Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9470
Peer-review started: April 30, 2022
First decision: May 30, 2022
Revised: June 12, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: September 16, 2022
Processing time: 124 Days and 12.4 Hours
Burkitt-like lymphoma with 11q aberration (BLL-11q) is a rare provisional lymphoma, and the majority of cases are usually diagnosed by excisional lymph node biopsy. Here we report a case of BLL-11q diagnosed by needle biopsy of the liver in order to improve further understanding of the disease, reduce misdiagnosis, and identify treatment regimens.
The patient was a 67-year-old male. He complained of increased frequency of stools for more than one year, periumbilical pain and discomfort exceeding 3 mo. A computed tomography scan suggested an appendiceal malignant tumor with multiple metastases of the peritoneum, omentum, and liver. Needle biopsy of liver nodules showed that the tumor cells were of median size, the shape was consistent, a small number of tumor cells were large, the “starry sky” pattern was evident, and some tissue cells showed multiple apoptotic debris with coarse particles. Immunohistochemistry was positive for CD20, CD10, BCL6, and MYC. The Ki-67 proliferation index was more than 95%. Molecular biological detection indicated a lack of MYC, BCL2 and BCL6 gene rearrangement with 11q aberration. Therefore, the diagnosis was BLL-11q of the liver. After eight courses of chemotherapy, the abdominal and pelvic peritoneal masses and liver nodules had almost disappeared. The patient recovered well after a follow-up period of more than 13 mo.
BLL-11q is rare, but patients treated with standard chemotherapy for Burkitt lymphoma can have a good prognosis. Reducing the dose of chemotherapy or developing specific therapies to prevent overtreatment may be considered, but more case studies are needed.
Core Tip: Burkitt-like lymphoma with 11q aberration (BLL-11q) is an uncommon lymphoma and is diagnosed by lymph node biopsy. We report a patient with BLL-11q, who presented with predominant digestive tract symptoms. The clinical consideration was colorectal cancer with multiple metastases. The diagnosis was confirmed by needle biopsy of liver nodules, and phagocytosis with a large number of coarse particles was found on pathological morphology, which suggested the diagnosis of BLL-11q. The patient was cured with chemotherapy and recovered well.
- Citation: Yang HJ, Wang ZM. Burkitt-like lymphoma with 11q aberration confirmed by needle biopsy of the liver: A case report. World J Clin Cases 2022; 10(26): 9470-9477
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9470.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9470
Burkitt-like lymphoma with 11q aberration (BLL-11q) was proposed as a provisional subtype in the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues[1]. Its morphology and immunophenotype are similar to burkitt lymphoma (BL). However, it lacks MYC gene rearrangement but shows characteristic 11q alterations (11q23.2-23.3 increase and 11q24.1-qter telomere deletion). This tumor is found infrequently, with less than 100 cases reported according to a PubMed search[2-6]. The demographical and pathological findings in the reported cases are shown in Supplementary Table 1. Only one patient diagnosed by needle biopsy of the liver has been reported[7]. Here, we report a case of BLL-11q diagnosed by liver biopsy.
The patient was a 67-year-old male. On January 26, 2021, he was hospitalized due to an increase in stool frequency for more than one year, periumbilical pain, and discomfort for more than 3 mo.
The frequency of stools increased one year ago without obvious inducement, 4-10 times every day, and a little at a time. He did not have black stool, vomiting, chills or fever. He did not pay attention to his stool frequency and did not see a doctor. Three months ago, he developed periumbilical pain and discomfort without an apparent reason.
The patient had no previous medical history.
The patient had no family history of genetic diseases and tumors, no history of surgery, and no history of immune deficiency.
On physical examination, his blood pressure was 130/85 mmHg, temperature was 36.8 °C, heart rate was 82 bpm, and respiratory rate was 17 breaths/min. No abnormalities were found in the cardiopulmonary region. No palpable masses were detected in the abdominal region or enlarged lymph nodes in the cervical and inguinal regions.
Routine blood testing showed that leukocytes were 4.25 × 109/L, erythrocytes were 4.12 × 1012/L, platelets were 279 × 109/L, and lactate dehydrogenase (LDH) was 214 U/L. Blood tumor markers were normal. There were no obvious abnormalities in the bone marrow cytology smear and biopsy. Routine fecal tests and occult blood test were normal.
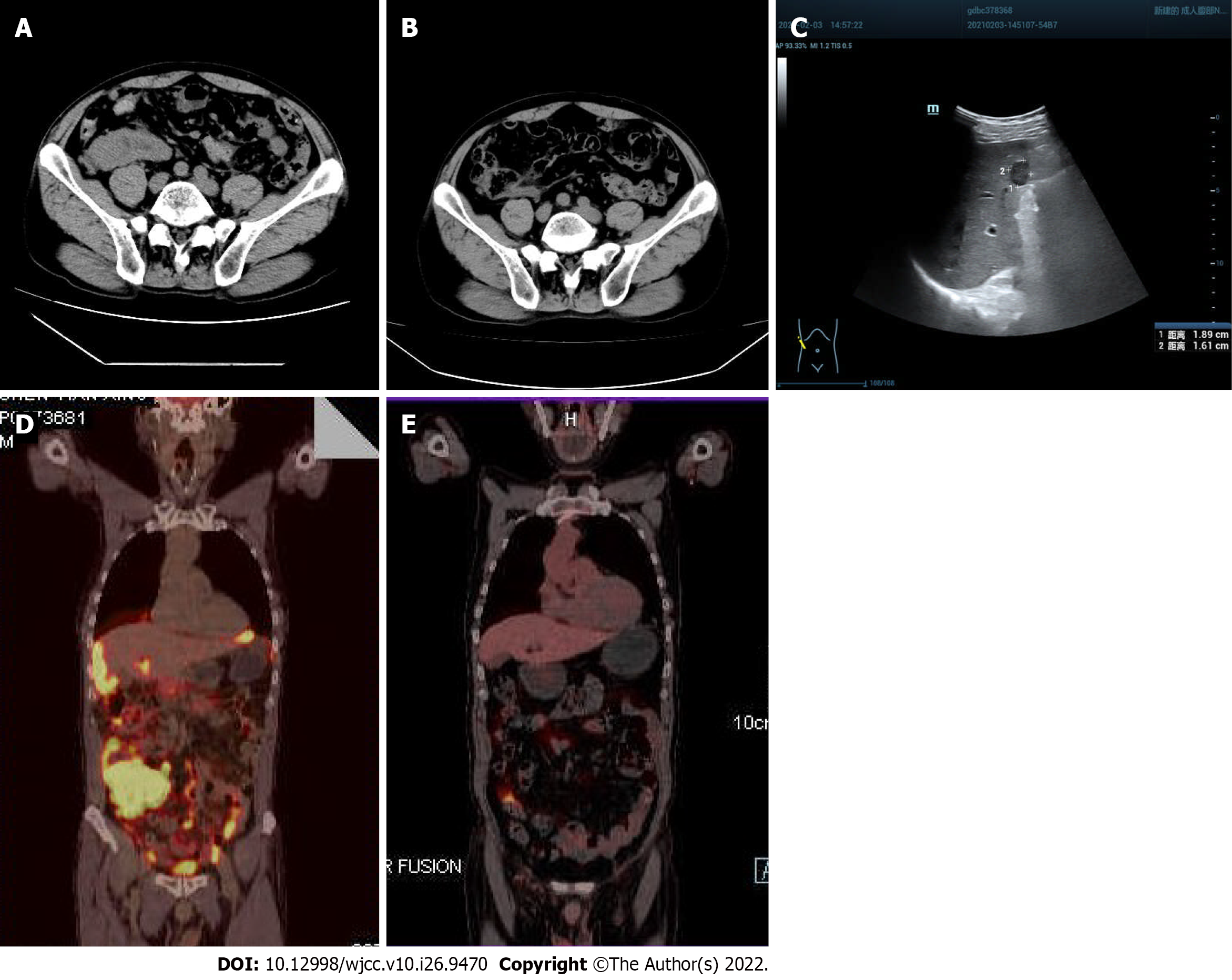
On January 29, 2021, an abdominal computed tomography scan at our hospital showed a mass in the right lower abdomen, suggesting a malignant tumor of the appendix with multiple metastases in the peritoneum, omentum, and liver. The boundary between the mass and the right psoas major muscle was unclear (Figure 1A). On February 3, 2021, B-ultrasound showed a solid hypoechoic mass in the lower segment of the right anterior lobe of the liver, 1.89 cm × 1.61 cm in size, with a clear boundary (Figure 1C). On March 12, 2021, positron emission tomography-computed tomography (PET-CT) showed a soft tissue mass in the liver and right lower abdomen, surrounding the ileocecal junction, with a maximum size of 6.6 cm × 9.4 cm, and a maximum standardized uptake value of 30.3; radioactive uptake in the hepatic capsule margin, abdominal and pelvic mesangium, and omentum was significantly increased (Figure 1D).
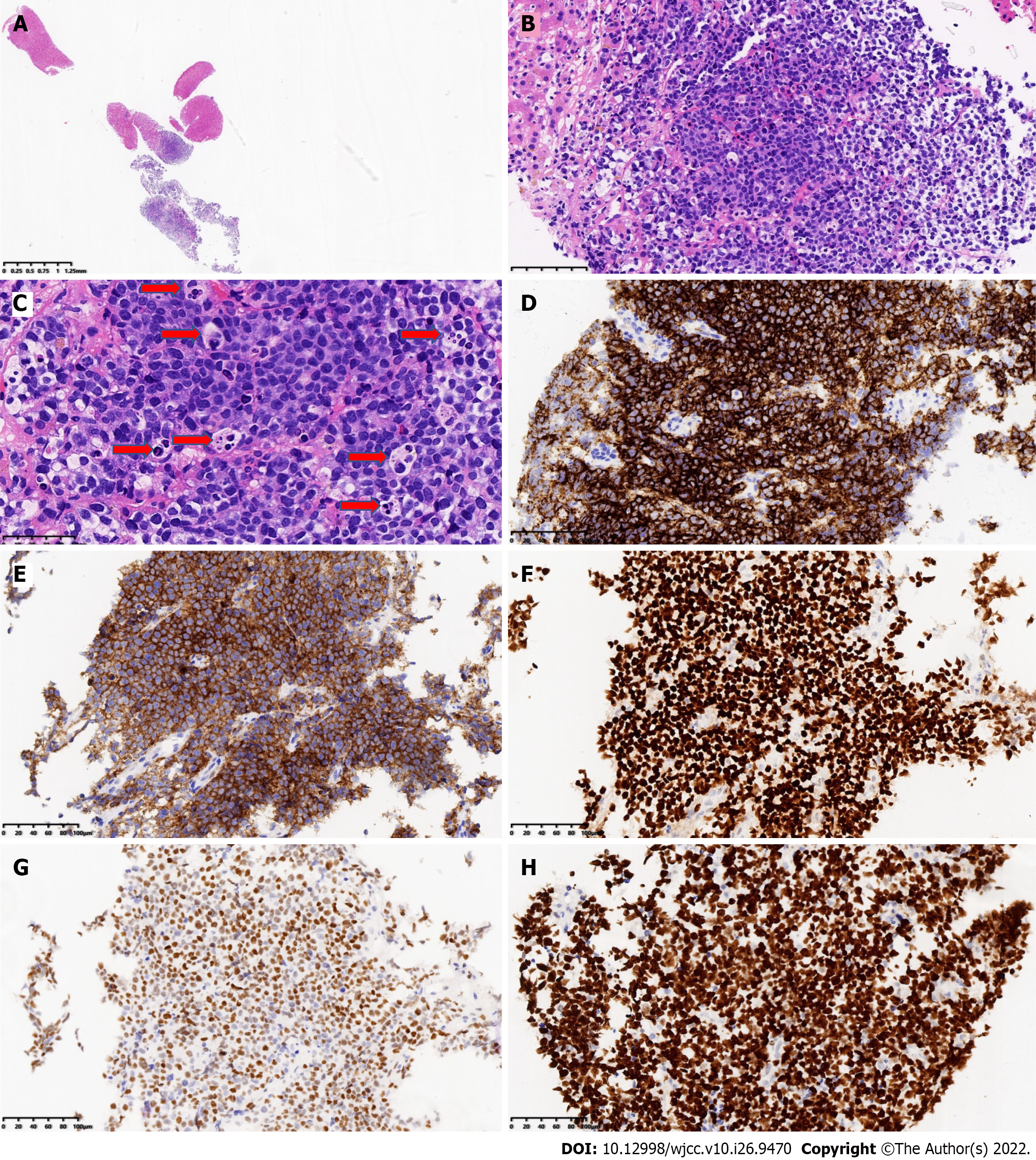
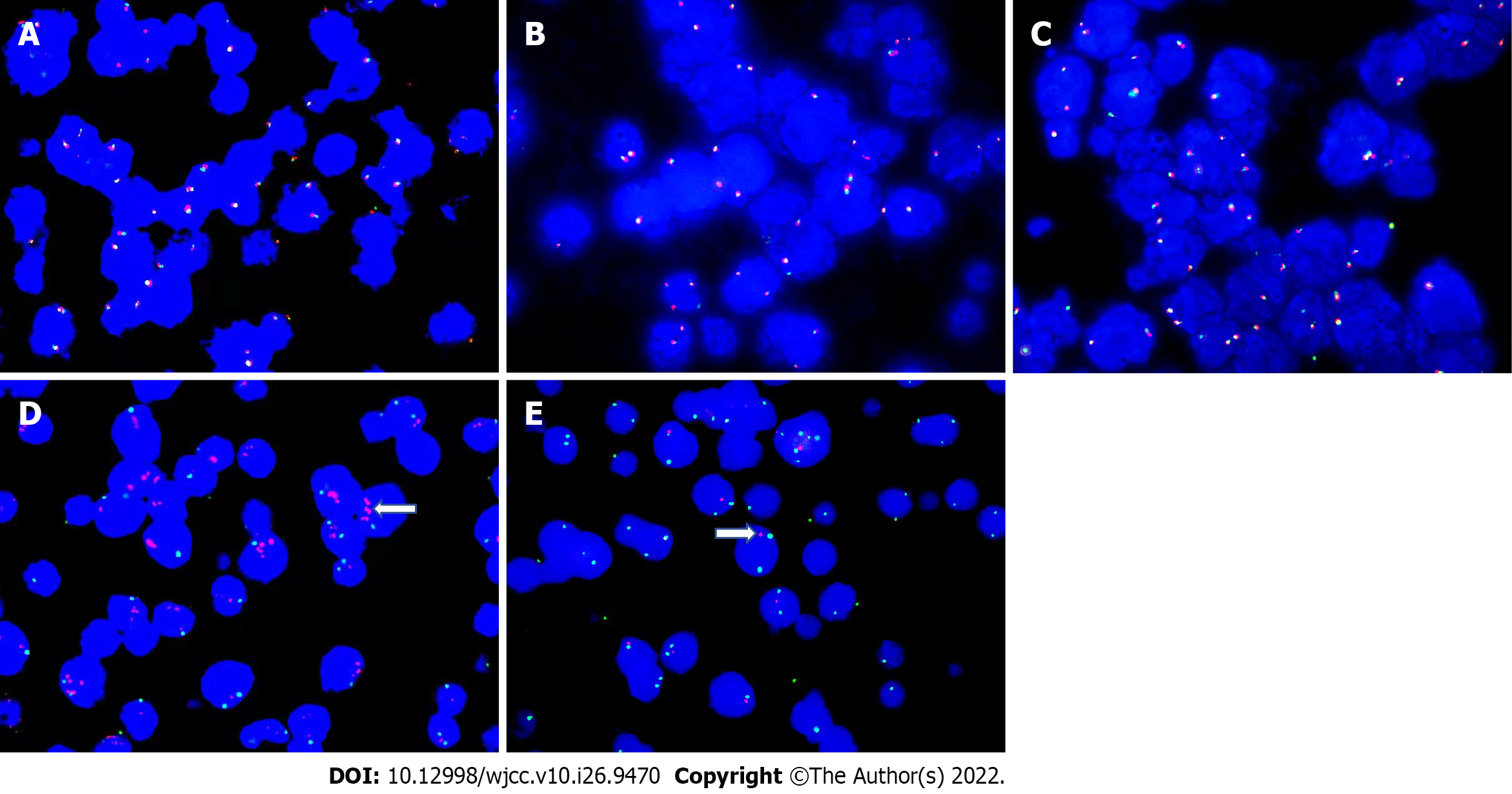
A liver nodule biopsy was performed on February 3, 2021. Tumor tissue was diffusely infiltrated. The tumor cells were medium-large, consistent in shape, little cytoplasm, basophilic, with fine granular nuclear chromatin, and occasionally small nucleoli. A few tumor cells were large, with obvious nucleoli and a “starry sky” pattern. There were scattered tissue cells that phagocytized apoptotic debris and nuclear fragments. Multiple (six) apoptotic debris were partially engulfed, with coarse particles. Scattered eosinophils, residual bile ducts, and peripheral degenerative hepatocytes were seen. Immunohistochemical markers CD20, CD10 and BCL6 were positive, MYC (positive, 70%), and the Ki-67 proliferation index > 95% (Figure 2). CD3, BCL2, MUM1, TDT, CD30, CD38, CyclinD1, EBER, and CK (Pan) were negative. Fluorescence in situ hybridization (FISH) detected the MYC gene, BCL2 gene, and BCL6 gene, counted 200 tumor cells, no red and green signal separated the cells, and the results were negative (Figure 3A-C). FISH analysis of chromosome 11 showed that 11q23 was amplified, and 11q24 was deleted (Figure 3D and E). Antibodies and probe information are shown in Supplementary Table 2.
The patient was diagnosed as BLL-11q of the liver.
The patient received the R-Hyper CVAD part A treatment protocol (Rituximab 700 mg on d0, cyclophosphamide 0.57 g q12 h on d1-3, dexamethasone 40 mg on d1-4 and d11-14, liposome doxorubicin 55 mg on d4, vindesine 4 mg on d4 and d11) d0-d4 chemotherapy, supplemented by stomach protection, anti-nausea, alkalization, and hydration treatment. After three courses of chemotherapy, according to the CT scan, the tumor in the right lower abdomen had significantly shrunk, and the boundary between the tumor and the right psoas major muscle was obvious (Figure 1B). After six courses of chemotherapy, PET-CT indicated that there was no obvious mass and no abnormally increased fluorodeoxyglucose metabolism in the abdominal, pelvic and peritoneal mesangium. There was also no noticeable increased radioactive uptake in the liver (Figure 1E).
The patient completed eight courses of chemotherapy. He recovered well after a follow-up period of more than 13 mo.
Since BLL-11q was named, cases have been reported continuously, but analysis has mainly been based on individual cases or minor cases, and the data on incidence rate and clinical manifestations are very limited. The age at onset of the tumor ranges widely from 2 to 83 years, but it is more common in young patients[2,8]. In children and adolescents under the age of 18 years, patients with BLL-11q have a median age of 13.9 years, relatively older than those with BL, high-grade B-cell lymphoma (HGBCL) and diffuse large B-cell lymphoma (DLBCL)[2]. The tumor is found more often in males than in females, and it tends to occur in lymph nodes[2,3]. In addition to cervical, abdominal, retroperitoneal, and pelvic lymph nodes, tumors in the breast, ileocecum, tonsil, bone, soft tissue, and testis have also been reported. They can invade the liver and pancreas and produce pleural effusions and ascites[2,7,9,10]. Recently, a case of BLL-11q which originated in the spleen was reported[11]. The size of the mass differs with the diameter ranging from 2-11 cm[3]. A tumor greater than 20 cm has also been reported[6]. Serum LDH may be normal or slightly elevated[2,6]. Clinical Ann Arbor stages are mostly stage I/II, and less frequently stage III/IV[2,3]. The bone marrow and hydrocephalus are rarely involved[8]. In addition to those with normal immune function, BLL-11q in patients with HIV infection, organ transplantation, and immunosuppression have been reported[10,12-14]. In the present case, the liver, ileocecal, abdominal and pelvic mesangium, and omentum were involved. The International Prognostic Index (IPI) score of group A in clinical stage IV was 4, and was in the high-risk group.
Morphologically, tumor cells mainly grow diffusely, similar to BL, or between BL and DLBCL. They may have a certain degree of cell pleomorphism, and are occasionally nodular[1,3,8]. Horn et al[5] first proposed that when tissue cells phagocytize 5-9 apoptotic debris and apoptotic fragments with coarser particles, it is speculated that the specificity for 11q abnormality is 91% and the sensitivity is 85%. This has been confirmed by Yu YT and colleagues[7]. They emphasized that when > 50% of tissue cells engulf apoptotic debris and thicker apoptotic fragments, this can predict 11q abnormalities. Although our case underwent a small liver tissue puncture, this morphological change was also observed, which confirmed its strong diagnostic potential. It is speculated that 11q abnormality may have a specific mechanism to drive the increase in apoptosis frequency, but the specific molecular mechanism requires further confirmation[5]. Therefore, we believe that when the “starry sky” pattern is obvious, and the tissue cells engulf apoptotic debris and coarse apoptotic fragments, 11q molecular detection should be carried out.
The typical immunophenotype of BL was CD20 +, CD10 +, BCL2 −, and Ki-67 > 90%. There was no significant difference in the expression of CD10, BCL6, MUM1, Ki-67, and MYC proteins between 11q negative and 11q positive cases. However, more 11q positive cases had negative BCL2 than 11q negative cases, and this difference was statistically significant[11]. It is reported that flow cytometry is positive for CD16/CD56 and lacks high expression of CD38, which supports the diagnosis of BLL-11q[6]. No flow cytometry samples were obtained from our case, and the immunohistochemical phenotype was similar to BL, which prompted us to further detect the MYC gene. In our case, MYC, BCL2, and BCL6 were not translocated, but the increase in 11q23 and the deletion of 11q24 were detected.
BLL-11q is easily confused with some highly invasive B-cell lymphomas. Differential diagnosis: Classic BL: Both morphology and immunophenotype overlap[1,15], but MYC gene rearrangement is present in BL. HGBCL is a double strike or triple strike lymphoma, that is, HGBCL with MYC and BCL2 and/or BCL6 translocation, and its identification mainly depends on molecular genetic detection. It is described in the literature that a few HGBCLs with positive BL and MYC gene translocation can also have an 11q aberration[9,13]. The diagnosis of BLL-11q requires the lack of MYC, BCL2, and BCL6 translocation. DLCBL of germinal center origin: The acquisition or amplification of 11q large fragment can occur, rather than the acquisition of 11q proximal region with deletion of the distal region[15].
As 11q aberration is not unique to BLL-11q, and other invasive lymphomas such as BL and MYC positive or negative HGBCL and DLBCL also exist, the clinical disease spectrum of malignant tumors with 11q aberration is broader than previously assumed[2,5,9,13]. Whether this lymphoma is a distinct category or a particular variant of other recognized entities is controversial. To improve the understanding of BLL-11q, Gonzalez-Farre et al[16] performed an analysis of copy number alterations and targeted sequencing of a large panel of B-cell lymphoma-related genes in 11 cases. Potential driver mutations were found in 27 genes, particularly involving BTG2, DDX3X, ETS1, EP300, and GNA13. These results suggest that BLL-11q is a germinal center-derived lymphoma closer to HGBCL or DLBCL than to BL. They think that the term aggressive B-cell lymphoma with 11q aberration captures their pathological features[16]. Recently, Kim et al[17] reported a composite lymphoma of BLL-11q and BL. They suggest BLL-11q as a distinct entity from BL. The sequence of molecular detection is to first detect MYC gene rearrangement, and then 11q chromosome detection when MYC gene rearrangement is negative, 11q proximal acquisition and distal deletion are required at the same time[1,8,9,13]. Only by combining morphology, immunophenotype and molecular biology can the correct diagnosis be made. The most sensitive method reported in the literature is DNA microarray[8]. Some authors suggest that all MYC rearrangement negative HGBCLs with BL/BL-11q morphology should be detected by microarray or FISH detection with 11q aberration, while others support a step-by-step method, starting with FISH detection. If FISH detection is difficult or negative, then microarray detection is performed[18].
It has been reported that prognosis of the tumor is good, most are completely resolved after BL standard chemotherapy, Au-Yeung et al[2] reported a two-year event-free survival rate of 100%[2,3,6,8]. Disease-free survival after 67 mo of follow-up was reported following chemotherapy[9]. A patient who was suffering from AIDS, after receiving one course of rituximab, cyclophosphamide, pirarubicin, vincristine, and prednisone (R-CTOEP), refused to continue chemotherapy and was followed up for 34 mo, no recurrence or new lesions were found[12]. The disease course in our case was more than 1 year before diagnosis. The IPI score of group A in clinical phase IV was 4, and was in the high-risk group. He was followed up for more than 13 mo after chemotherapy. The abdominal and pelvic masses and liver nodules disappeared, and the patient is now in a good condition. Therefore, we can consider that BLL-11q is a separate lymphoma subtype[1,18], and the chemotherapy dose can be reduced, or specific treatment methods can be formulated to prevent overtreatment[2,18]. However, more case-cohort studies and clinical comparisons at the same stage and risk are needed to determine the best treatment regimen.
BLL-11q can occur in both intra- and extra-lymph nodes. In addition to surgical excision of the specimen, a definite diagnosis can be made when the tissue volume in the needle biopsy specimen is sufficient. In terms of pathomorphology, the tumor cells are of medium size, consistent shape, and the significant “starry sky” phenomenon is accompanied by a large number of phagocytic reactions with coarse particles suggestive of BLL-11q diagnosis. Pathological diagnosis can only be confirmed by immunohistochemistry and molecular detection. Following chemotherapy, patients can achieve satisfactory results, and their prognosis is good. Reducing the dose of chemotherapy or developing specific therapies to prevent overtreatment may be considered, but more case studies are needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adam CA, Romania; Elpek GO, Turkey; Haddadi S, Algeria; Ni X, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Leoncini L, Campo E, Stein H, Harris NL, Jaffe E. S, Kluin RM. Burkitt-like lymphoma with 11q aberration. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised, 4th ed, Lyon, France: IARC, 2017: 334. |
| 2. | Au-Yeung RKH, Arias Padilla L, Zimmermann M, Oschlies I, Siebert R, Woessmann W, Burkhardt B, Klapper W. Experience with provisional WHO-entities large B-cell lymphoma with IRF4-rearrangement and Burkitt-like lymphoma with 11q aberration in paediatric patients of the NHL-BFM group. Br J Haematol. 2020;190:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Zhang YP, Zhang L, Zhang DD, Wang GN, Zhao WG, Jian XY, Li WC. [Clinicopathological and molecular genetic features of Burkitt-like lymphoma with 11q aberration]. Zhonghua Bing Li Xue Za Zhi. 2021;50:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Wang L, Jin YP, Gao G, Wu DY, Zhou XJ, Liu YY, Xia QX. [Clinicopathological features and molecular genetics of Burkitt-like lymphoma with 11q aberration]. Zhonghua Bing Li Xue Za Zhi. 2021;50:655-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Horn H, Kalmbach S, Wagener R, Staiger AM, Hüttl K, Mottok A, Bens S, Traverse-Glehen A, Fontaine J, Siebert R, Rosenwald A, Ott G. A Diagnostic Approach to the Identification of Burkitt-like Lymphoma With 11q Aberration in Aggressive B-Cell Lymphomas. Am J Surg Pathol. 2021;45:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Rymkiewicz G, Grygalewicz B, Chechlinska M, Blachnio K, Bystydzienski Z, Romejko-Jarosinska J, Woroniecka R, Zajdel M, Domanska-Czyz K, Martin-Garcia D, Nadeu F, Swoboda P, Rygier J, Pienkowska-Grela B, Siwicki JK, Prochorec-Sobieszek M, Salaverria I, Siebert R, Walewski J. A comprehensive flow-cytometry-based immunophenotypic characterization of Burkitt-like lymphoma with 11q aberration. Mod Pathol. 2018;31:732-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Yu YT, Takeuchi K, Baba S, Chang KC. Morphologically Suspected Burkitt-like Lymphoma With 11q Aberrations Confirmed by Fluorescence In Situ Hybridization. Am J Surg Pathol. 2022;46:576-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ganapathi KA, Brown LE, Prakash S, Bhargava P. New developments in non-Hodgkin lymphoid malignancies. Pathology. 2021;53:349-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Grygalewicz B, Woroniecka R, Rymkiewicz G, Rygier J, Borkowska K, Kotyl A, Blachnio K, Bystydzienski Z, Nowakowska B, Pienkowska-Grela B. The 11q-Gain/loss Aberration Occurs Recurrently in MYC-Negative Burkitt-like Lymphoma With 11q Aberration, as Well as MYC-Positive Burkitt Lymphoma and MYC-Positive High-Grade B-Cell Lymphoma, NOS. Am J Clin Pathol. 2017;149:17-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Ferreiro JF, Morscio J, Dierickx D, Marcelis L, Verhoef G, Vandenberghe P, Tousseyn T, Wlodarska I. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/Loss pattern. Haematologica. 2015;100:e275-e279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Okwan-Duodu D, Huang Q. Primary splenic Burkitt-like lymphoma with 11q aberration. Blood. 2021;138:1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Wang J, Ma L, Guo J, Xi Y, Xu E. Burkitt-like lymphoma with 11q aberration in a patient with AIDS and a patient without AIDS: Two cases reports and literature review. Open Med (Wars). 2021;16:428-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Gebauer N, Witte HM, Merz H, Oschlies I, Klapper W, Caliebe A, Tharun L, Spielmann M, von Bubnoff N, Feller AC, Murga Penas EM. Aggressive B-cell lymphoma cases with 11q aberration patterns indicate a spectrum beyond Burkitt-like lymphoma. Blood Adv. 2021;5:5220-5225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | de Nattes T, Camus V, François A, Dallet G, Ferrand C, Guerrot D, Lemoine M, Morin F, Thieblemont C, Veresezan EL, Candon S, Latouche JB, Bertrand D. Kidney Transplant T Cell-Mediated Rejection Occurring After Anti-CD19 CAR T-Cell Therapy for Refractory Aggressive Burkitt-like Lymphoma With 11q Aberration: A Case Report. Am J Kidney Dis. 2022;79:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Salaverria I, Martin-Guerrero I, Wagener R, Kreuz M, Kohler CW, Richter J, Pienkowska-Grela B, Adam P, Burkhardt B, Claviez A, Damm-Welk C, Drexler HG, Hummel M, Jaffe ES, Küppers R, Lefebvre C, Lisfeld J, Löffler M, Macleod RA, Nagel I, Oschlies I, Rosolowski M, Russell RB, Rymkiewicz G, Schindler D, Schlesner M, Scholtysik R, Schwaenen C, Spang R, Szczepanowski M, Trümper L, Vater I, Wessendorf S, Klapper W, Siebert R; Molecular Mechanisms in Malignant Lymphoma Network Project; Berlin-Frankfurt-Münster Non-Hodgkin Lymphoma Group. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014;123:1187-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Gonzalez-Farre B, Ramis-Zaldivar JE, Salmeron-Villalobos J, Balagué O, Celis V, Verdu-Amoros J, Nadeu F, Sábado C, Ferrández A, Garrido M, García-Bragado F, de la Maya MD, Vagace JM, Panizo CM, Astigarraga I, Andrés M, Jaffe ES, Campo E, Salaverria I. Burkitt-like lymphoma with 11q aberration: a germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica. 2019;104:1822-1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Kim M, Hwang HS, Yoon DH, Chun SM, Go H. Distinct genetic alterations in Burkitt-like lymphoma with 11q aberration and Burkitt lymphoma: a novel case report of composite lymphoma. Haematologica. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Brown LE, Bowen LM, McCarthy LC, Cooley LD, Repnikova E, Gener MA, Garola R, August KJ, Hays JA, Zwick DL, Li W. Application of 2016 WHO classification in the diagnosis of paediatric high-grade MYC-negative mature B-cell lymphoma with Burkitt-like morphological features. J Clin Pathol. 2020;73:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |