Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9462
Peer-review started: April 26, 2022
First decision: June 8, 2022
Revised: June 20, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: September 16, 2022
Processing time: 128 Days and 14.9 Hours
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare and potentially life-threatening condition after receiving coronavirus disease vaccines. It is characterized by symptom onset at 5 to 30 d postvaccination, thrombocytopenia, thrombosis, high D-dimer level, and antiplatelet factor 4 (anti-PF4) antibody positivity. VITT can progress very fast, requiring urgent management. Only few studies have described its detailed clinical course and imaging changes. We report a typical VITT case in a patient who underwent regular repeated brain imaging examinations.
A young woman presented with headaches at 7 d after the ChAdOx1 nCoV-19 vaccine (AZD1222) injection. She then showed progressive symptoms of left upper limb clumsiness. Brain computed tomography revealed venous infarction at the right parietal lobe with a hyperacute thrombus in the cortical vein. Two hours later, brain magnetic resonance imaging revealed hemorrhage at the same area. Magnetic resonance venography showed an irregular contour of the right transverse sinus. Laboratory examination revealed a high D-dimer level, thrombocytopenia, and a high titer for anti-PF4 antibodies. She was treated with anticoagulants, intravenous immunoglobulin, and steroids and analgesic agents were administered for pain control. She had a marked improvement on headaches and clumsiness after treatment along with radiological thrombus resolution. During follow-up at the outpatient department, her modified Rankin scale at 90 d was 1.
Clinicians should be alerted whenever patients present with persistent and progressive headaches or focal motor/sensory deficits postvaccination.
Core Tip: Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare and potentially life-threatening condition. Only few studies have described the detailed clinical course and imaging changes in patients with VITT. We report a typical case of VITT in a patient presenting with headaches 7 d postvaccination with progressive left upper limb clumsiness. A series of brain imaging examinations revealed venous infarction, which can progress very fast. Our case demonstrated that VITT diagnosis can be delayed. Clinicians should be alerted whenever a patient with a persistent and progressive headaches or focal motor/sensory deficits after vaccination also presents with high D-dimer level and thrombocytopenia.
- Citation: Jiang SK, Chen WL, Chien C, Pan CS, Tsai ST. Rapid progressive vaccine-induced immune thrombotic thrombocytopenia with cerebral venous thrombosis after ChAdOx1 nCoV-19 (AZD1222) vaccination: A case report. World J Clin Cases 2022; 10(26): 9462-9469
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9462.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9462
Coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been an ongoing worldwide pandemic since 2019[1]. Vaccines can be powerful weapons in ending this pandemic. Several vaccines against SARS-CoV-2 obtained Emergency Use Authorization, and billions of people worldwide have been vaccinated. Since March 22, 2021, people in Taiwan have started to receive vaccines, with most of them have received ChAdOx1 nCoV-19 (AZD1222) vaccinations initially. Unfortunately, in April 2021, several articles have reported that patients developed vaccine-induced immune thrombotic thrombocytopenia (VITT) after being vaccinated with AZD1222 and Ad26.COV2.S (Johnson and Johnson) in Europe, with antiplatelet factor 4 (anti-PF4) antibodies being the pathological mechanism[2-5].
The study of Pavord et al[6] involving 220 patients from the United Kingdom demonstrated that VITT has an overall mortality rate of 22% and the incidence of VITT was at least 1:100000 among patients aged ≥ 50 years and at least 1:50000 among patients aged < 50 years. Even if the initial studies reported mostly female patients, VITT had no gender predominance. Half of the 220 patients had a thrombus in the cerebral veins, which was the most common thrombotic site. They also descripted the Case Definition Criteria for VITT. These patients were diagnosed with definite VITT after meeting all of the following five criteria: symptom onset at 5-30 d postvaccination, thrombocytopenia, thrombosis, high D-dimer level, and anti-PF4 antibody positivity[6]. Cerebral venous thrombosis (CVT) is also a rare disease with an incidence of 1.32 per 100000 per person-years[7]. The symptoms of CVT vary and depend on the site of thrombosis. Headache, which is usually the initial and most frequent symptom, might be the sole manifestation of CVT. Other symptoms include seizures, motor or sensory deficits, aphasia, and the function deficits involving the brain[8].
The previously published reports mainly mentioned the symptom and hematology panel of this syndrome. The progression was not discussed in detail. However, VITT could be a rapid progressive disease. Here we present a case of VITT-related CVT with rapid disease progression after receiving the first dose of the AZD1222. We described her symptom progression, diagnostic imaging results, treatment, and outcome chronologically.
A 36-year-old Asian woman presented to the emergency department (ED) of our hospital complaining of progressive headaches for 10 d accompanied by left upper limb clumsiness.
The patient received her first dose of the AZD1222 without experiencing side effects, such as fever, headache, and myalgia, initially. However, on the 7th day after vaccination, she experienced a headache over the bilateral occipital regions accompanied by dizziness. On the 13th day after vaccination, the pain worsened and extended to the left side of the neck. On the 17th day after vaccination, she still had severe headaches and she noted left upper limb clumsiness, prompting consultation at our ED. During the whole course, there was no slurred speech, double vision, fever, or trauma history.
The patient had a history of iron deficiency anemia, possibly caused by menorrhagia, with a normal platelet count. She denied having chronic headaches or other systemic diseases.
The patient worked as a barber and was able to independently perform her activities of daily living. She did not take regular maintenance medications, such as oral contraceptives and iron supplements. She also denied tobacco smoking, alcohol drinking, and substance abuse. Her family history is unremarkable in her situation.
Neurological examination showed that she had agraphesthesia and poor proprioception over the left hand. Her Glasgow Coma Scale score was 15 with no obvious weakness over the upper and lower limbs. There were no other specific physical and neurological findings.
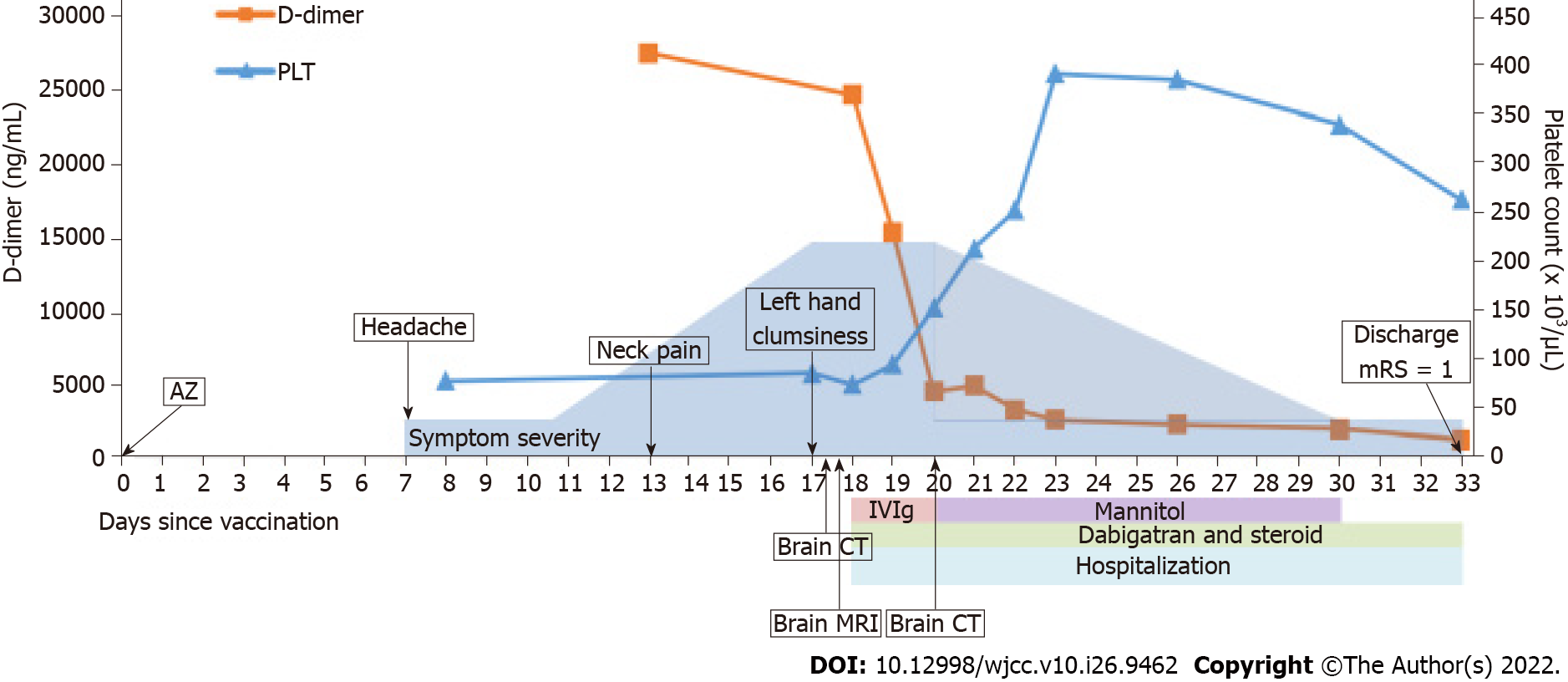
Before the patient visited our ED, on the 7th day after vaccination, thrombocytopenia (platelet count: 77 × 103/µL, reference range: 130 × 103/µL to 400 × 103/µL) and anemia (hemoglobin: 8.3 g/dL, reference range: 11.1-15.0 g/dL) were noted, where the D-dimer level was unknown at that time. On the 13th day after vaccination, while the pain worsened, a high D-dimer level (27500.8 ng/mL, reference range: < 500 ng/mL) was also noted.
On the 17th day after vaccination, while she visited our ED, a series of laboratory evaluations and autoimmune profile tests for the possible etiology of the CVT revealed normal findings in most tests, but weak positivity for anti--Sjögren’s-syndrome-related antigen A antibody (anti-SSA antibody: 12 U/mL, reference range: ≤ 7 U/mL) and antinuclear antibody (ANA: 1:80, reference range: negative). The fibrinogen level was 178.1 mg/dL (reference range: 200-400 mg/dL) and the international normalized ratio was 1.1 (reference range: 0.9-1.2). She still had low iron levels (14 µg/dL, reference range: 50-212 µg/dL) with normal transferrin levels. Anti-PF4 antibodies were also checked, which later showed positivity on ELISA (optical density: 3.4165, reference range: ≤ 0.4, Immucor GTI Diagnostics, Waukesha, WI, United States)[9]. The finding of the polymerase chain reaction test for SARS-CoV-2 from the nasal swab was negative.
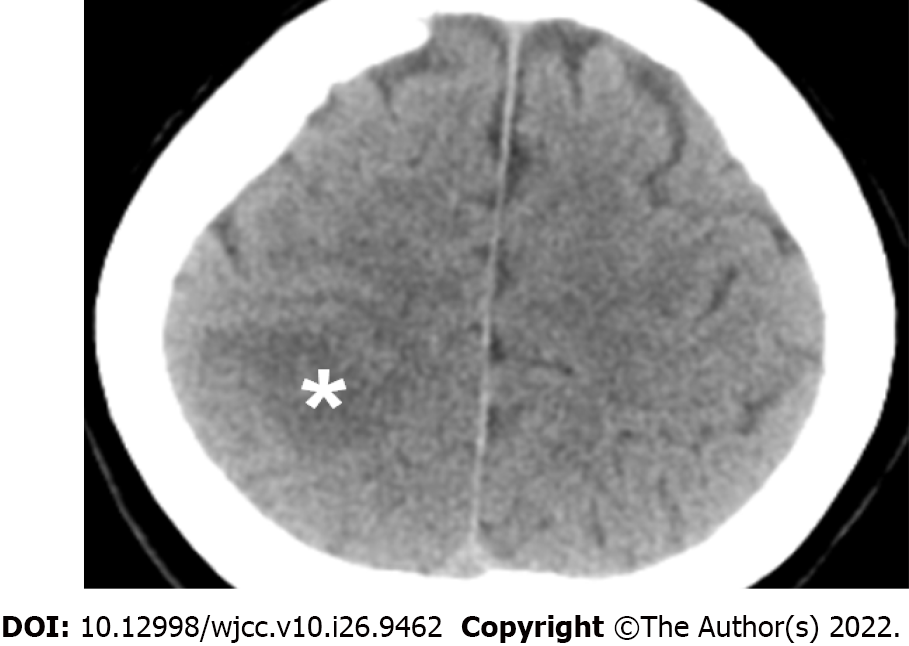
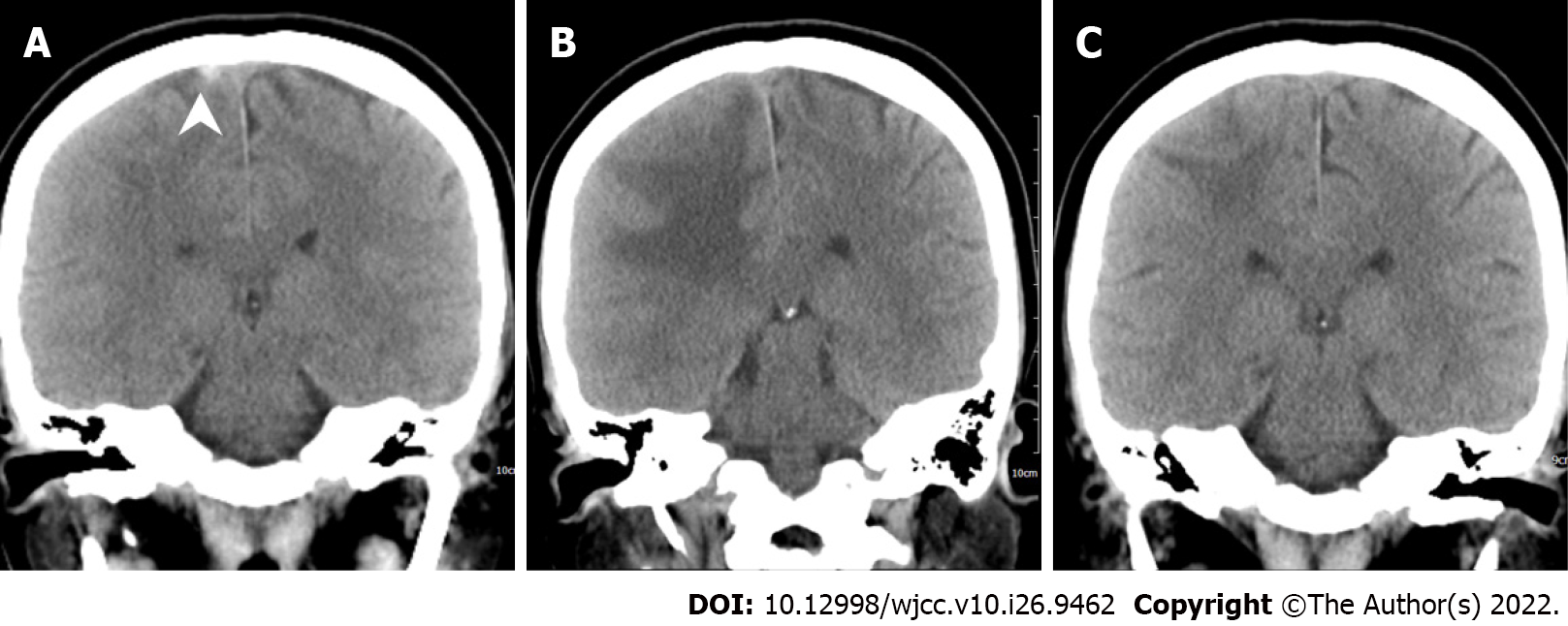
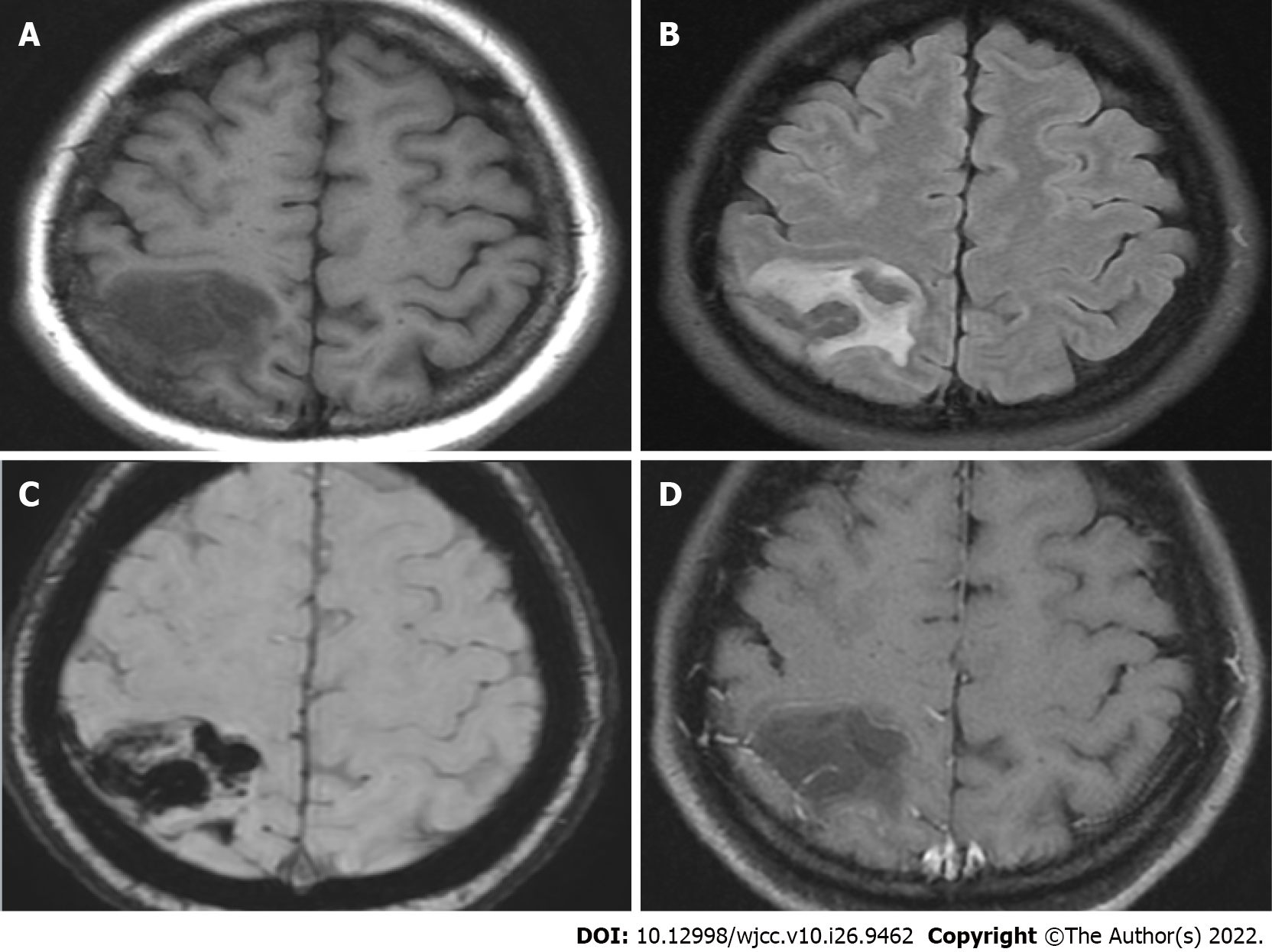
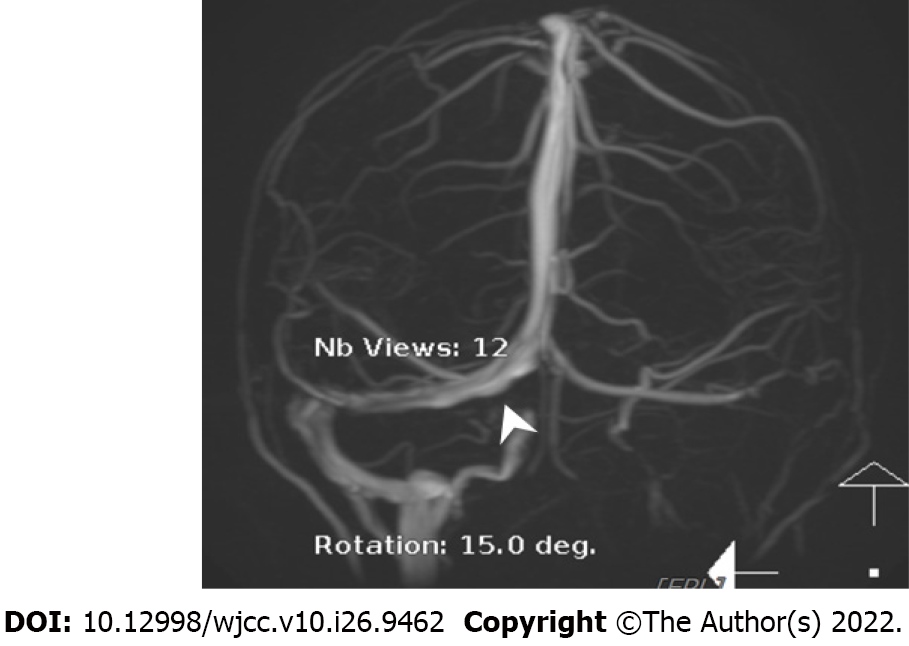
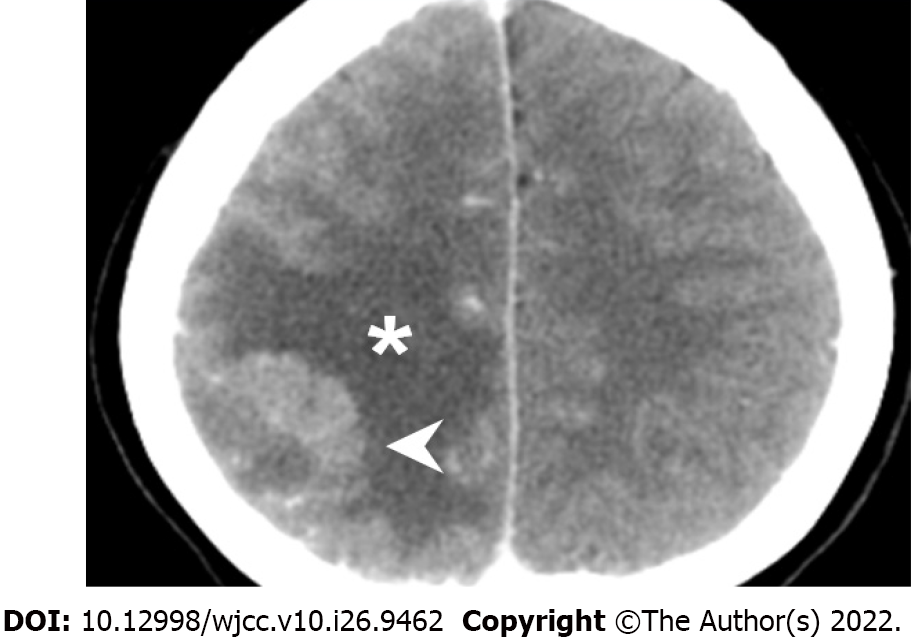
The initial brain CT at the ED revealed focal edema over the right parietal lobe without hemorrhage (Figure 1) and a hyperacute thrombus in her right cortical vein (Figure 2). Two hours later, brain magnetic resonance imaging (MRI) revealed an irregular contour of the right transverse sinus, which was unusual in female patients with this age, and hyperacute hemorrhage at the right parietal lobe (Figures 3 and 4).
We consulted a neuroradiologist (WLC) for endovascular thrombectomy (EVT) or thrombolysis. Both procedures are invasive treatments; they are treatment options for cases with continued neurological worsening despite best medical treatment[8]. We did not perform the EVT because the patient became stable after the administration of intravenous immunoglobulin (IVIG) and oral anticoagulant.
The final diagnosis of the presented case is VITT with CVT and hemorrhagic transformation after ChAdOx1 nCoV-19 (AZD1222) vaccination.
After the brain imaging was performed and all the blood tests were arranged, she was admitted to the intensive care unit and started on oral dabigatran (110 mg twice daily) and IVIG infusion (total dose: 2 g/kg, intravenous drip for 2 d); steroids with methylprednisolone were also used for right parietal lobe vasogenic edema. After IVIG therapy on 20th day after vaccination, brain CT showed increased edema of the right parietal lobe, as compared with the initial brain CT (Figure 5). Thus, mannitol infusion was started. Analgesic agents for pain control were also used.
After treatment, her headache and neurological deficits improved, her D-dimer level decreased, and her platelet count gradually increased. On 33th day after vaccination, she was discharged, but she still had mild headaches and mild proprioception deficit of the left hand. However, no agraphesthesia was noted. Figure 6 presents the clinical time course. During following-up at outpatient department, her modified Rankin scale at 90 d is 1 (could return to work). The follow-up brain CT on 61th day showed resolution of the cerebral edema. After 5 mo, the follow-up anti-PF4 antibody titer was much decreased, and the optical density decreased from 3.4165 to 0.5845 (reference range: ≤ 0.4, Immucor GTI Diagnostics, Waukesha, WI, United States).
We present a case of definite VITT, according to the Case Definition Criteria[6], with detailed clinical course and serial brain imaging examinations. She experienced headaches at 7th day after receiving AZD1222 and was diagnosed with CVT with hemorrhagic transformation with high titer anti-PF4 antibodies, thrombocytopenia, and high D-dimer levels. This case demonstrates that the diagnosis of VITT can be easily delayed, rapid progressive disease and hemorrhagic transformation could be noted within 2 h after initial brain CT revealed no hemorrhage.
Early diagnosis and treatment are important in achieving a good prognosis. Our patient was diagnosed with VITT and received treatment at 11 d after experiencing the first symptom, even if thrombocytopenia was already present. Clinicians should be alerted and should check the D-dimer level and platelet count, if patients present with a persistent headache after vaccination. If thrombocytopenia and high D-dimer levels are noted, diagnostic brain imaging should be performed. Imaging in other sites based on the patient’s symptoms may be considered to screen for thrombosis. It is worth noting that the initial brain images may be normal; if symptoms persist and laboratory data are still remarkable, imaging examinations need to be repeated[10]. In our case, the progression of the lesion was remarkable as the initial brain CT did not show any hemorrhage, whereas the brain MRI 2 h later showed hemorrhagic transformation.
VITT treatment could be divided into immune therapy and anticoagulation. IVIG is recommended to interrupt VITT antibody-induced platelet activation, which was the reason such treatment was initiated as soon as possible, even if the result of the anti-PF4 antibodies was still unknown. Plasma exchange can also be used for refractory diseases, such as multiple thrombosis with evidence of excessive platelet activation and low platelet count (< 30000/µL)[6]. Systemic steroids were also used in some patients, especially in severe cases with platelet counts of < 30000/µL[6]; in our case, systemic steroid was used to control the cerebral vasogenic edema. For anticoagulants, non-heparin-based anticoagulants such as argatroban, fondaparinux, and non-vitamin K antagonist oral anticoagulants are recommended. In our case, dabigatran was chosen for anticoagulation. However, in one study, heparin did not appear to be harmful in patients who received it[6]. Regardless, it may be reasonable to avoid heparin in cases of diagnostic uncertainty in which heparin-induced thrombocytopenia is a possible differential diagnosis. For the treatment of CVT, anticoagulant is the most important medication even if the patient had intracranial hemorrhage at baseline[11]. Endovascular intervention is indicated for patients with continued neurological deterioration despite intensive anticoagulation treatment[11,12]. Decompressive surgery is performed for patients with impending herniation and it is used for life saving[11]. In our case, we used anticoagulants even though the patient had some hemorrhagic transformations. Both endovascular intervention and decompressive surgery were not performed because her symptom improved gradually without clinical signs of brain herniation.
Several risk factors for CVT include infection, pregnancy, puerperium, trauma, medication, genetic and autoimmune disorders, and malignancy[8]. In a previous population cohort study, a higher-than-expected CVT rate was confirmed in Danish and Norwegian individuals aged 18-65 years who received the AZD1222, with ≥ 2.5 events per 100000 vaccinations[13]. Additionally, there were no identifiable medical risk factors for VITT and no gender predominance; however, it seemed more prevalent in younger age groups[6]. Our patient did not have any of the aforementioned risk factors, aside from having received the AZD1222, weakly positive anti-SSA and ANA. She had no symptoms of Sjögren’s-syndrome and systemic lupus erythematosus. She also had iron deficiency anemia, which may be related to her menorrhagia. There was no evidence of hemolysis, and the levels of total bilirubin and lactate dehydrogenase were normal. She had not taken any regular medications, such as oral contraceptives and hormone replacement therapy, which are known causes for CVT.
CVT may also be caused by genetic disorders, such as protein C, protein S, antithrombin deficiencies, factor V Leiden, and prothrombin mutations. In our case, the protein C, protein S, and antithrombin levels were normal. However, we did not check for mutations in factor V Leiden and prothrombin because of their low prevalence in Asians[14]. This is also the reason the risk of venous thromboembolism in Asians is low[15].
VITT is a rare condition with a high mortality rate. Early diagnosis and treatment are important in achieving a favorable prognosis. Clinicians should be alerted whenever a patient presents with persistent and progressive headaches or focal motor/sensory deficits after vaccination. D-dimer level and platelet count are important clues for initial screening.
We thank Jiunu-An Lin, the group leader of Laboratory Department in China Medical University Hospital for the examination of anti-PF4 antibody.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bao Y, China; Gessler F, Germany S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368:m1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 2. | Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384:2092-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1614] [Cited by in RCA: 1670] [Article Influence: 417.5] [Reference Citation Analysis (0)] |
| 3. | Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021;384:1964-1965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 329] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 4. | Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattør TH, Tjønnfjord GE, Holme PA. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 946] [Cited by in RCA: 1100] [Article Influence: 275.0] [Reference Citation Analysis (0)] |
| 5. | Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384:2202-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 753] [Article Influence: 188.3] [Reference Citation Analysis (0)] |
| 6. | Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, Rampotas A, Ambler G, Makris M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N Engl J Med. 2021;385:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 425] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 7. | Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43:3375-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16:523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Desai D, Smythe M, Sykes E. 69 The Ability of the Immucor IgG-Specific ELISA Optical Density Result to Predict SRA Positivity for the Diagnosis of Heparin-Induced Thrombocytopenia (HIT). Am J Clin Pathol. 2018;149:S30-S30. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Salih F, Schönborn L, Kohler S, Franke C, Möckel M, Dörner T, Bauknecht HC, Pille C, Graw JA, Alonso A, Pelz J, Schneider H, Bayas A, Christ M, Kuramatsu JB, Thiele T, Greinacher A, Endres M. Vaccine-Induced Thrombocytopenia with Severe Headache. N Engl J Med. 2021;385:2103-2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, Aguiar de Sousa D, Stam J; European Stroke Organization. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 398] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 12. | Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 1229] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 13. | Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PL, Watle SV, Mikkelsen AP, Pedersen L, Sørensen HT, Thomsen RW, Hviid A. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 274] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 14. | Stein PD, Matta F. Epidemiology and incidence: the scope of the problem and risk factors for development of venous thromboembolism. Clin Chest Med. 2010;31:611-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Nicole Tran H, Klatsky AL. Lower risk of venous thromboembolism in multiple Asian ethnic groups. Prev Med Rep. 2019;13:268-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |