turbinate, nasal septum, or orbit. T1WI of this tumor displays a slightly hypointense signal relative to the gray matter. In contrast, T2WI exhibits a relatively hypointense signal compared to that of the retained mucus. In addition, T2WI indicates that the edge of the tumor is not clear and that there is an inhomogeneous signal. Cystic degeneration and bleeding have also been reported. Enhanced T1WI displays more than moderate and inhomogeneous enhancement[10]; and (3) Approximately 20% of schwannomas occur in the anterior skull base. In most cases, T2WI findings indicate a hyperintense signal with variable enhancement[9]. Schwannoma is usually found firmly attached to the cribriform plate or olfactory groove and is characterized by poor vascularization. In some patients, cranial nerves are involved, manifesting as thinning and displacement[11].
Although HB in the anterior skull base is rare, the aforementioned imaging features should serve as one of the criteria for differential diagnosis. The diagnosis of HB in the anterior skull base is essential for selection of the appropriate surgical procedure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garg P, India; Salim J, Indonesia S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Duan M, Yang L, Kang J, Wang R, You H, Feng M. Neuroimaging Features of Optic Nerve Hemangioblastoma Identified by Conventional and Advanced Magnetic Resonance Techniques: A Case Report and Literature Review. Front Oncol. 2021;11:763696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Pamela Ferreira Neto B, Martins Barreto Santana J, Dornellys da Silva Lapa J, Cristina de Souza Melo T, Maynart Pereira Oliveira A. Noncystic cerebellopontine angle hemangioblastoma: A case of an atypical location. Int J Surg Case Rep. 2020;74:234-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Rocha L, Noronha C, Taipa R, Reis J, Gomes M, Carvalho E. Supratentorial hemangioblastomas in von Hippel-Lindau wild-type patients - case series and literature review. Int J Neurosci. 2018;128:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Qu L, Lv C, Ji T, Wang Y, Yu J. Cerebral Hemangioblastoma Without Von Hippel-Lindau Syndrome: A Report of 6 Cases. Int J Surg Pathol. 2021;29:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Matsusue E, Inoue C, Tabuchi S, Yoshioka H, Nagao Y, Matsumoto K, Nakamura K, Fujii S. Advanced magnetic resonance imaging findings of cerebellar hemangioblastomas: A report of three cases and a literature review. Acta Radiol Open. 2022;11:20584601221077074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Lahkim M, Andour H, Laamrani FZ, Allaoui M, Saouab R, El Fenni J, En-Nouali H. Cerebellar hemangioblastoma: Case report with review of the literature. Radiol Case Rep. 2021;16:3109-3112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Sánchez-Ortega JF, Claramonte M, Martín M, Calatayud-Pérez J. Sporadic supratentorial hemangioblastoma with meningeal affection: A case report and literature review. Surg Neurol Int. 2021;12:394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Tabibkhooei A, Fattahi A, Rahatlou H. Presentation of a Hemangioblastoma in cavernous sinus: An extremely rare case report. Int J Surg Case Rep. 2018;44:54-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Francies O, Makalanda L, Paraskevopolous D, Adams A. Imaging review of the anterior skull base. Acta Radiol Open. 2018;7:2058460118776487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Veyrat M, Vérillaud B, Fiaux-Camous D, Froelich S, Bresson D, Nicolai P, Herman P. Olfactory Neuroblastoma. Adv Otorhinolaryngol. 2020;84:154-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Figueroa-Ángel V, Rodríguez-Aceves CA, Calderon-Miranda WG, Escobar-Hernandez N, Joaquim AF, Moscote-Salazar LR. Subfrontal Schwannoma: Case Report and Review of Literature. World Neurosurg. 2018;111:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |