Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9454
Peer-review started: May 5, 2022
First decision: May 31, 2022
Revised: June 4, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: September 16, 2022
Processing time: 119 Days and 17.2 Hours
Rhabdomyosarcoma is a soft tissue tumor of primitive mesenchymal cells origin, occurring predominantly in children and adolescents, but extremely rare in adults and the data regarding its treatment are sparse. Here, we would like to share our experience in the treatment of a locally advanced primary embryonal rhabdomyosarcoma of cervix in a 39-year-old female.
The patient was admitted with symptoms of intermenstrual bleeding and postcoital bleeding for six months. Physical examination revealed a friable, polyp-like mass (5 cm × 5 cm) in her cervix protruding into the vagina, while the uterus was mobile and normal-sized. Colposcopy-directed biopsy was performed, and a pathological diagnosis of embryonal rhabdomyosarcoma was made. Magnetic resonance imaging of the pelvis showed that the cervical volume was significantly increased, with a hypointense and hyperintense soft tissue mass on the right side, invading the cervical stroma; the mass was 5 cm × 5 cm with a clear boundary and confined to the cervix; there were no obvious findings indicating tumor invasion in the vaginal wall, parametrium, or pelvic wall; no enlarged lymph nodes were observed in the pelvic cavity. Based on our findings, the tumor was classified as stage IA according to the intergroup rhabdomyosarcoma studies criteria and IB3 stage according to The International Federation of Gynecology and Obstetrics 2018. The patient underwent two courses of neoadjuvant chemotherapy and a partial remission was achieved. Subsequently, she underwent laparoscopic radical hysterectomy, bilateral salpingo-oophrectomy and pelvic lymph node dissection and there were no risk factors revealed by postoperative pathological exami
Our experience suggests that neoadjuvant vincristine, dactinomycin, and cyclophosphamide chemotherapy followed by radical surgery and adjuvant chemotherapy might be reasonable therapeutic option for bulky cervical rhabdomyosarcoma in adults without fertility desire. Since large-scale studies on such rare conditions are rather impossible, further case reports and systematic reviews could help optimize the treatment of primary, bulky cervical rhabdomyosarcoma in adults.
Core Tip: Because of the extreme rarity of adult primary cervical rhabdomyosarcomas, their treatment remains challenging. Accurate diagnosis is critical for a good prognosis since the treatment of cervical rhabdomyosarcoma differs from that of other cervical tumors, particularly in radicality of surgery and chemotherapy regimens. Our experience suggests that neoadjuvant vincristine, dactinomycin, and cyclophosphamide chemotherapy followed by radical surgery and adjuvant chemotherapy might be reasonable therapeutic option for bulky cervical rhabdomyosarcoma in adults without fertility desire. Since large-scale studies on such rare conditions are rather impossible, further case reports and systematic reviews could help optimize the treatment of primary, bulky cervical rhabdomyosarcoma in adults.
- Citation: Xu LJ, Cai J, Huang BX, Dong WH. Locally advanced cervical rhabdomyosarcoma in adults: A case report. World J Clin Cases 2022; 10(26): 9454-9461
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9454.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9454
Rhabdomyosarcoma is a soft tissue tumor of primitive mesenchymal cells origin, occurring predominantly in children and adolescents, but extremely rare in adults[1,2]. Approximately 30% of rhabdomyosarcomas originate in the genitourinary system, mostly in the vagina; less than 0.5% arise in the uterine cervix. Adult patients appear to have worse prognosis, that may be partially due to their rarity and the absence of standardized treatment protocols or guidelines[3,4]. We present a case of primary, locally advanced embryonal rhabdomyosarcoma of the cervix in a 39-year-old woman with a good prognosis, sharing our experience regarding treatment.
A 39-year-old woman was admitted with symptoms of intermenstrual bleeding and postcoital bleeding.
She had intermenstrual bleeding and postcoital bleeding for six months. No medical treatment was performed.
She reported a history of two normal vaginal deliveries (G2P2A0).
Physical examination revealed a friable, polyp-like mass (5 cm × 5 cm) in her cervix protruding into the vagina, while the uterus was mobile and normal-sized.
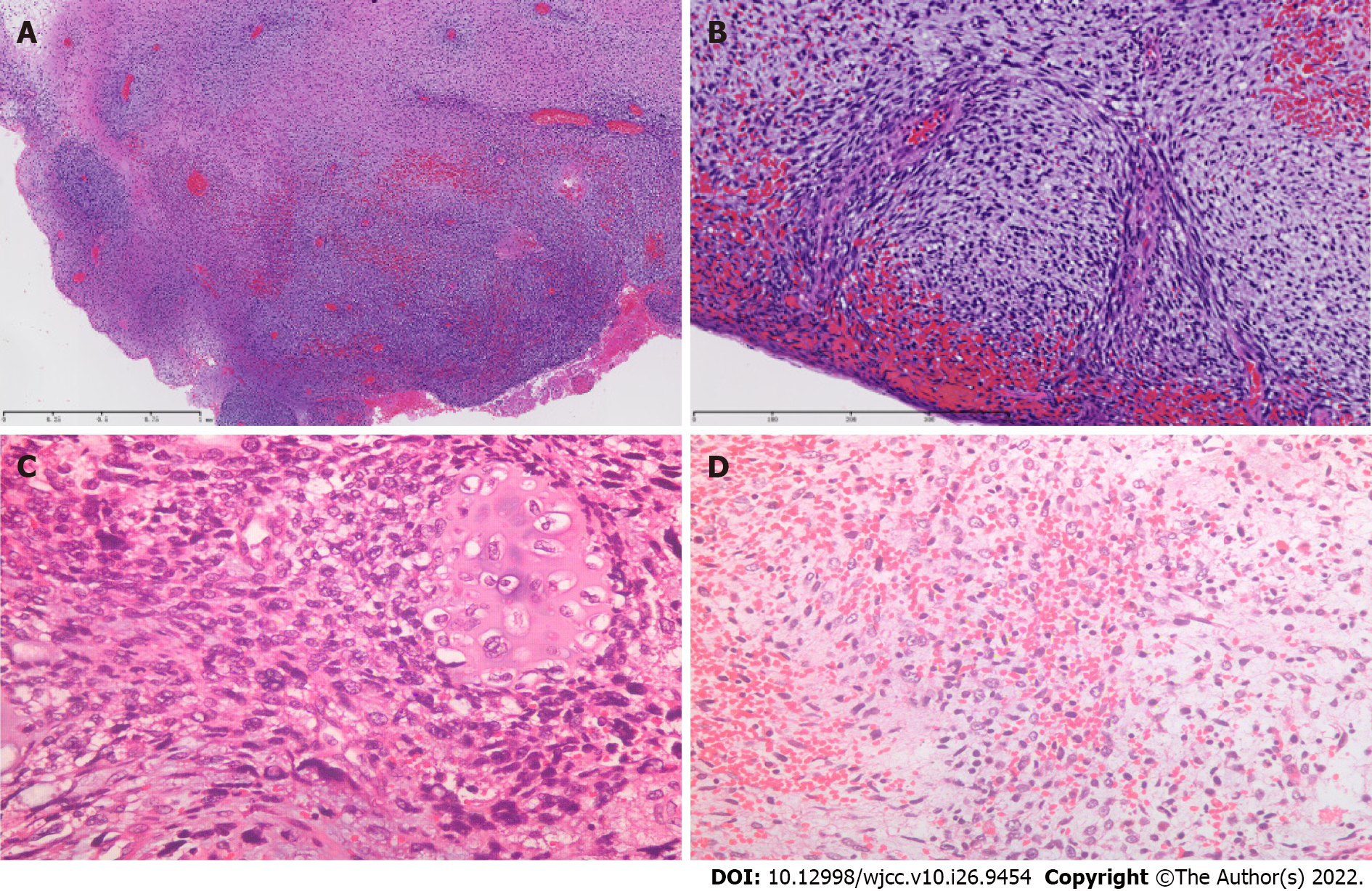
Colposcopy-directed biopsy was performed, and a pathological diagnosis of embryonal rhabdomyosarcoma was made (Figure 1). The tumor cells of embryonal rhabdomyosarcoma have various shapes, which basically reproduce the cells of various stages of the embryonic development of skeletal muscle. But spindle cell rhabdomyosarcoma is mainly composed of spindle cells, with inconspicuous or few rhabdomyoblasts. In addition, the embryonic type is more common in the genital tract, whereas spindle cell subtype is more common in the extremities.
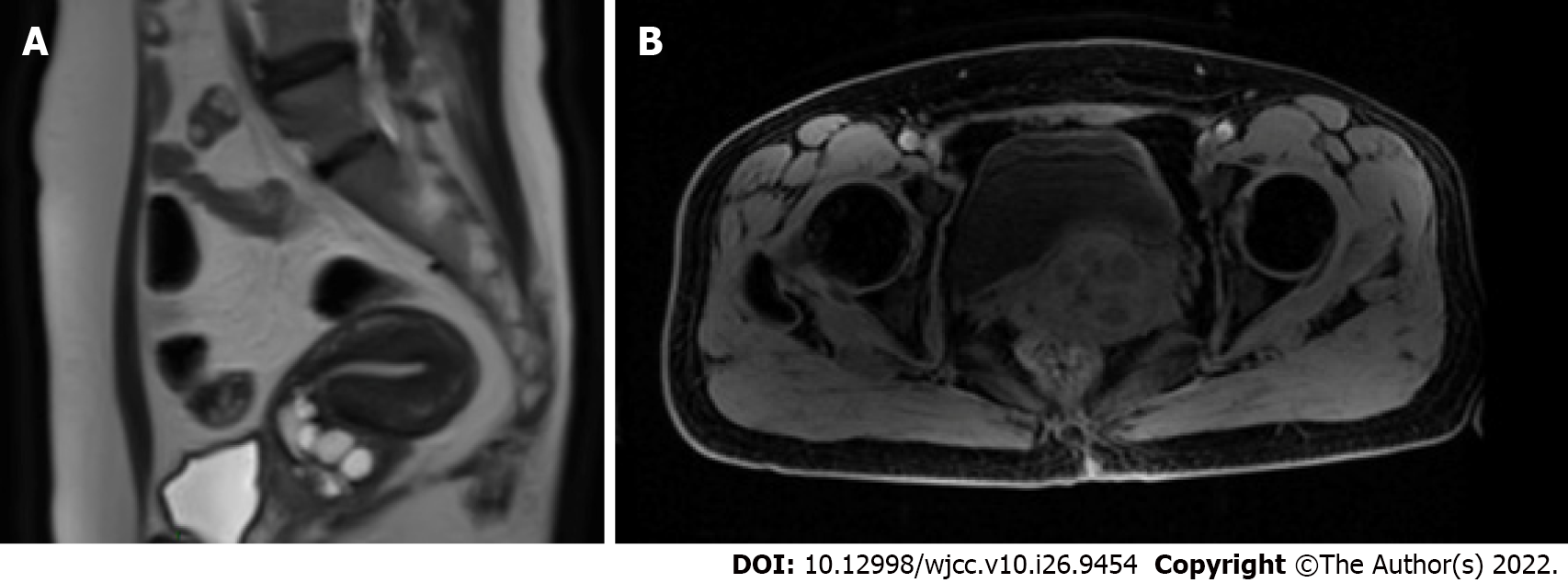
Magnetic resonance imaging (MRI) of the pelvis showed that the cervical volume was significantly increased, with a hypointense and hyperintense soft tissue mass on the right side, invading the cervical stroma; the mass was 5 cm × 5 cm with a clear boundary and confined to the cervix; there were no obvious findings indicating tumor invasion in the vaginal wall, parametrium or pelvic wall; no enlarged lymph nodes were observed in the pelvic cavity (Figure 2A). Chest radiography revealed clear lung fields. There was no abnormality in the liquid-based cytology test or high-risk human papillomavirus (HPV) test of exfoliated cervical cells. The results of blood chemistry (routine blood tests and renal and liver function tests) and serum tumor markers, including squamous cell carcinoma antigen, cancer antigen (CA) 125, CA199, and carcinoembryonic antigen, were within normal limits.
Based on these findings, the tumor was classified as stage IA according to the intergroup rhabdomyosarcoma studies (IRS) criteria and IB3 stage according to The International Federation of Gynecology and Obstetrics 2018.
Radical surgery was planned as the treatment strategy. Considering a tumor size greater than 4 cm, the patient underwent neoadjuvant chemotherapy with two cycles of vincristine, dactinomycin, and cyclophosphamide (VAC) every 3 wk before surgery. After neoadjuvant chemotherapy, the tumor size was reduced to 3 cm × 3 cm, as revealed by magnetic resonance imaging (Figure 2B). Subsequently, she underwent laparoscopic radical hysterectomy, bilateral salpingo-oophrectomy and pelvic lymph node dissection. The intraoperative blood loss volume was approximately 100 mL. There were no injuries to the major vessels or nerves, ureter, bladder, or intestinal tract. No postoperative urinary retention or other complications were observed.
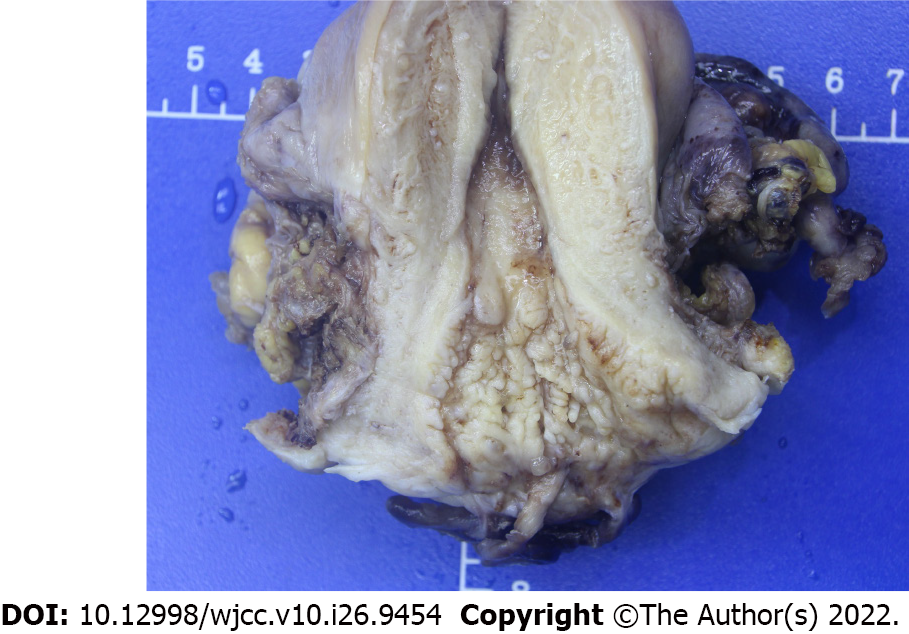
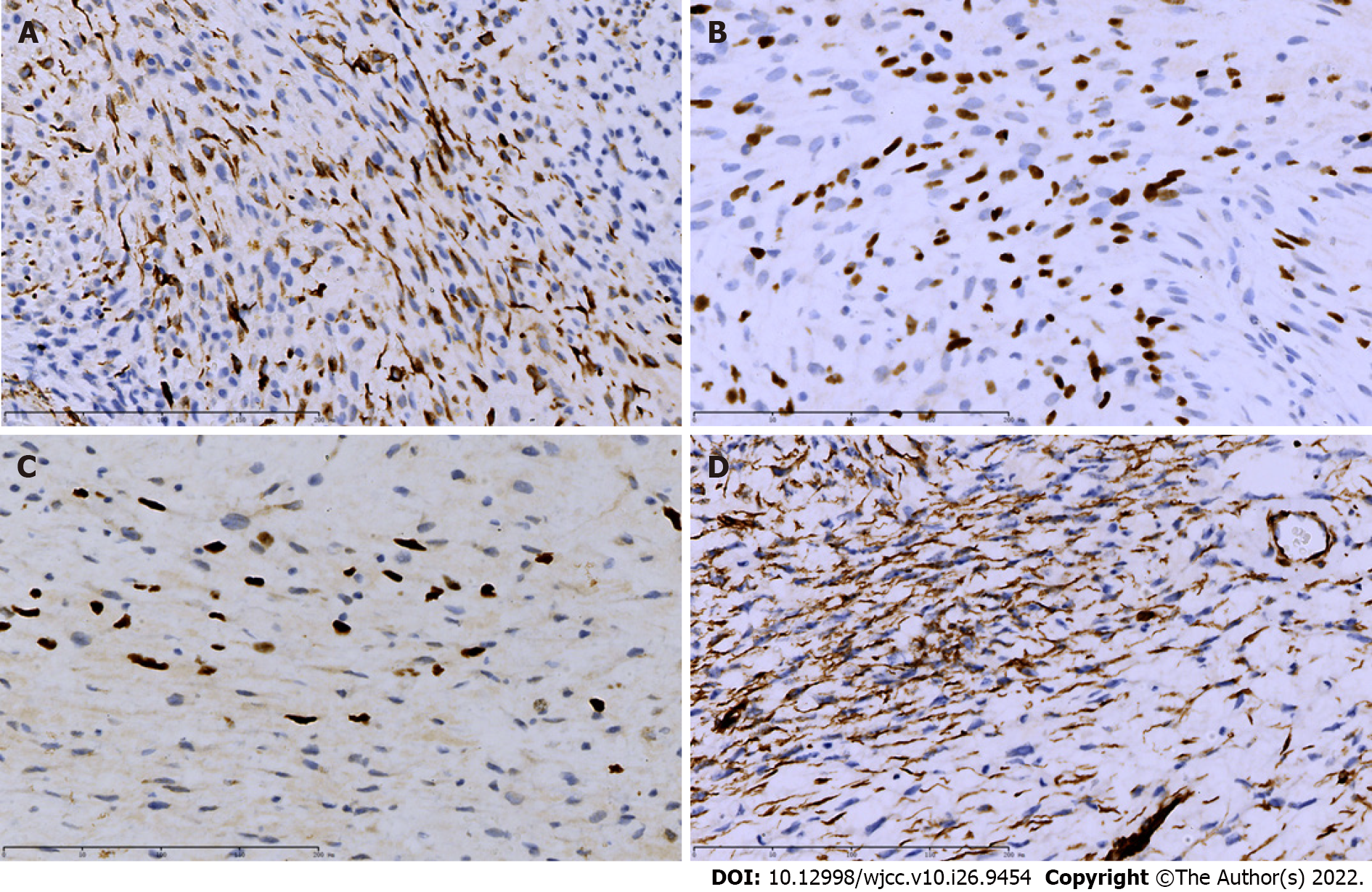
Postoperative pathological examination revealed a polyp-like cervical mass measuring 3.5 cm × 2 cm involving the posterior cervical lip and confirmed the histotype of embryonal rhabdomyosarcoma of the cervix with partial cartilage differentiation (Figure 3). The vaginal wall, parametrium, endometrium, ovaries, and fallopian tubes were tumor-free, with no invasion of the pelvic lymph nodes and lymphovascular space. The surgical margins were tumor-free. Immunohistochemistry showed that the tumor was positive for WT1 (100%), myogenin (100%), MyoD1 (focal positive), and desmin (focal positive) (Figure 4).
Following the surgery, adjuvant chemotherapy with VAC was administered for four cycles at an interval of 3 wk. The patient experienced grade I diarrhea and hematological toxicity but no grade III/IV chemotherapy-associated side effects.
The patient was then followed up regularly, every 3 mo in the first two years, every 6 mo in the third year, and once a year after the fourth year. At each visit, history taking and clinical examination were carried out to detect treatment complications, and recurrent disease. The follow-up exam included physical exam, vaginal vault cytology, chest X-ray, abdominal and pelvic computed tomography scan. The patient was disease-free until the last follow-up, 49 mo after completing the entire treatment.
According to the 2020 World Health Organization classification, rhabdomyosarcomas are subdivided into four histological types: embryonal, alveolar, pleomorphic, and spindle cell/sclerosing rhabdomyosarcoma[5]. Embryonic type is the most frequent histology, which accounts for slightly more than half of all rhabdomyosarcomas. In adults, embryonal rhabdomyosarcoma of the cervix is rare and characterized by unique pathological findings[3]. There is no standard treatment for patients with rhabdomyosarcoma of the cervix. Clinically, the primary treatment options are surgery with or without adjuvant chemotherapy and radiotherapy[6]. We present a 39-year-old woman with cervical rhabdomyosarcoma who underwent neoadjuvant chemotherapy, followed by surgery and adjuvant chemotherapy. The patient was disease-free until the last follow-up, 49 mo after completing the entire treatment.
Cervical rhabdomyosarcoma differs from cervical squamous cell carcinomas and adenocarcinomas in several aspects. The former is often located in the upper part of the cervix or vagina. It grows rapidly outward to fill the entire vagina or even protract from the vaginal opening, preferentially invading the bladder, rectum, or other pelvic organs, and occurs predominantly in children and young adults. Patients commonly complain of irregular vaginal bleeding or a mass prolapse from the vagina. In contrast, cervical squamous cell carcinomas and adenocarcinomas are predominantly located in the cervix and grow more slowly than cervical rhabdomyosarcoma with a tendency to invade the parametrium. They usually occur in adult women with a sexual history, and irregular vaginal bleeding or contact bleeding is the most common primary symptom[7,8].
The diagnosis of cervical rhabdomyosarcoma largely relies on biopsy and subsequent pathological examination with immunohistochemistry. Cervical masses often have varied appearances, such as polypoid or grape-like, and are frequently misdiagnosed as cervical polyps[9]. Immunohistochemical staining for muscle markers, such as desmin and myogenin, has been considered helpful for the diagnosis and differential diagnosis of cervical rhabdomyosarcoma[10,11]. MyoD1 and myogenin are the most commonly used positive markers with high specificity and sensitivity for rhabdomyosarcoma[12,13]. WT1 and desmin help confirm a diagnosis of cervical botryoid rhabdomyosarcoma, particularly when they are positive in a large proportion of cells[11]. As in our case, the tumor cells are positive for all four markers, while these markers are often negative in cervical squamous cell carcinoma, adenocarcinoma, or polyp. In addition, limited data indicate that cervical rhabdomyosarcoma is generally HPV-negative[12], while most cervical squamous cell carcinomas and adenocarcinomas are HPV-positive, suggesting that HPV status could serve as a marker for differential diagnosis, and HPV-based cervical cancer screening and vaccination are unlikely to prevent cervical rhabdomyosarcoma[14,15].
Most patients with primary cervical rhabdomyosarcoma were diagnosed at an early stage and did well with surgery and adjuvant chemotherapy, although the number of cases and duration of follow-up were limited[16,17]. Surgical procedures include polypectomy, loop electrosurgical excision, cervical conization, cervical excision, hysterectomy, or radical hysterectomy, which are generally less radical than those for cervical squamous cell carcinomas and adenocarcinomas because of the young age and fertility needs of the patients and less frequent involvement of the parametrium. However, a systematic pelvic lymphadenectomy appears to be critical for cervical rhabdomyosarcoma because 13.3% of patients with cervical rhabdomyosarcoma who underwent pelvic lymphadenectomy had a nodal disease[9]. As adjuvant therapy, considering the age of the patient and the long-term irreversible complications of radiation therapy caused by fibrosis of adjacent organs and tissues that can seriously impair the quality of life, chemotherapy seems to be more suitable because of improved quality of life and good responsiveness. Adjuvant chemotherapy is generally essential for the treatment of rhabdomyosarcoma[18], and the commonly used chemotherapy regimens include vincristine plus dactinomycin (VA), VAC, and VA with ifosfamide (IVA)[19,20,21], which are different from platinum-based chemotherapy for cervical cancer. Furthermore, the IRS clinical group recommends adjuvant radiation therapy[22] to treat tumors at advanced stages, such as those with nodal involvement, distant metastases, or recurrent diseases[23].
There are few reports on the treatment of bulky cervical rhabdomyosarcoma in adults. Baiocchi et al[24] presented a 47-year-old woman with a 10 cm rhabdomyosarcoma of the cervix in Group IA based on the IRS Group criteria. The patient underwent an upfront radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection. The pathology demonstrated a “grape-like” or “cauliflower-like” tumor based on the endocervix with more than 50% depth infiltration and uterine isthmus extension. Fifty lymph nodes were analyzed, and no metastases were observed. As the patient had localized disease, which was confined to the site of origin and was completely excised, she received 12 courses of adjuvant VAC chemotherapy over one year. However, detailed follow-up information was not available. Yuan et al[25] reported nine cases of stage I embryonal rhabdomyosarcoma of the female genital tract, one of which was a 36-year-old woman with a 5.7 cm rhabdomyosarcoma lesion of the cervix. She was treated with neoadjuvant chemotherapy, surgery, and adjuvant chemotherapy and was free of disease during a 9 mo follow-up period. In our study, the patient was 39 years old, with a bulky, cervix-confined tumor and no fertility needs. We performed two courses of VAC neoadjuvant chemotherapy to reduce the tumor size, increase the operability, and optimize the surgical outcome, and a radical surgery including hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection was performed laparoscopically. Pathology revealed that the tumor had invaded the inner 1/3 of the cervical muscle wall, and no clear intravascular tumor plugs were found around the tumor. The resection margins were negative; 64 lymph nodes were analyzed, and no metastases were found. After surgery, the patient received adjuvant chemotherapy. The patient was disease-free 49 mo after treatment completion, which indicated a high probability of cure. Our case, with a long follow-up period, provides valuable data on the oncologic outcome of bulky cervical rhabdomyosarcoma in adults after adequate treatment.
Because of the extreme rarity of adult primary cervical rhabdomyosarcomas, their treatment remains challenging. Accurate diagnosis is critical for a good prognosis since the treatment of cervical rhabdomyosarcoma differs from that of other cervical tumors, particularly in radicality of surgery and chemotherapy regimens. Our experience suggests that neoadjuvant VAC chemotherapy followed by radical surgery and adjuvant chemotherapy might be reasonable therapeutic option for bulky cervical rhabdomyosarcoma in adults without fertility desire. Since large-scale studies on such rare conditions are rather impossible, further case reports and systematic reviews could help optimize the treatment of primary, bulky cervical rhabdomyosarcoma in adults.
We would like to thank the Department of Obstetrics and Gynecology, Union Hospital, Wuhan, China.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Japan; Soe KK, United States S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Young JL Jr, Miller RW. Incidence of malignant tumors in U. S. children. J Pediatr. 1975;86:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 608] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Hartley AL, Birch JM, Blair V, Kelsey AM, Harris M, Jones PH. Patterns of cancer in the families of children with soft tissue sarcoma. Cancer. 1993;72:923-930. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Ferguson SE, Gerald W, Barakat RR, Chi DS, Soslow RA. Clinicopathologic features of rhabdomyosarcoma of gynecologic origin in adults. Am J Surg Pathol. 2007;31:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol. 2021;28:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 6. | Raney RB, Maurer HM, Anderson JR, Andrassy RJ, Donaldson SS, Qualman SJ, Wharam MD, Wiener ES, Crist WM. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major Lessons From the IRS-I Through IRS-IV Studies as Background for the Current IRS-V Treatment Protocols. Sarcoma. 2001;5:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143 Suppl 2:22-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 757] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 8. | Olawaiye AB, Baker TP, Washington MK, Mutch DG. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J Clin. 2021;71:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 9. | Ricciardi E, Plett H, Sangiorgio V, Paderno M, Landoni F, Aletti G, Prader S, du Bois A, Harter P, Colombo N. Adult primary cervical rhabdomyosarcomas: A Multicentric cross-national case series. Int J Gynecol Cancer. 2020;30:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | McCluggage WG, Longacre TA, Fisher C. Myogenin expression in vulvovaginal spindle cell lesions: analysis of a series of cases with an emphasis on diagnostic pitfalls. Histopathology. 2013;63:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Riedlinger WF, Kozakewich HP, Vargas SO. Myogenic markers in the evaluation of embryonal botryoid rhabdomyosarcoma of the female genital tract. Pediatr Dev Pathol. 2005;8:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Missaoui N, Mestiri S, Bdioui A, Zahmoul T, Hamchi H, Mokni M, Hmissa S. HPV infection and p16INK4A and TP53 expression in rare cancers of the uterine cervix. Pathol Res Pract. 2018;214:498-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Ditto A, Martinelli F, Carcangiu M, Solima E, de Carrillo KJ, Sanfilippo R, Haeusler E, Raspagliesi F. Embryonal rhabdomyosarcoma of the uterine cervix in adults: a case report and literature review. J Low Genit Tract Dis. 2013;17:e12-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Muñoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS, Shah KV, Meijer CJ, Bosch FX; International Agency for Research on Cancer. Multicentric Cervical Cancer Study Group. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 339] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 2779] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 16. | Bernal KL, Fahmy L, Remmenga S, Bridge J, Baker J. Embryonal rhabdomyosarcoma (sarcoma botryoides) of the cervix presenting as a cervical polyp treated with fertility-sparing surgery and adjuvant chemotherapy. Gynecol Oncol. 2004;95:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Stankovic ZB, Djuricić S, Stanković DS, Zdravković S, Gazikalović S, Sedlecki K. Minimal invasive treatment of cervical rhabdomyosarcoma in an adolescent girl. J BUON. 2007;12:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Raney RB, Anderson JR, Barr FG, Donaldson SS, Pappo AS, Qualman SJ, Wiener ES, Maurer HM, Crist WM. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001;23:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649-4655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 20. | Ogilvie CM, Crawford EA, Slotcavage RL, King JJ, Lackman RD, Hartner L, Staddon AP. Treatment of adult rhabdomyosarcoma. Am J Clin Oncol. 2010;33:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Baker KS, Anderson JR, Link MP, Grier HE, Qualman SJ, Maurer HM, Breneman JC, Wiener ES, Crist WM. Benefit of intensified therapy for patients with local or regional embryonal rhabdomyosarcoma: results from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2000;18:2427-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Arndt CA, Stoner JA, Hawkins DS, Rodeberg DA, Hayes-Jordan AA, Paidas CN, Parham DM, Teot LA, Wharam MD, Breneman JC, Donaldson SS, Anderson JR, Meyer WH. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803. J Clin Oncol. 2009;27:5182-5188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 23. | Walterhouse D, Watson A. Optimal management strategies for rhabdomyosarcoma in children. Paediatr Drugs. 2007;9:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Baiocchi G, Faloppa CC, Osório CA, Kumagai LY, Fukazawa EM, Cunha IW. Embryonal rhabdomyosarcoma of the uterine cervix in a 47-year-old woman. J Obstet Gynaecol Res. 2011;37:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Yuan G, Yao H, Li X, Li H, Wu L. Stage 1 embryonal rhabdomyosarcoma of the female genital tract: a retrospective clinical study of nine cases. World J Surg Oncol. 2017;15:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |