Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9493
Peer-review started: May 12, 2022
First decision: June 8, 2022
Revised: June 17, 2022
Accepted: August 11, 2022
Article in press: August 11, 2022
Published online: September 16, 2022
Processing time: 112 Days and 17.2 Hours
The bone is the second most common site of thyroid cancer metastasis, after the lung. Treatment options for bone metastasis of thyroid cancer include surgery, radioiodine therapy (RAIT), external radiation therapy, thyroid-stimulating hormone (TSH) inhibition, bisphosphonates, and small-molecule targeted therapies. In most cases, thyroid carcinoma is found in the thyroid tissue; reports of follicular thyroid carcinoma with a single metastasis to the lumbar spine are rare.
We report a case of bone metastasis as the only clinical manifestation of thyroid cancer. The patient was a 67-year-old woman with lumbar pain for 7 years and aggravation with intermittent claudication who had previously undergone partial thyroidectomy of a benign thyroid lesion. No abnormal nodules were found in the bilateral thyroid glands. However, imaging studies were consistent with a spinal tumor, and the lesion was diagnosed as a metastatic follicular carcinoma of thyroid origin. We adopted a multidisciplinary collaboration and comprehensive treatment approach. The patient underwent lumbar spine surgery, total resection of the thyroid, postoperative TSH suppression therapy, and RAIT. There were no complications associated with the operation, and the patient had good posto
Follicular thyroid carcinoma is associated with early hematogenous metastasis, and the bone is a typical site of metastasis. Single bone metastasis is not a contraindication to medical procedures, and providing the appropriate therapy can result in better outcomes and quality of life for these patients.
Core Tip: Although bone metastasis from differentiated thyroid cancer is common, it is very rare for bone metastasis to be the solitary presentation of thyroid cancer. Here, we present a case of bone metastasis of follicular thyroid carcinoma with no indication of primary cancer. The patient had undergone partial thyroidectomy 20 years previously for thyroid nodules, but the pathological diagnosis at that time was a benign thyroid lesion. We employed a multi-institution, multidisciplinary team to diagnose and treat this patient, and she has had a good outcome thus far. This case highlights several important issues, such as the importance of follow-up for patients with seemingly indolent lesions and the utility of a comprehensive treatment approach.
- Citation: Chen YK, Chen YC, Lin WX, Zheng JH, Liu YY, Zou J, Cai JH, Ji ZQ, Chen LZ, Li ZY, Chen YX. Follicular carcinoma of the thyroid with a single metastatic lesion in the lumbar spine: A case report. World J Clin Cases 2022; 10(26): 9493-9501
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9493.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9493
The global incidence of thyroid cancer has risen over the past 30 years. In particular, the incidence of thyroid cancer in Chinese women has increased significantly[1]. Although thyroid cancer is known as an indolent carcinoma, because of its insidious and anatomic characteristics, many people die of thyroid cancer every year, or have their quality of life affected. The majority of thyroid cancers (86%) are differentiated thyroid cancers (DTCs), and these can be divided into papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC). PTC accounts for 75% of all thyroid cancers and FTC for 15%-20%[2]. Bone metastasis occurs in various cancers, including lung, breast, and prostate cancer. The bone is the second most common site of thyroid cancer metastasis after the lung[3], with a poorer prognosis. The spine is the most common site of bone metastasis for differentiated thyroid cancer, accounting for approximately 50% of cases[4,5]. Spinal metastasis of differentiated thyroid cancer is most often characterized by local intractable pain and neurological symptoms[6]. FTC is more likely to develop spinal metastasis than PTC and is the most common histological type of bone metastasis[7]. This may be because FTC tends to spread hematogenously, whereas PTC tends to spread through the lymphatic system. A previous study found that, of 26 cases of malignant transformation of struma ovarii related to FTC, seven were related to bone metastasis. Identifying the optimal treatment plan for such patients has become a perplexing problem for clinicians. Here, we describe our encounter with a case of FTC in which we provided a more reasonable treatment plan for the patient through preoperative comprehensive evaluation and multidisciplinary and multi-institution consultation, which significantly improved the patient's quality of life and prognosis.
The patient was a 67-year-old woman who was admitted to the clinic in September 2019.
The patient was suffering lumbar pain for 7 years and aggravation with interspace claudication since the preceding February.
Twenty years previously, the patient had undergone partial thyroidectomy for thyroid nodules at a primary hospital. Post-operative examination of the resected specimen suggested a benign thyroid lesion.
The patient had no history of food or drug allergies, and genetic diseases.
Physical examination revealed mild limitation of lumbar flexion and extension activity, L4 paravertebral tenderness, no obvious abnormalities in sensation/movement, and normal blood circulation in both lower limbs. The results of the straight leg raising test, strength test, and bilateral Patrick's sign were negative. No abnormal nodules were touch in the bilateral thyroid glands, and cervical lymph nodes showed no enlargement bilaterally.
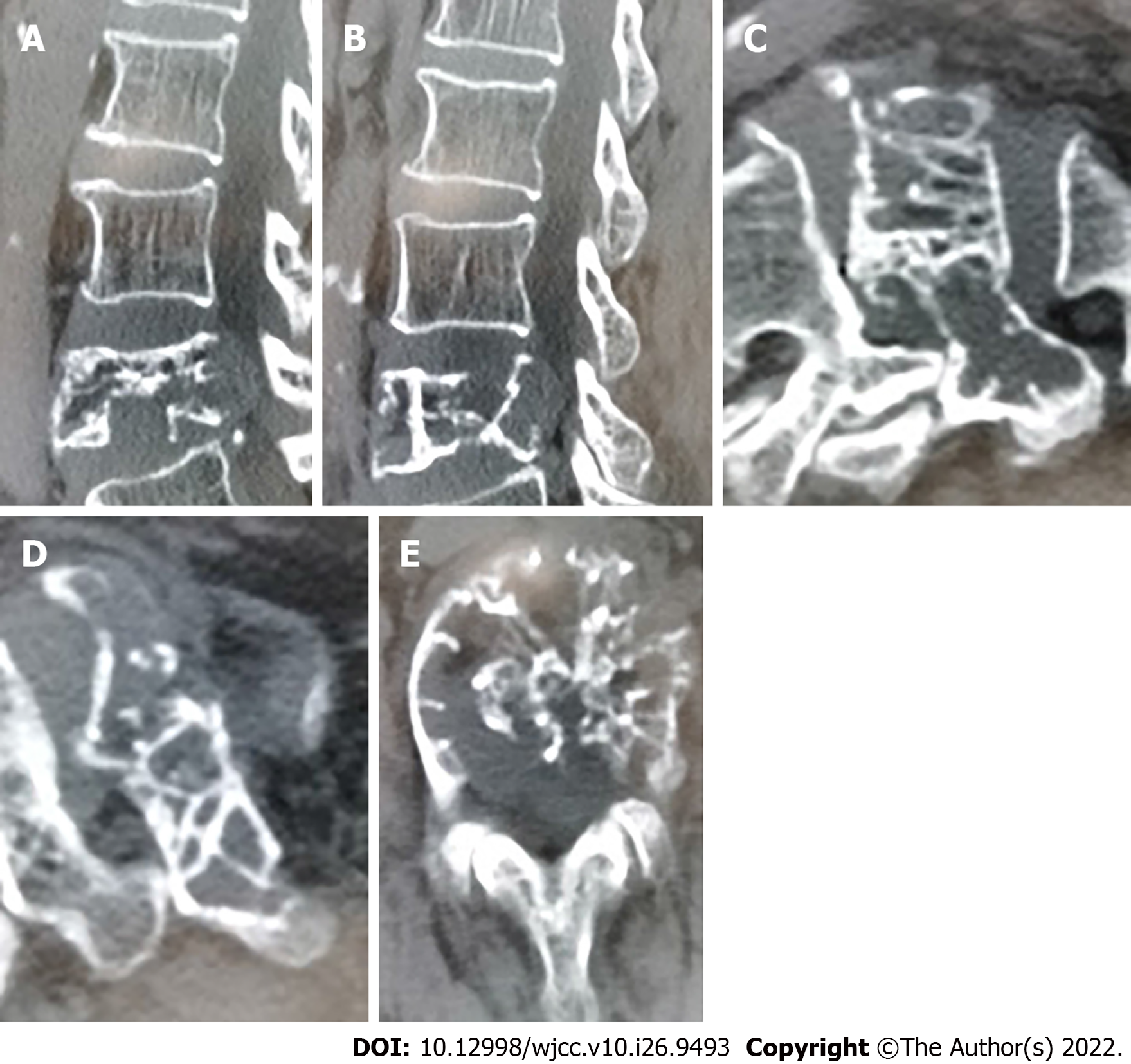
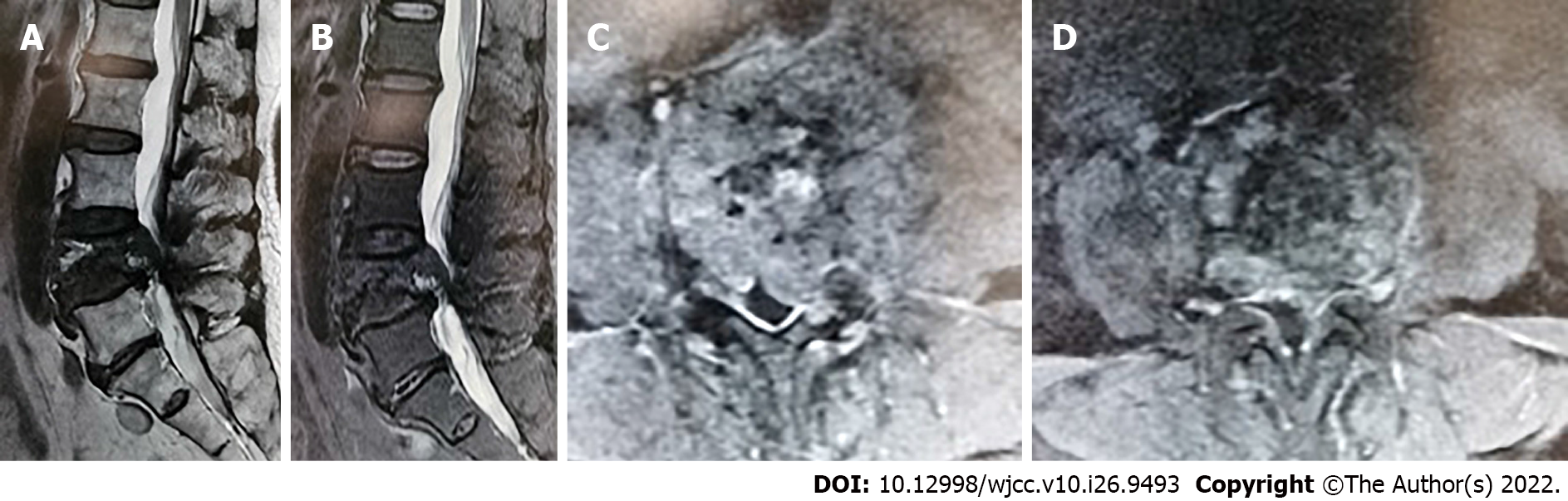
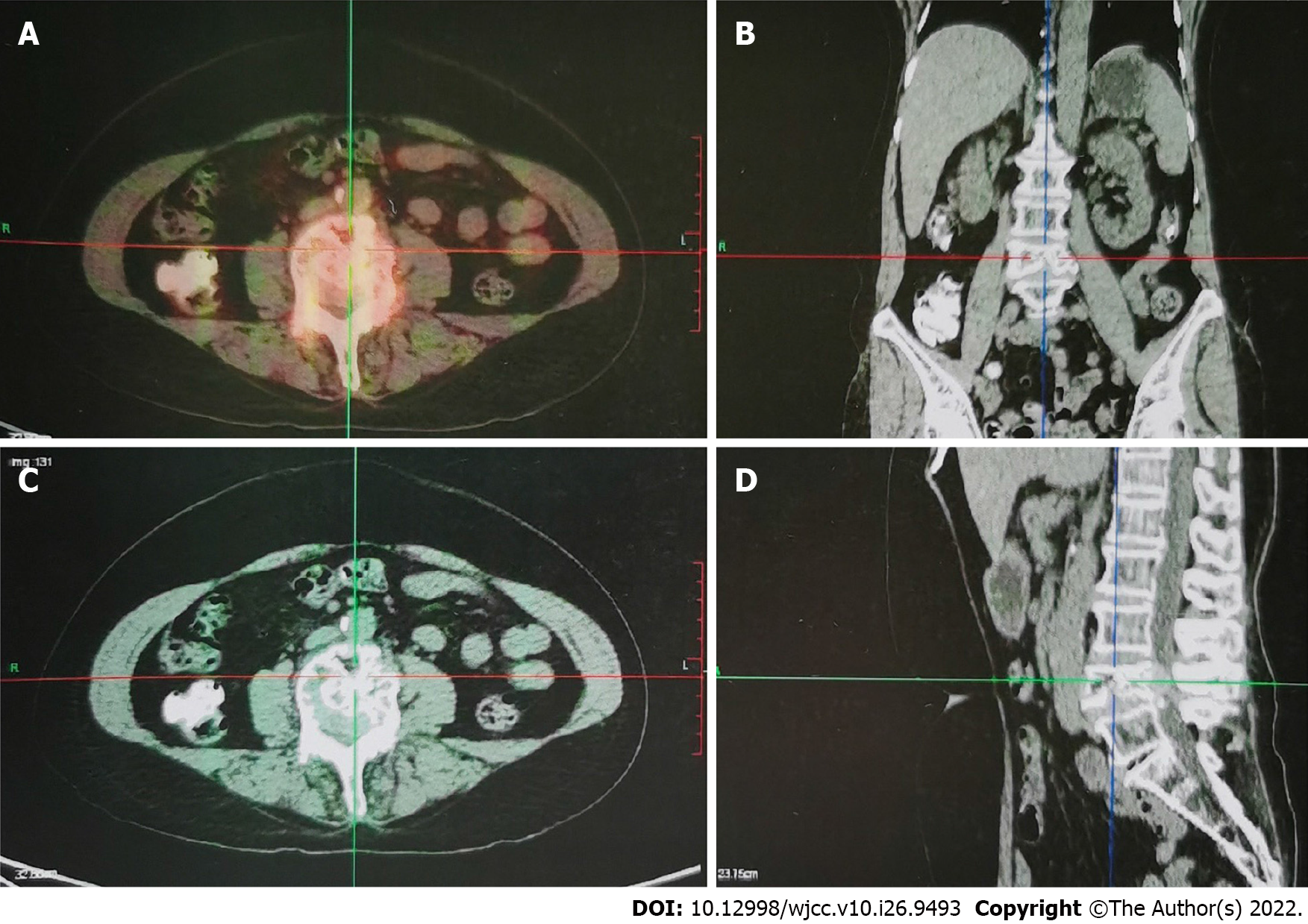
Color ultrasonography indicated solid nodules in the middle of the right lobe of the thyroid, thyroid imaging reporting and data system (TI-RADS) category 4A, and hypoechoic nodules in the bilateral lobes of the thyroid gland, TI-RADS category 3. Therefore, a nodular goiter was considered. No abnormal enlargement of the bilateral cervical lymph nodes was observed. Lumbar computed tomography (CT) and magnetic resonance imaging (MRI) results suggested metastatic or malignant tumors (Figures 1 and 2). Thyroid fine-needle aspiration demonstrated a large number of red blood cells (RBCs) with benign proliferative follicular epithelial cells. Thyroid needle biopsy results indicated a small number of thyroid follicles with nodular hyperplasia. Pathological examination of the L4 vertebral puncture biopsy indicated fibrinoid necrosis, broken bone, and thyroid tissue, which suggested metastatic thyroid cancer. Therefore, we sent the sample for pathological consultation at the Shantou University Medical College. Their examination indicated that bone marrow tissue, bone trabecula, and thyroid follicular tissue were observed in the puncture tissue of the L4 vertebral body. Considering the potential for ectopic thyroid tissue, we wanted to exclude FTC. Thyroid function was normal. However, MRI of the lumbar spine indicated malignancy, and whole-body positron emission tomography (PET)-CT was consistent with spinal tumors. No other metastases were found (Figure 3).
The patient was considered to have follicular carcinoma of the thyroid with metastasis of the lumbar spine.
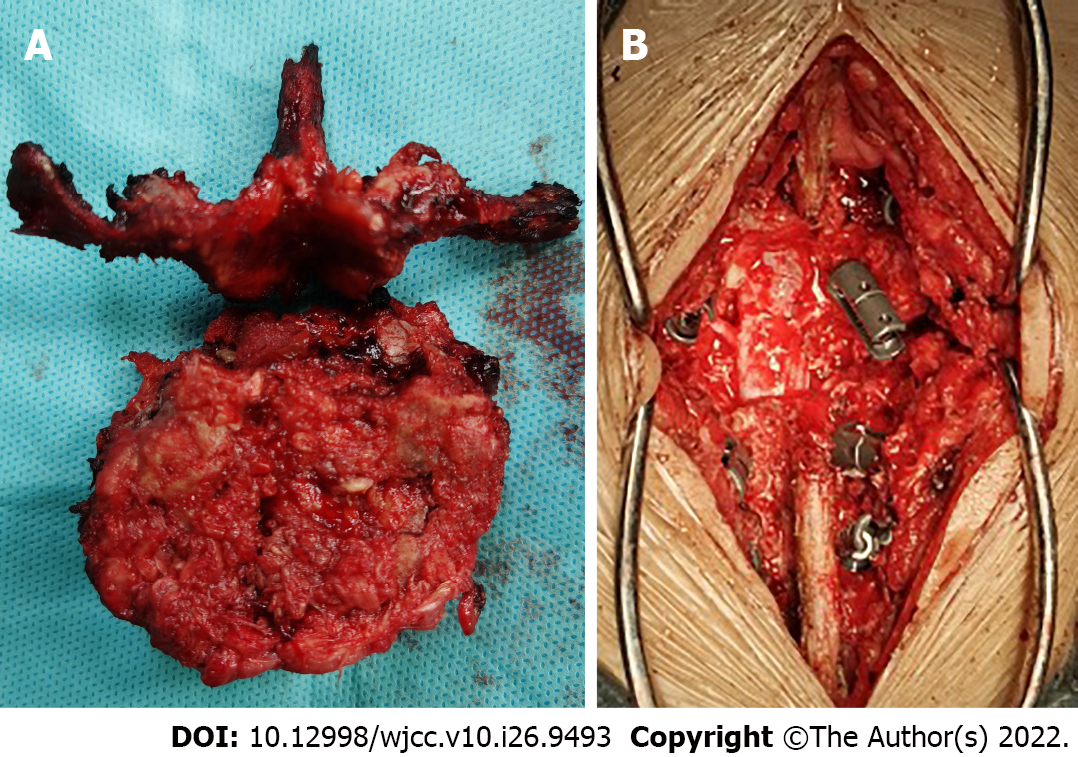
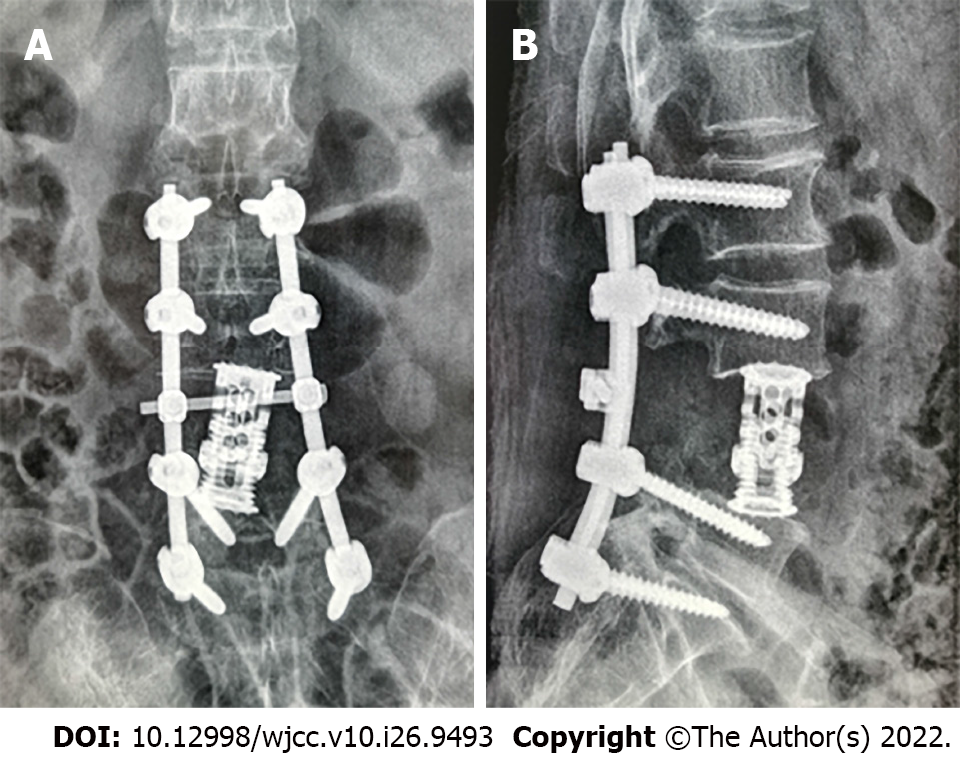
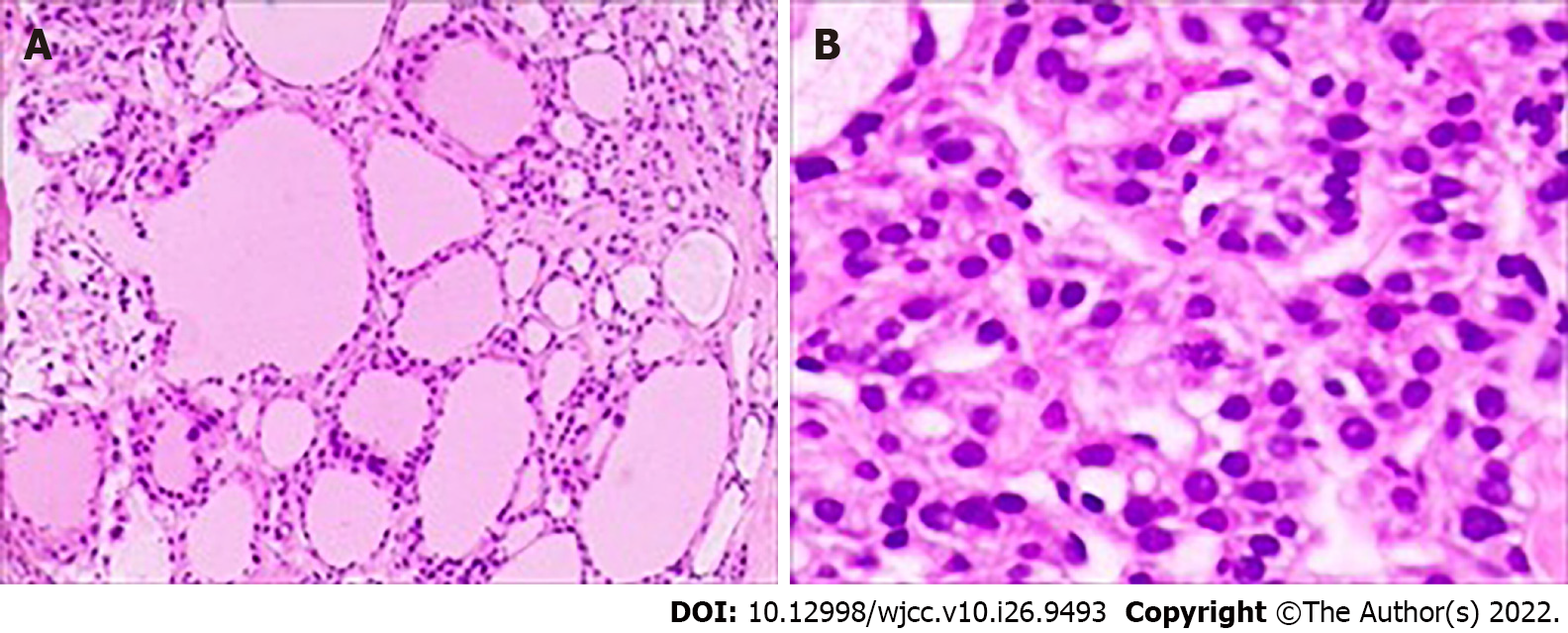
After diagnosis, the patient was treated using anterior and posterior combined lumbar spine surgery with artificial vertebral body replacement to L4, and internal fixation with a pedicle screw and rod system (Figures 4 and 5). The post-operative recovery was smooth. Post-operative pathological analysis was conducted in consultation with the Affiliated Cancer Hospital of Sun Yat-sen University. The tumor was positive for galectin-3 and negative for TTF-1, partially positive for CD19 and HBME-1, mostly positive for CD56 and TPO, and positive for thyroglobulin (Tg). The Ki-67 index was < 5% (Figure 6).
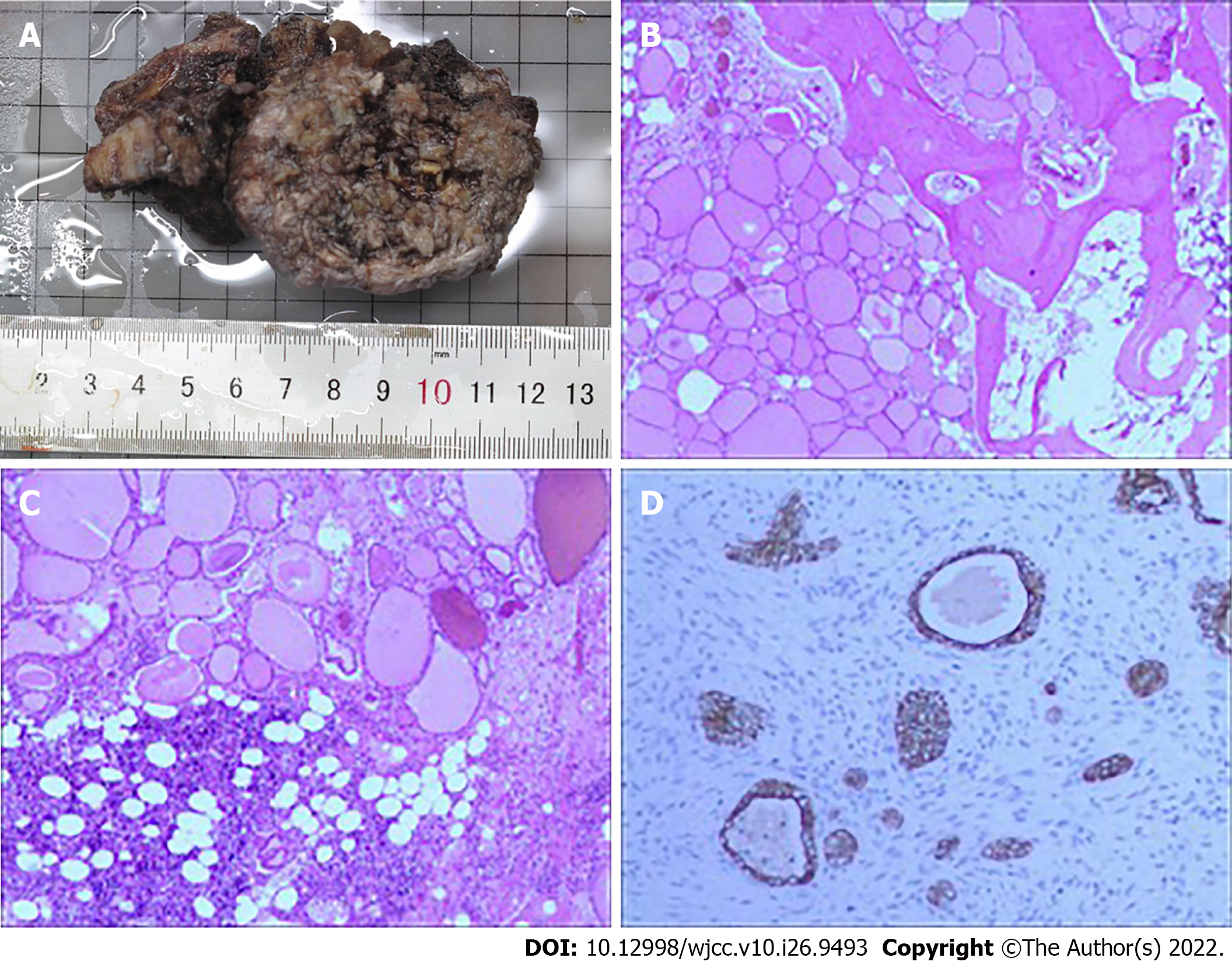
After the spinal surgery was stable, the patient was transferred to general surgery for total thyroidectomy. No primary lesions were found in intraoperative freezing pathology, nor using the thin section technique after surgery (Figure 7). Due to the difficult diagnosis of the patient, the bone specimen was sent to the Dean-Hopkins International Remote Pathological Consultation on January 3, 2020. They found that the L4 vertebra had highly differentiated thyroid carcinoma metastases involving the vertebra, cartilage, and bone marrow space. This lesion was a metastatic follicular carcinoma of thyroid origin. RAIT and TSH inhibition therapy were subsequently performed, and 50 μg of levothyroxine was administered orally every morning. Owing to the lack of radioactive iodine, 200 mCi of I-131 was administered orally on December 22, 2020.
Follow-up lumbar CT and I-131 body imaging on October 14, 2021, showed no recurrence. Currently, the patient’s daily movement capacity is comparable to that of healthy individuals.
Distal bone metastasis is a common complication of FTC, with most cases occurring in patients with advanced disease[8]. Spinal metastases usually affect the thoracic (60%-80%), lumbar (15%-30%), and cervical vertebrae (< 10%). The main symptoms of spinal metastases are pain (83%-95%), spinal cord compression (28%), and pathological fracture (13%). Patients may have incomplete paralysis or paraplegia[9-11]. Among the cases of FTC bone metastasis reported in the literature, the thyroid lesions were detected by preoperative imaging or rough examination of surgical specimens. However, there is a case of lumbar spinal metastasis in which lumbar metastatic lesions was the only symptom, and the pathologist was unable to identify primary FTC in the surgical thyroid section.
Regarding the survival of bone metastasis of DTC, a total of 444 patients diagnosed with DTC between 1953 and 1994 were followed up and analyzed in previous studies. Among them, 115 had bone metastases. The 20-year survival rate of these 115 patients was 8%, and all patients received timely treatment with I-131[12].
The differential diagnosis of FTC from follicular thyroid adenoma depends mainly on the capsule and vascular evaluation of thyroidectomy specimens[13]. In this case, considering the patient’s previous medical history, it cannot be completely ruled out that FTC with slow progression and malignant tendency, low malignant degree, undetermined malignant potential, or early atypical follicular carcinoma may be misdiagnosed or undiagnosed because of the lack of pathological diagnostic ability and effective immunohistochemical means at the initial presentation.
Malignant tumors are characterized by recurrence and distant metastasis. This patient had undergone partial thyroidectomy > 20 years previously and had not received any postoperative treatment. Although no primary thyroid cancer was found, long-term distant metastasis of FTC was considered because of her previous medical history and the current diagnosis and treatment level.
PET-CT is the most common examination method to identify distant metastases, and 124I-PET-CT is targeted for bone metastasis[14] due to its sensitivity to hypermetabolic lesions. CT is used to assess the stability of the spine, and MRI is used to assess the relationship between the tumor and the spinal cord to guide the choice between radiotherapy and surgery[10]. Cervical lymph node dissection should be considered based on the preoperative evaluation.
Treatment of patients with FTC spinal metastases involves a multidisciplinary approach, including surgery (total thyroidectomy, excision of metastatic lesions, palliative resection, and total vertebrotomy), RAIT, external radiation therapy (EBRT), TSH suppressive therapy, and systemic therapy (bisphosphonates and small-molecule targeted therapy for tumors)[6,8,10]. For our patient, total thyroidectomy provided the following benefits: (1) One-time treatment of any potential cancer focus; (2) postoperative monitoring of Tg for tumor recurrence and metastasis; (3) postoperative treatment with I-131; (4) reduction in the probability of tumor recurrence and reoperation to avoid an increase in the incidence of serious complications caused by reoperation; and (5) accurate evaluation of postoperative staging and risk stratification. Resection of metastatic foci is helpful in improving survival and quality of life[11]. Postoperative imaging is also helpful for monitoring the emergence of new hypermetabolic lesions.
I-131 is the basic therapy for bone metastases in DTC and is suitable for the vast majority of patients who are sensitive to radiation iodine therapy. Response to therapy is also an independent prognostic factor[9,11]. EBRT is a palliative treatment for patients with insensitivity to I-131, those with incomplete surgery or distant metastasis, and the elderly. TSH suppressive therapy aims to reduce the potential for further tumor growth[11,15]. Bisphosphonates (such as zoledronic acid) effectively reduce the occurrence of pain, spinal cord compression, pathological bone fractures, hypercalcemia, and other skeletal iliac-related events by inhibiting the activity of DTC-induced osteoclasts. Denosumab, a human monoclonal antibody newly approved by the Food and Drug Administration, prevents osteoclasts from resorbing the bone by inhibiting the NF-κB receptor activator ligand[16]. Vascular endothelial cell growth factor receptor therapy effectively controls angiogenesis and tumor growth in patients who are not sensitive to I-131. Gene therapy includes oncogene silencing, anti-transcriptional ribozyme therapy, tumor suppressors, and lentiviral vectors[9,17].
Hematogenous metastasis is the most common metastatic route for FTC. Tg synthesis and secretion is commonly monitored as an indicator of thyroid cancer recurrence in patients after total thyroid resection and resection of metastatic lesions[18,19]. Therefore, if Tg is detected, tumor metastasis and recurrence should be considered. Here, the metastatic lesion needed to be differentiated from ectopic thyroid tissue. Ectopic thyroid tissue is a rare developmental abnormality involving abnormal embryonic development of the thyroid gland from the endoderm to the base of the primordial foregut or the final anterior trachea. It is the result of failed thyroid migration not only along the nail tongue canal but also in the subphrenic organ. The ectopic thyroid distribution includes the tongue, jaw, endotracheal tube, lateral neck, axilla, neck artery, iris, pituitary, esophagus, intrathoracic region, mediastinum, lung, heart, gallbladder, adrenal glands, ovaries, pancreas, stomach, duodenum, small intestine, and mesentery. Double ectopic distribution has also been described (usually nearly tubular and involving the tongue). The most common site is the base of the tongue, especially the blind foramen, which accounts for approximately 90% of reported cases[20]. Histopathologically, the bone is derived from the mesoderm; therefore, thyroid tissue from the endoderm cannot be ectopic to the lumbar spine of the mesoderm. Cases such as this one should also be distinguished from lumbar teratomas. The most common site of lumbar teratoma is the sacrococcygeal region (57%), followed by the gonadal region (29%), mediastinal region (7%), retroperitoneal region (3%), cervical region, and skull; spinal teratomas are mostly found in fetuses or infants[21]. Based on the pathological results, the spine lesion was considered to be a metastatic thyroid lesion.
For patients with differentiated thyroid cancer with bone metastases, the main symptoms are bone related events[6]; timely treatment can provide better outcomes for these patients. Timely treatment before serious bone-related events occur, the doctor patient and meticulous actively cooperate with the diagnosis and treatment and patients, complete lumbar surgery and thyroid surgery, postoperative treatment with I-131 reduced the risk of recurrence. We believe that this is the reason for the better prognosis of this patient and the advantage of this case in diagnosis and treatment. This case report has a few limitations, such as difficulties in diagnosis and a long diagnosis time. There was no Tg monitoring before the operation and postoperative loss of follow-up, which were shortcomings of this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharaf MM, Syria; Sugiyama Y, Japan; Tangsuwanaruk T, Thailand S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Chen F, Yin S, Zhu J, Jia L, Zhang H, Yang C, Liu C, Deng Z. Effects of nuclear factorκB on the uptake of 131iodine and apoptosis of thyroid carcinoma cells. Mol Med Rep. 2018;17:4959-4964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Zhu X, Bai Q, Lu Y, Zhu L, Zhou X, Wu L. Expression and function of CXCL12/CXCR4/CXCR7 in thyroid cancer. Int J Oncol. 2016;48:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Nervo A, Ragni A, Retta F, Gallo M, Piovesan A, Liberini V, Gatti M, Ricardi U, Deandreis D, Arvat E. Bone metastases from differentiated thyroid carcinoma: current knowledge and open issues. J Endocrinol Invest. 2021;44:403-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Kushchayeva YS, Kushchayev SV, Carroll NM, Felger EA, Links TP, Teytelboym OM, Bonichon F, Preul MC, Sonntag VK, Van Nostrand D, Burman KD, Boyle LM. Spinal metastases due to thyroid carcinoma: an analysis of 202 patients. Thyroid. 2014;24:1488-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol. 2013;20:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Farooki A, Leung V, Tala H, Tuttle RM. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2012;97:2433-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Schlumberger MJ, Torlantano M. Papillary and follicular thyroid carcinoma. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Dong P, Chen N, Li L, Huang R. An upper cervical cord compression secondary to occult follicular thyroid carcinoma metastases successfully treated with multiple radioiodine therapies: A clinical case report. Medicine (Baltimore). 2017;96:e8215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Ramadan S, Ugas MA, Berwick RJ, Notay M, Cho H, Jerjes W, Giannoudis PV. Spinal metastasis in thyroid cancer. Head Neck Oncol. 2012;4:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006;15:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Kushchayeva YS, Kushchayev SV, Wexler JA, Carroll NM, Preul MC, Teytelboym OM, Sonntag VK, Van Nostrand D, Burman KD, Boyle LM. Current treatment modalities for spinal metastases secondary to thyroid carcinoma. Thyroid. 2014;24:1443-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1113] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 13. | Li Y, Huo Z, Chen J. [Problems in pathologic diagnosis of thyroid follicular cell carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2014;43:348-352. [PubMed] |
| 14. | Weber M, Binse I, Nagarajah J, Bockisch A, Herrmann K, Jentzen W. The role of 124I PET/CT lesion dosimetry in differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2019;63:235-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9688] [Article Influence: 1076.4] [Reference Citation Analysis (1)] |
| 16. | Koizumi M, Gokita T, Toda K. Impending Atypical Femoral Fracture in Patients With Medullary Thyroid Cancer With Skeletal Metastasis Treated With Long-term Bisphosphonate and Denosumab. Clin Nucl Med. 2017;42:463-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Wexler JA. Approach to the thyroid cancer patient with bone metastases. J Clin Endocrinol Metab. 2011;96:2296-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Yang SP, Koh LCW, Kong KW, Parameswaran R, Loke KSH, Ngiam KY, Tan WB, Loh T, Ng DCE, Goh BC, Ngeow J, Tai ES. A Multiplex Thyroid-Specific Assay for Quantification of Circulating Thyroid Cell-Free RNA in Plasma of Thyroid Cancer Patients. Front Genet. 2021;12:721832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Chung SR, Baek JH, Choi YJ, Sung TY, Song DE, Kim TY, Lee JH. Diagnostic Algorithm for Metastatic Lymph Nodes of Differentiated Thyroid Carcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Guerra G, Cinelli M, Mesolella M, Tafuri D, Rocca A, Amato B, Rengo S, Testa D. Morphological, diagnostic and surgical features of ectopic thyroid gland: a review of literature. Int J Surg. 2014;12 Suppl 1:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Faheem M, Syed HH, Kardam D, Maheshwari V, Khan R, Sharma A. Teratoma of the lumbosacral region: a case report. J Med Case Rep. 2011;5:370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |