Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9484
Peer-review started: May 6, 2022
First decision: May 31, 2022
Revised: June 12, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: September 16, 2022
Processing time: 119 Days and 2.4 Hours
Salivary gland cancer is a rare disease in which cancer cells form in the tissues of the salivary glands. It mostly occurs in the glands that have secretion functions, such as the parotid gland, sublingual gland and submandibular gland. This is very rare when it occurs in other nonsecreting glands. Here, we report one case of salivary gland carcinoma occurring in the thymus and discuss related diagnoses and treatment progress.
One 33-year-old middle-aged man presented with a thymus mass without any clinical symptoms when he underwent regular physical examination. Later, the patient was admitted to the hospital for further examination. Computed tomography (CT) showed that there was a mass of 3 cm × 2.8 cm × 1.5 cm in the thymus area. The patient had no symptom of discomfort or tumor- related medical history before. After completing the preoperative examinations, it was confirmed that the patient had indications for surgery. The surgeon performed a transthoracoscope "thymectomy + pleural mucostomy" for him. During the operation, the tumor tissue was quickly frozen, and the symptomatic section showed a malignant tumor. The final pathological result suggested thymus salivary gland carcinoma- mucoepidermoid carcinoma (MEC). In the second month after surgery, we performed local area radiotherapy for the patient, with a total radiation dose of 50.4 Gy/28Fx. After 12 mo of surgery, the patient underwent positron emission tomography-CT examination, which indicated that there was no sign of tumor recurrence or metastasis. After 16 mo of operation, CT scan re-examination showed that there was no sign of tumor recurrence or metastasis. As of the time of publication, the patient was followed up for one and a half years. He had no sign of tumor recurrence and continued to survive.
The incidence of MEC in the thymus is low, and its diagnosis needs to be combined with clinical features and imaging methods. Histopathological analysis plays a key role in the diagnosis of the disease. Patients with early-stage disease have a good prognosis and long survival period. In contrast, patients with advanced-stage disease have a poor prognosis and short survival period. Combining radiotherapy and chemotherapy in inoperable patients may prolong survival.
Core Tip: In this report, we showed one one 33-year-old middle-aged man presented with a thymus mass without any clinical symptoms when he underwent regular physical examination. After completing the pre-operative examinations, it was confirmed that the patient had indications for surgery. The surgeon performed a transthoracoscope "thymectomy + pleural mucostomy" for him. The final pathological result suggested: Thymus salivary gland carcinoma-mucoepidermoid carcinoma, which was rarely reported in literatures. The patient underwent a local radiotherapy for total dose of 50.4 Gy after the surgery. He had no sign of recurrence and continued to survive as of time of publication.
- Citation: Deng R, Li NJ, Bai LL, Nie SH, Sun XW, Wang YS. Postoperative radiotherapy for thymus salivary gland carcinoma: A case report. World J Clin Cases 2022; 10(26): 9484-9492
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9484.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9484
Similar to tumors of major salivary glands in other parts of the body, salivary gland tumors that originate in the thoracic cavity are also widely known. Tumors of the thoracic salivary glands most often occur in the lungs[1,2]. Salivary gland tumors that occur in the thymus are extremely rare, with few reported cases in the literature. As early as the 1980s, salivary carcinoma of the thymus was first reported in Japan. This patient was a 59-year-old female patient. Due to the limited diagnosis and treatment technology at that time, the patient eventually died of tumor compressing on the heart[3]. Among the various pathological types of thymic salivary gland tumors, mucoepidermoid carcinoma is the most common, followed by adenoid cystic carcinoma. Because of the rarity of cases, it is more difficult to diagnose. Both thoracoscopic biopsy and image-guided percutaneous needle biopsy have limited diagnostic value for the disease[4]. Therefore, the accurate identification of salivary gland-type tumors of the thymus, even if rare, is critical for proper treatment and prognosis. Here, we report a case of thymus primary salivary gland carcinoma and discuss the diagnosis and treatment strategies.
Physical examination found a thymus mass for half a year.
The patient’s chest computed tomography (CT) revealed a thymic mass before 11 mo. The patient had no chest pain, chest tightness, cough, expectoration, exertion, hot flashes or night sweats, and he was observed and followed up. Half a month ago, the patient re-examined the chest CT. The CT showed that the enhanced scan of the anterior mediastinal nodule showed mild enhancement, and the size was not significantly changed. Thymoma? The patient went to our clinic and was admitted to our department with a "thymus mass".
The patient had no history of chronic diseases, such as hypertension, cancers, diabetes, heart diseases, chronic bronchitis, or emphysema. He also had no history of infectious diseases, such as hepatitis, tuberculosis, typhoid fever, and malaria.
The patient had no history of chronic diseases, such as hypertension, cancers, diabetes, heart diseases, chronic bronchitis, or emphysema. He also had no history of infectious diseases, such as hepatitis, tuberculosis, typhoid fever, and malaria.
Body temperature: 36.3°; Respiratory rate: 18/min; Heart rate: 69/min; Blood pressure: 125/69 mmHg. The superficial lymph nodes of the whole body were not palpated. The neck was soft and without resistance, the trachea was in the middle, the thorax was not deformed, and the breathing was uniform.
The laboratory tests were as follows: blood routine test: RBC: 7.37 × 10 12/L, HCT: 51.3%, PCT: 0.29%, MCV: 69.6 fL, MCH: 21.0 pg, MCHC: 302 g/L. Biochemical tests: Uric acid: 583 umoL. Thyroid function: FT3: 4.52 pmo1/L, TsH: 1.908 uIU/mL, FT4: 1 L. 90 pmol/L, TPOAb: 0.47 IU/mL.
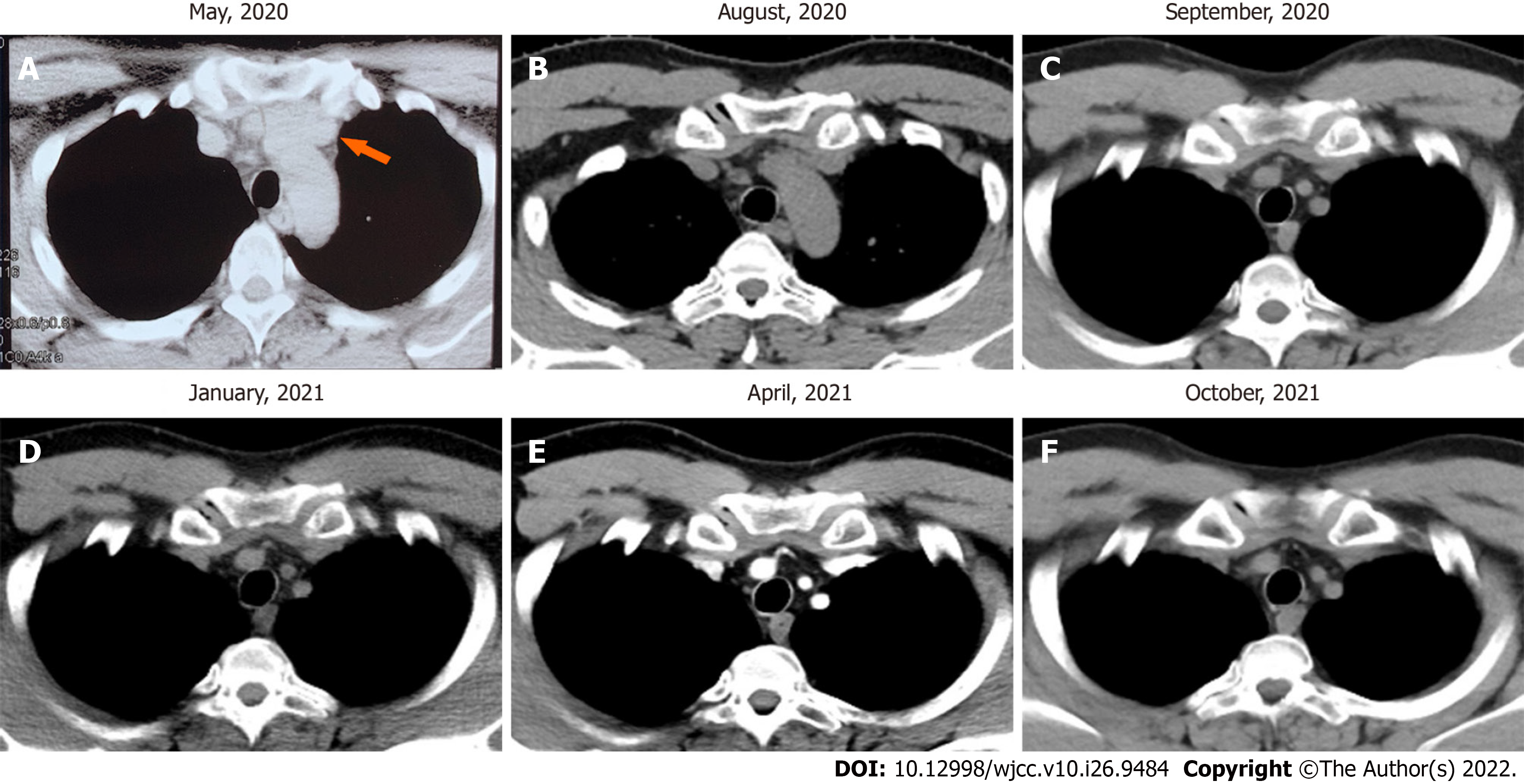
Chest CT revealed "anterior mediastinal nodules, enhanced scan showed mild enhancement, thymoma? small nodules in the upper lobes of the lungs, a few chronic inflammatory foci in the dorsal segment of the lower lobe of the left lung (Figure 1)".
The laboratory tests were as follows: Blood routine test: RBC: 7.37 × 10 12/L, HCT: 51.3%, PCT: 0.29%, MCV: 69.6 fL, MCH: 21.0 pg, MCHC: 302 g/L. Biochemical tests: Uric acid: 583 umoL. Thyroid function: FT3: 4.52 pmo1/L, TsH: 1.908 uIU/mL, FT4: 1 L.90 pmol/L, TPOAb: 0.47 IU/mL.
Body temperature: 36.3°; Respiratory rate: 18/min; Heart rate: 69/min; Blood pressure: 125/69 mmHg. The superficial lymph nodes of the whole body were not palpated. The neck was soft and without resistance, the trachea was in the middle, the thorax was not deformed, and the breathing was uniform.
Thymus salivary gland cancer-mucoepidermoid carcinoma.
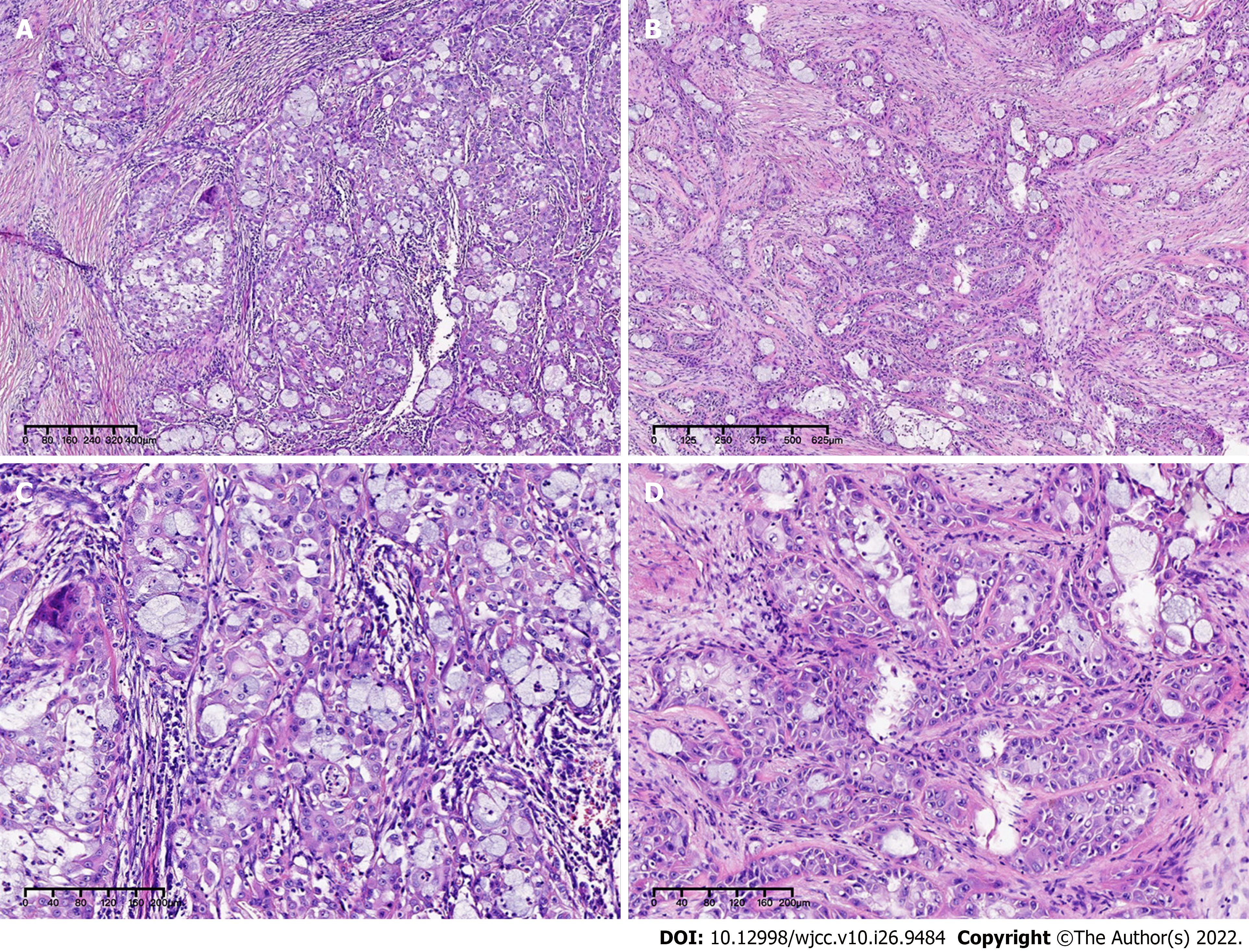
After evaluation, there were indications for surgery for this patient, and no surgical contraindications were observed. Then, this patient underwent thymectomy + pleural adhesion cauterization surgery. The intraoperative freezing mass tissue pathology revealed “malignant tumors, prone to salivary carcinoma. The final diagnosis depends on paraffin section analysis and immunohistochemical tests”. After the operation, the patient had a small amount of pleural effusion symptoms and recovered after thoracentesis and drainage operation.
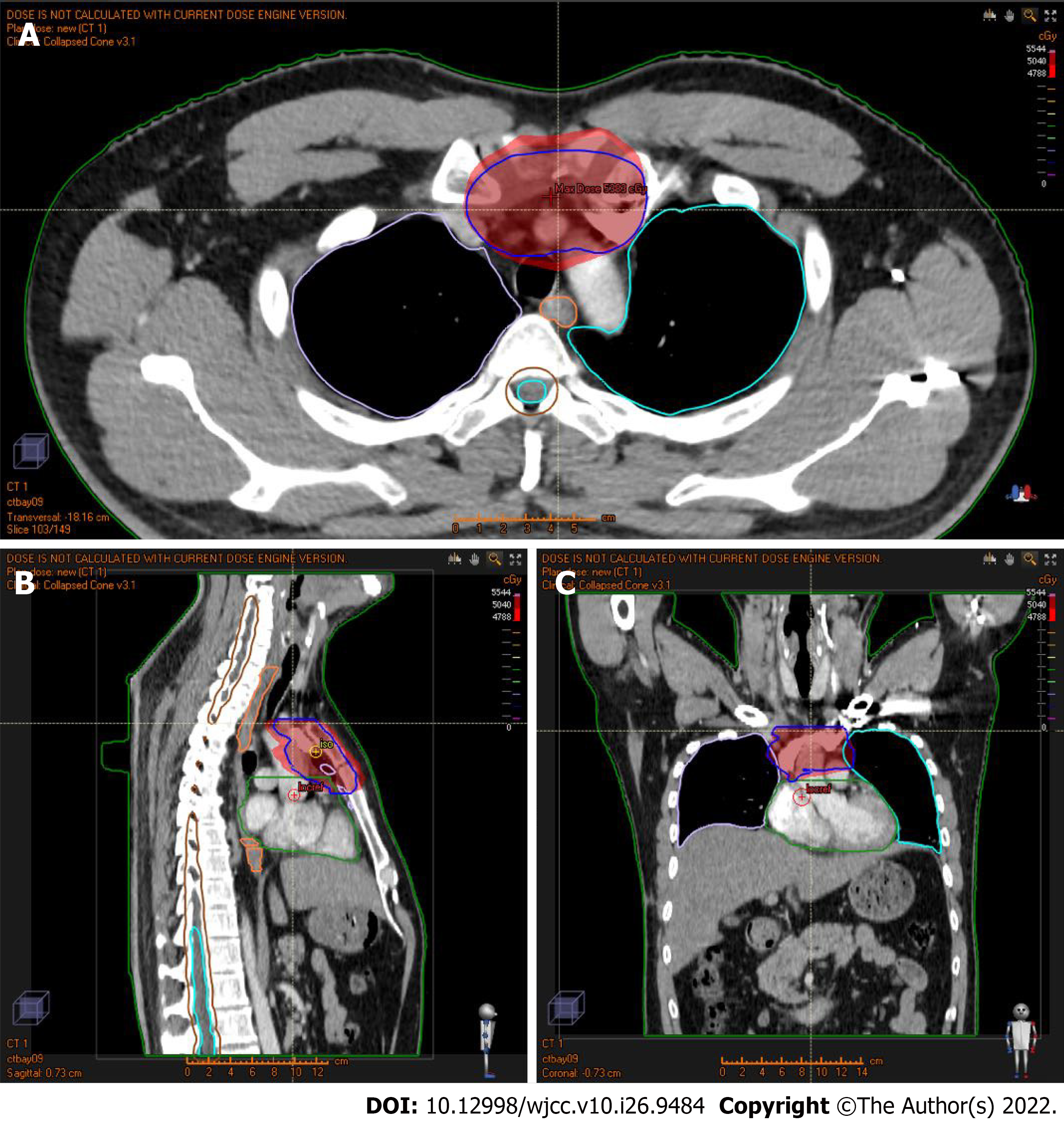
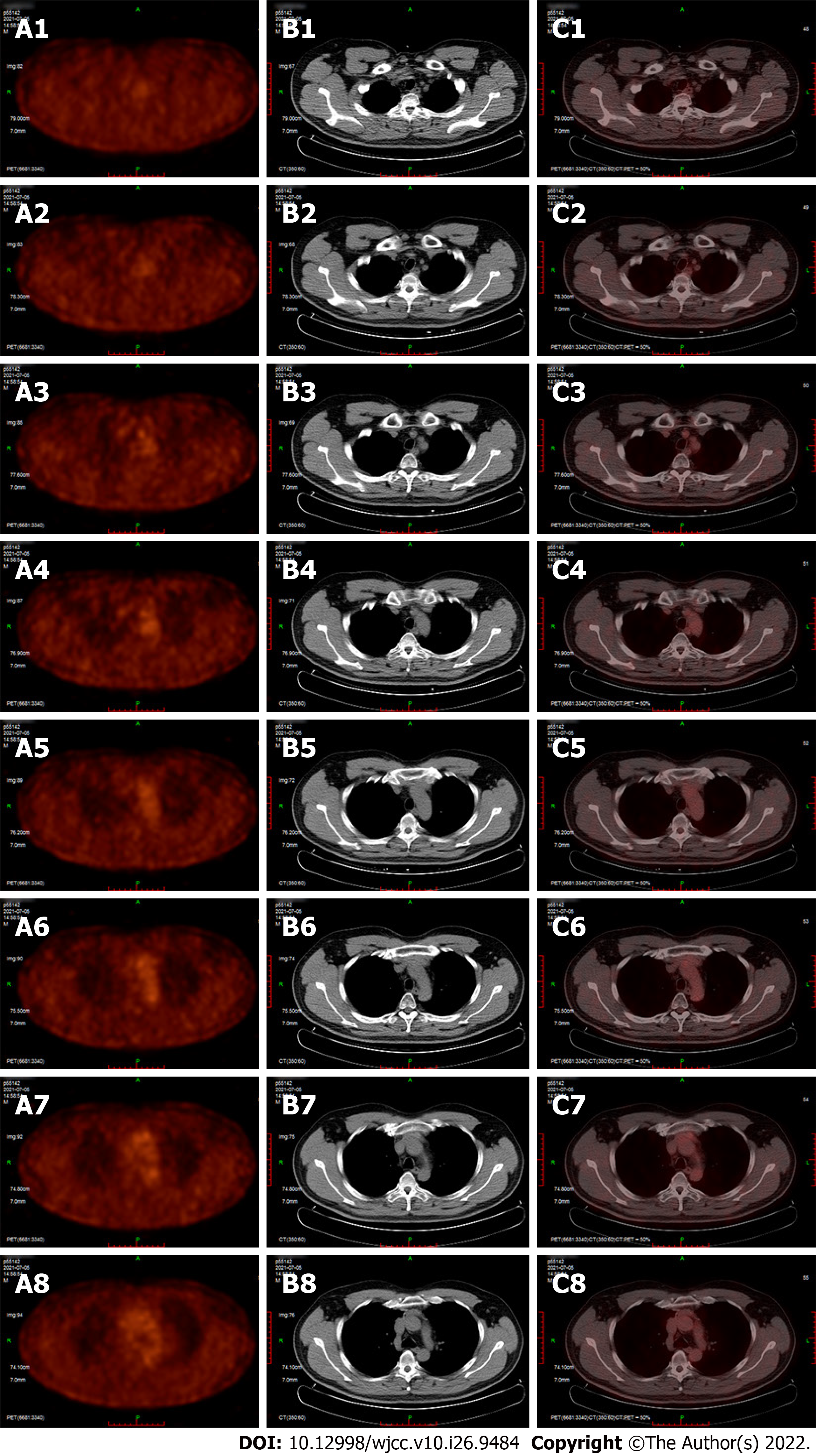
The patient received a CT scan (Figure 2) 2 mo after the surgery. After 2 mo, the patient underwent radiotherapy with an intensity-modulated radiotherapy technique. The total dose for radiotherapy was 50.4 Gy/28 Fx. The dose distribution chart is shown in Figure 3. The patient received a positron emission tomography-CT scan at the first year after surgery, and the result showed no increased fluorodeoxyglucose metabolism area (Figure 4). In the next year, the patient regularly returned to the hospital to receive a physical follow-up examination. The repeated examination presented no recurrence or metastasis signs (Figure 1).
Mucoepidermoid carcinoma (MEC) is the most common malignant tumor of the salivary glands and usually occurs in the parotid gland. MEC accounts for approximately 12% of salivary gland epithelial tumors and approximately 30% of their malignant tumors[5]. MEC of the thymus is currently mostly reported in individual cases universally. According to the clinical symptoms of MEC of the thymus, the imaging findings have no obvious specificity. Its clinical behavior is highly variable and can be divided into two types, highly differentiated and poorly differentiated, according to its morphological and cytological characteristics. Poorly differentiated MEC has stronger invasion and metastasis ability than well-differentiated MEC, recurrence and distant metastasis often occur, and the prognosis is poor[6,7]. The histological diagnosis of mucoepidermoid carcinoma of the salivary gland mainly relies on HE-stained sections. There is no specific marker for the diagnosis or estimation of prognosis. Therefore, research on the diagnosis and prognosis specificity of mucoepidermoid carcinoma of the salivary gland is very important. With the advancement of research in the field of tumor molecular biology, some new biomarkers continue to appear, which not only provide help for the pathological diagnosis of mucoepidermoid carcinoma of the salivary glands but also improve its prognosis and treatment options[8]. MECs have a unique structure and cell heterogeneity, with a characteristic t(11;19)(q21;p13) chromosomal translocation, resulting in the MECT1-MAML2 fusion gene. MECT1 protein can activate cyclic adenosine monophosphate (cAMP) response element binding to mediate transcription[9]. The MECT1-MAML2 fusion protein can upregulate the expression of vascular endothelial cell growth factor receptor 1 downstream of the cAMP/CREB pathway and the expression of hair split-related enhancer 1 and hair split-related enhancer 5 downstream of the Notch pathway and promote tumorigenesis[10]. The positive detection rate of MECT1 was as high as 88%, and it was translocation positive in all low- or intermediate-level MECs. The positive detection rate of MECT1-MAML2 fusion transcripts in high-level salivary gland MECs is 60%, and the MECT1/MAML2 translocation is likely the main oncogenic driver in these tumors[11]. Otherwise, the most recent research indicated that CRTC1-MAML2 was the major oncogenic driver in MEC[12]. The positive expression rate of NP63 in salivary epithelial malignant tumors is higher than that in benign tumors, and the positive expression rate is higher in poorly differentiated and highly malignant salivary epithelial tumors, suggesting that P63 can promote the proliferation and dedifferentiation of salivary gland cancer cells[13,14]. Research has shown that the positive expression of P63 increases with decreasing MEC differentiation, and P63 helps the differential diagnosis of salivary MEC and acinar cell carcinoma and pathological typing[15]. Additionally, Bcl-2 may participate in the process angiogenesis and angiogenic mimicry (VM) formation[16,17]. The detection results of VE-cadherin are helpful to assess the prognosis of MEC to a certain extent. VE- cadherin may be an ideal target for VM targeted therapy of MEC[18]. Although the relevant mechanism of VE-cadherin in MECs is still unclear, as one of the regulatory factors involved in the control of angiogenesis, it may be used as a molecular marker for evaluating the prognosis or therapeutic effect of MECs.
In the lungs, analyzing the immunohistochemical results of 26 cases of lung MEC, it was found that 26 patients had positive expression of CK7, Muc5AC, P63 and P40 and negative expression of TTF-1, and the variation in Ki-67 ranged from 2%-80%, with an average of 9.7%; the average high-grade lung MEC Ki-67 value was 22.4%, and the average low-grade lung MEC Ki-67 value was 4.1%[19]. A report indicated that 25 patients had P63 expression without TTF-1 and Napsin A expression, and 23 patients had P40 expression[20]. Researchers conducted a comprehensive genome analysis of 48 MECs and found a total of 183 genomic abnormalities. Compared with low-grade tumors, gene mutations occur more frequently in high-grade tumors. In high- level MEC, the most frequently observed mutations occur in TP53, the PI3K/mTOR pathway, and CDKN2A. In addition, ERBB2, BRCA, and FGFR were also amplified in some cases[21]. However, their variation has nothing to do with poor prognosis. However, these markers cannot be used as independent diagnostic criteria for MEC and are not specific. More specific and diagnostic markers require more in-depth exploration by researchers. Considering the situation of our patient, this patient had no specific clinical symptoms or imaging characteristics. Enhanced CT also had no obvious enhancement characteristics. The diagnosis of this patient completely relied on his unique H-E path- morphological features, and his immunohistochemical molecular features only played a weak auxiliary role. After repeated comparison and verification by pathologists, the diagnosis of MEC was finally made. The first choice for the treatment of MEC of the thymus is complete resection of the lesion, local lymph node dissection, and the removal of the surrounding tissues and organs as much as possible. However, surgical resection is the first choice for the treatment of low-grade tumors. Considering high-grade (low-differentiated) local invasion and easy distant metastasis, for high-grade (low-differentiated) or recurring MEC of the thymus, surgical resection of the lesion is still active radiotherapy, and chemotherapy is needed to prolong the survival time of patients. Researchers have reported that thymic cancer is highly sensitive to radiotherapy, and postoperative radiotherapy can reduce the local recurrence rate to 17%[22]. Radiotherapy can be used for MEC of the thymus to prolong the survival of patients. A report stated that the combined chemotherapy regimen of irinotecan and cisplatin resulted in a 3-mo complete remission[23]. Therefore, for inoperable patients, a combination of radiotherapy and chemotherapy is used to prolong the survival of patients.
In summary, the incidence of mucoepidermoid carcinoma of the thymus is low, and the clinical symptoms, signs and imaging examinations have no obvious specificity. The diagnosis mainly depends on the results of postoperative pathology and immunohistochemistry. At present, the first choice for the treatment of thymic mucoepidermoid carcinoma is complete surgical resection. The prognosis of MEC of the thymus is generally poor, and the survival time of high- and middle-differentiated types is long. Therefore, surgical treatment, radiotherapy, and chemotherapy are used for MEC of the thymus to improve the quality of life of patients and prolong the survival time of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ni W, United States; Vahedi M, Iran S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Fechner RE, Bentinck BR. Ultrastructure of bronchial oncocytoma. Cancer. 1973;31:1451-1457. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Moran CA, Suster S, Koss MN. Acinic cell carcinoma of the lung ("Fechner tumor"). A clinicopathologic, immunohistochemical, and ultrastructural study of five cases. Am J Surg Pathol. 1992;16:1039-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Tanaka M, Shimokawa R, Matsubara O, Aoki N, Kamiyama R, Kasuga T, Hatakeyama S. Mucoepidermoid carcinoma of the thymic region. Acta Pathol Jpn. 1982;32:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Kalhor N, Weissferdt A, Moran CA. Primary Salivary Gland Type Tumors of the Thymus. Adv Anat Pathol. 2017;24:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Taghavi N, Yazdani F, Akbarzadeh Baghban A, Sargolzaei S, Kardouni Khoozestani P. Comparative Analysis of P63, Maspin and Matrix Metalloproteinase 2 Expression in Mucoepidermoid Carcinoma and Adenoid Cystic Carcinoma of Salivary Glands. J Dent (Shiraz). 2020;21:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Gill S, Mohan A, Aggarwal S, Varshney A. Mucoepidermoid carcinoma of hard palate. Indian J Pathol Microbiol. 2018;61:397-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Granic M, Suton P, Mueller D, Cvrljevic I, Luksic I. Prognostic factors in head and neck mucoepidermoid carcinoma: experience at a single institution based on 64 consecutive patients over a 28-year period. Int J Oral Maxillofac Surg. 2018;47:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lopes MA, da Cruz Perez DE, de Abreu Alves F, de Almeida OP, Kowalski LP. Clinicopathologic and immunohistochemical study of intraoral mucoepidermoid carcinoma. Otolaryngol Head Neck Surg. 2006;134:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Conroy T, Hebbar M, Bennouna J, Ducreux M, Ychou M, Llédo G, Adenis A, Faroux R, Rebischung C, Kockler L, Douillard JY. Quality-of-life findings from a randomised phase-III study of XELOX vs FOLFOX-6 in metastatic colorectal cancer. Br J Cancer. 2010;102:59-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H, Griffin JD. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 2005;24:2391-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Kang H, Tan M, Bishop JA, Jones S, Sausen M, Ha PK, Agrawal N. Whole-Exome Sequencing of Salivary Gland Mucoepidermoid Carcinoma. Clin Cancer Res. 2017;23:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Chen Z, Ni W, Li JL, Lin S, Zhou X, Sun Y, Li JW, Leon ME, Hurtado MD, Zolotukhin S, Liu C, Lu J, Griffin JD, Kaye FJ, Wu L. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Foschini MP, Gaiba A, Cocchi R, Pennesi MG, Pession A. p63 expression in salivary gland tumors: role of DeltaNp73L in neoplastic transformation. Int J Surg Pathol. 2005;13:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Edwards PC, Bhuiya T, Kelsch RD. Assessment of p63 expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and basal cell and canalicular adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 15. | Sams RN, Gnepp DR. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013;7:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Zhao N, Sun BC, Sun T, Ma YM, Zhao XL, Liu ZY, Dong XY, Che N, Mo J, Gu Q. Hypoxia-induced vasculogenic mimicry formation via VE-cadherin regulation by Bcl-2. Med Oncol. 2012;29:3599-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Biroccio A, Candiloro A, Mottolese M, Sapora O, Albini A, Zupi G, Del Bufalo D. Bcl-2 overexpression and hypoxia synergistically act to modulate vascular endothelial growth factor expression and in vivo angiogenesis in a breast carcinoma line. FASEB J. 2000;14:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Irani S, Dehghan A. Expression of Vascular Endothelial-Cadherin in Mucoepidermoid Carcinoma: Role in Cancer Development. J Int Soc Prev Community Dent. 2017;7:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Huo Z, Wu H, Li J, Li S, Wu S, Liu Y, Luo Y, Cao J, Zeng X, Liang Z. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One. 2015;10:e0143169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Roden AC, García JJ, Wehrs RN, Colby TV, Khoor A, Leslie KO, Chen L. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol. 2014;27:1479-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Schvartsman G, Pinto NA, Bell D, Ferrarotto R. Salivary gland tumors: Molecular characterization and therapeutic advances for metastatic disease. Head Neck. 2019;41:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Liu Q, Gu Z, Yang F, Fu J, Shen Y, Wei Y, Tan L, Zhang P, Han Y, Chen C, Zhang R, Li Y, Chen K, Chen H, Liu Y, Cui Y, Wang Y, Pang L, Yu Z, Zhou X, Xiang J, Fang W; Members of the Chinese Alliance for Research in Thymomas. The role of postoperative radiotherapy for stage I/II/III thymic tumor-results of the ChART retrospective database. J Thorac Dis. 2016;8:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Fuse ET, Kamimura M, Takeda Y, Kawaishi M, Kimura S, Niino H, Saito K, Kobayashi N, Kudo K. Response of a thymic mucoepidermoid carcinoma to combination chemotherapy with cisplatin and irinotecan: a case report. Lung Cancer. 2008;59:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |