Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9447
Peer-review started: April 23, 2022
First decision: June 16, 2022
Revised: July 6, 2022
Accepted: August 11, 2022
Article in press: August 11, 2022
Published online: September 16, 2022
Processing time: 132 Days and 3.9 Hours
Ascites, pleural effusion and raised CA-125 in the absence of malignancy in systemic lupus erythematosus is known as Tjalma syndrome.
We report a special case of a systemic lupus erythematosus patient presenting with Tjalma syndrome. She presented with ascites and elevated CA-125 in the absence of benign or malignant ovarian tumor and no pleural effusions, which is an unusual presentation for this rare condition.
Tjalma syndrome can present with massive ascites alone without pleural or pericardial effusions.
Core Tip: We report a special case of a systemic lupus erythematosus patient presenting with pseudo- pseudo Meigs’ syndrome. She presented with ascites and elevated CA-125 in the absence of benign or malignant ovarian tumor and no pleural effusions, which is an unusual presentation for this rare condition: Tjalma syndrome can present with massive ascites alone without pleural or pericardial effusions.
- Citation: Wang JD, Yang YF, Zhang XF, Huang J. Systemic lupus erythematosus presenting with progressive massive ascites and CA-125 elevation indicating Tjalma syndrome? A case report. World J Clin Cases 2022; 10(26): 9447-9453
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9447.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9447
Systemic lupus erythematosus (SLE) is a chronic, autoimmune disease with multiple systemic disorders. Tjalma syndrome, also known as pseudo–pseudo Meigs’ syndrome, is a clinical manifestation of SLE that is characterized by ascites, pleural effusions and elevated CA-125 in the absence of benign or malignant ovarian tumor[1]. Massive ascites are rare in SLE patients without any other complications. Herein we report a special case of an SLE patient presenting with Tjalma syndrome. She presented with ascites and elevated CA-125 but no pleural effusions.
A 23-year-old woman presented with nausea, vomiting and distention for 2 wk without abdominal pain, diarrhea, rashes or arthralgia.
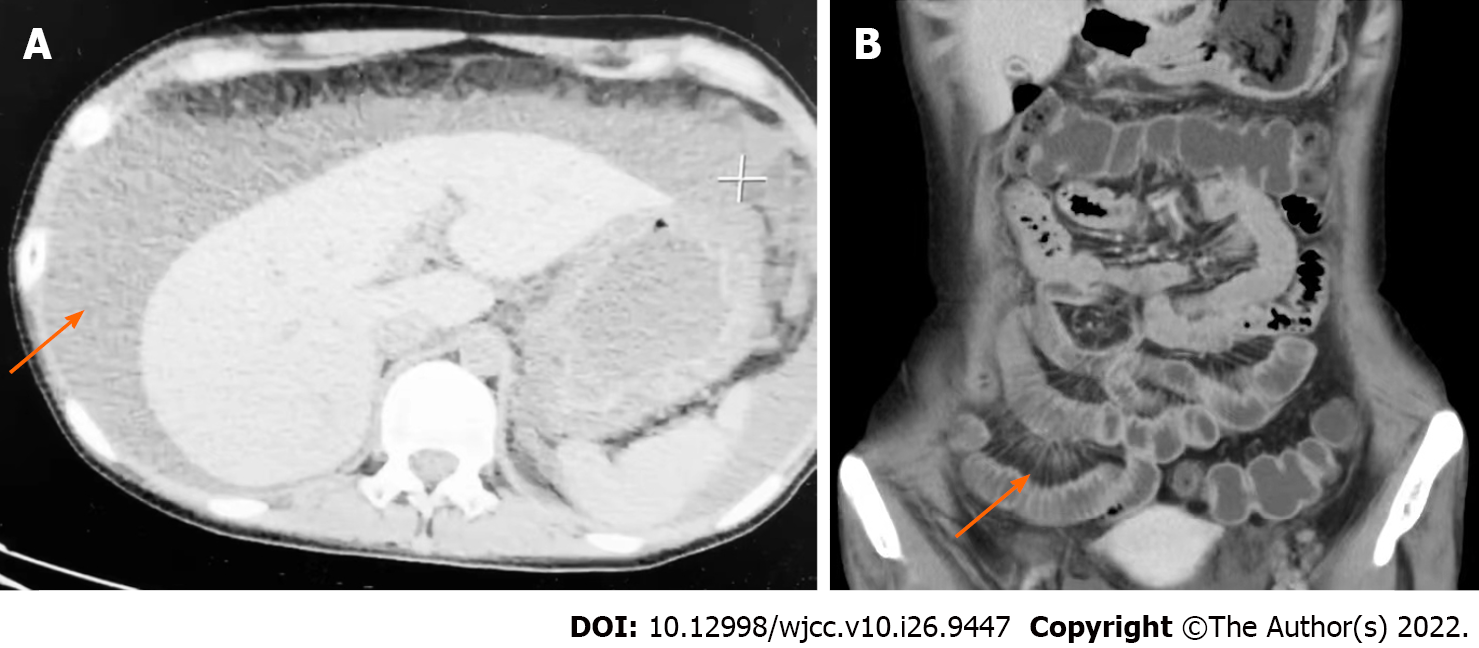
The patient had presented herself to an outside hospital 10 d ago where physical examination revealed a distended abdominal wall, while abdominal computed tomography scan revealed massive ascites (Figure 1A). Laboratory examinations at the outside hospital showed markedly elevated CA-125 at 1685 U/mL (0-35 U/mL). Ascitic fluid analyses revealed negative results from Rivalta tests. After diuresis treatment for 7 d, the amount of ascites in the patient was gradually reduced. However, there were no changes in nausea or vomiting.
The patient had a history of immune thrombocytopenia for 2 years and was administered with a long-term maintenance dose of 5 mg/d prednisone.
The patient denied any family history.
Patient temperature and blood pressure were 37.2 ºC and 123/82 mmHg, respectively, whereas her heart and respiratory rates were 89 beats/min and 20 breaths/min, respectively. No rales were heard in lung auscultation, and her heart beat was regular without murmurs. Her abdomen was distended, shifting dullness was positive, and neither her liver nor spleen were palpable. Physical examination of other parameters did not reveal any abnormalities.
The following is the patient’s laboratory examination results: White blood cell count, 6.8 × 109/L; neutrophil%, 77.9%; hemoglobin, 100 g/L; platelet, 130 × 109/L; total protein, 58 g/L; albumin, 31.6 g/L; d-dimer, 1910 μg/L; and ferritin, 37.7 μg/L. The 24-h urine protein quantitate was 74 mg/24 h. Antinuclear antibody 1:100 (+), Anti-Sjogren’s syndrome A antigen antibody (+), C3 0.46 g/L (0.79–1.52) and C4 0.11 g/L (0.12–0.36). Lymphocyte subset findings were: Total lymphocytes, 500 × 106/L; T-cell lymphocytes (CD3), 226 × 106/L; B-cell lymphocytes (CD19), 265.9 × 106/L; T-helper lymphocytes (CD4), 54.4 × 106/L; natural killer cells (CD16/56), 5.6 × 106/L; and CD4/CD8, 0.71. In addition, the tumor marker (CA-125) was 439.9 U/mL, whereas other tumor markers, including AFP, CEA, NSE, CA153, CA199 and β-HCG were normal. Moreover, erythrocyte sedimentation rate, C-reactive protein, ANCA, index of autoimmune liver diseases, immunoglobulin G4, hepatitis B surface antigen and HIV were all found to be within normal ranges. T-SPOT showed negative results.
Small bowel enhanced computed tomography revealed a swollen gastric wall. Small bowel wall and colon wall were slightly thickened with abnormal bowel enhancement. The number of mesenteric vessels was increased, and mesenteric vessels were engorged exhibiting a “comb sign” appearance (Figure 1B). Enhanced magnetic resonance imaging scans of the pelvic tumor showed bilateral ovaries with enlarged multifocal cystic lesions; thus, endometriotic cysts were considered. Abdominal ultrasound showed abdominal effusions, while portal ultrasound observations were normal. Ultrasonic examinations did not reveal any pleural or pericardial effusions. Gastrointestinal endoscopy revealed diffuse edema of gastric and colon walls.
Tjalma syndrome, protein-losing enteropathy and lupus cystitis.
The patient was treated with 20 mg intravenous methylprednisolone and 0.2 g hydroxychloroquine per day.
There was a subsequent improvement in nausea and vomiting during her hospital stay while her ascites were reduced. However, she later presented with violent vomiting, and 7 d after admission, she was vomiting moderate amounts of a coffee-like liquid. Then, the patient started presenting with yellow watery diarrhea. Ultrasonographic examinations and abdominal computed tomography scans showed bilateral hydronephrosis and hydroureter in addition to bladder wall thickening and small abdominal effusions. The fecal occult blood test was positive, and stool cultures revealed an infection of Clostridium difficile. Tests for Clostridium difficile toxins A and B were positive. Then, she was treated with 80 mg intravenous methylprednisolone twice daily and oral vancomycin for 10 d, which resulted in symptomatic improvement and the absence of any pathogens from her repeat stool microbiological investigations. Prior to discharge, her renal ultrasound was normal and CA-125 was 21.8 U/mL. The patient was discharged from the hospital with 12 mg oral prednisolone and 0.2 g hydroxychloroquine. At follow up 1 mo later, there was no vomiting or diarrhea.
CA-125 is a biomarker for gynecological malignancy. Clinically, CA-125 can be elevated by various benign diseases. Elevated CA-125 levels in SLE patients are attributed to mesothelial cell activation. In SLE patients, elevated serum CA-125 levels are independently associated with serositis[2,3]. Pleural and pericardial effusions are common among SLE patients. However, massive ascites are rare in SLE patients without any other complications[4]. Ascites in SLE are attributed to nephrotic syndrome, constrictive pericarditis, lupus peritonitis, protein-losing enteropathy or Budd–Chiari syndrome. A rapid onset of massive ascites can be an initial manifestation of SLE[5].
Our patient presented with painless massive ascites coexisting with low complement and hypoproteinemia. However, she did not show any overt proteinuria, and heart ultrasound as well as hepatic hilum ultrasound were normal. Therefore, lupus peritonitis, nephrotic syndrome, constrictive pericarditis and Budd–Chiari syndrome were ruled out. We postulated that hypoproteinemia was due to protein-losing enteropathy, resulting in intestinal damage caused by SLE (diarrhea, bowel wall edema and mesenteric vasculitis), consistent with previous studies[6,7]. However, 99m-labeled human serum albumin is required for definite diagnosis[8], which is not available at our hospital.
Lupus cystitis is a rare complication of SLE that generally presents with lower urinary tract symptoms and gastrointestinal symptoms, such as vomiting, nausea and abdominal pain[9,10]. Ultrasonographic examination of the patient showed bilateral hydronephrosis and hydroureter in addition to bladder wall thickening, which conforms to manifestations of lupus cystitis. Yuan et al[11] reported that lupus mesenteric vasculitis and lupus cystitis concurrently occurred in 22.7% of patients, thus lupus cystitis should be suspected in SLE patients, especially those with lower urinary tract and gastrointestinal symptoms.
We summarized the clinical features of previous 20 cases of Tjalma syndrome and current cases (Table 1). All patients were female, and their mean age was 36.5 ± 10.7 (mean ± SD) years. A decrease in serum C3 and C4 levels was reported in all Tjalma syndrome patients, which was attributed to complement consumption caused by complement system activation[12]. The patient was clinically diagnosed with SLE with elevated CA-125, but there were no benign or malignant tumors. A review of previous studies revealed ascites and pleural effusions in all cases, but only 10 patients presented with pericardial effusions. Although there were no pleural effusions, just as pericardial effusions were not found in some previous cases, the clinical features of this case fit the Tjalma syndrome, which can be a specific finding. Tjalma syndrome can present with massive ascites alone without pleural or pericardial effusions, which requires further clinical attention. Generally, Tjalma syndrome has good prognostic outcomes after administration of methylprednisolone and immunosuppressants, with resolution of ascites and pleural effusions and normalization of CA-125.
| Year | Ref. | Gender | Age | Naive SLE | CA1251
| Ascites | Pleural effusion | Pericardial effusion | Nausea/ | Dyspnea | Hypopro | Prote | ANA | dsDNA | SSA | Low complement | Leuko | Ane | Thrombocy | APS | Treatment | Outcome |
| 2005 | Tjalma[13] | Female | 38 | Yes | 887 | + | + | + | + | + | + | + | MP + AZA | Remission | ||||||||
| 2005 | Schmitt et al[1] | Female | 33 | Yes | 2287 | + | + | + | + | + | + | + | + | + | + | + | + | MP + MMF + HCQ | Remission | |||
| 2008 | Ural et al[14] | Female | 38 | Yes | 1229 | + | + | + | + | + | + | + | MP + HCQ | Remission | ||||||||
| 2011 | Bes and Soy[15] | Female | 47 | Yes | 233 | + | + | + | + | + | + | + | + | + | + | MP + HCQ | Remission | |||||
| 2012 | Dalvi et al[3] | Female | 56 | No | 70.1 | + | + | + | + | + | + | + | MP + MMF | Remission | ||||||||
| 2013 | Bes et al[16] | Female | 42 | Yes | 91.3 | + | + | + | + | + | + | + | + | + | + | + | MP + CYC + AZA | Remission | ||||
| 2013 | Lee et al[17] | Female | 29 | Yes | 345 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | MP + HCQ | Remission | |
| 2013 | Lee et al[17] | Female | 54 | No | 344.9 | + | + | + | + | + | + | + | + | + | + | MP + MMF | Remission | |||||
| 2016 | Cheah et al[7] | Female | 34 | No | 1613.8 | + | + | + | + | + | + | MP + HCQ | Remission | |||||||||
| 2016 | McVorran et al[18] | Female | 40 | Yes | 307 | + | + | + | + | + | + | + | MP | Remission | ||||||||
| 2019 | Torres Jiménez et al[19] | Female | 14 | Yes | 59 | + | + | + | + | + | + | + | + | + | + | MP + CYC + MMF + RTX | Remission | |||||
| 2018 | Zampeli et al[20] | Female | 40 | No | 85 | + | + | + | + | + | + | + | + | + | + | + | MP + CYC + MMF | Remission | ||||
| 2019 | Awad et al[21] | Female | 43 | No | 80 | + | + | + | + | + | + | + | + | MP + MMF + HCQ | Remission | |||||||
| 2019 | Tansir et al[22] | Female | 22 | Yes | 2025 | + | + | + | + | + | + | + | + | + | + | + | MP + CYC + HCQ + AZA | Remission | ||||
| 2019 | Li et al[23] | Female | 24 | No | 949 | + | + | + | + | + | + | + | + | MP + MMF | Remission | |||||||
| 2019 | Ahmed et al[24] | Female | 44 | Yes | 227 | + | + | + | + | + | + | + | + | MP + AZA | Remission | |||||||
| 2019 | Gao et al[6] | Female | 44 | Yes | 360.8 | + | + | + | + | + | + | + | + | + | + | + | MP + HCQ + LEF | Remission | ||||
| 2021 | Quintero-Muñoz et al[25] | Female | 33 | No | 187 | + | + | + | + | + | + | + | + | + | + | + | + | MP + MMF + HCQ + CYC | Death | |||
| 2021 | Meena et al[26] | Female | 23 | No | 230.5 | + | + | + | + | + | + | + | + | + | MP + HCQ + AZA | Remission | ||||||
| 2022 | Karadeniz et al[27] | Female | 33 | No | 476 | + | + | + | + | + | + | + | + | + | + | MP + MMF + HCQ | Remission | |||||
| 2022 | Current case | Female | 23 | Yes | 1685 | + | + | + | + | + | + | + | MP + HCQ | Remission |
In conclusion, massive ascites with increased CA-125 do not always indicate the presence of malignancy, especially in patients with SLE. Although rare, Tjalma syndrome has been increasingly reported in recent years. Therefore, there is a need for increased awareness of this condition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Gupta T, India; Tanaka H, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Schmitt R, Weichert W, Schneider W, Luft FC, Kettritz R. Pseudo-pseudo Meigs' syndrome. Lancet. 2005;366:1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Yang Z, Liang Y, Li C, Zhong R. Serum CA125 elevation is independently associated with serositis in SLE patients. Clin Exp Rheumatol. 2012;30:93-98. [PubMed] |
| 3. | Dalvi SR, Yildirim R, Santoriello D, Belmont HM. Pseudo-pseudo Meigs' syndrome in a patient with systemic lupus erythematosus. Lupus. 2012;21:1463-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Weinstein PJ, Noyer CM. Rapid onset of massive ascites as the initial presentation of systemic lupus erythematosus. Am J Gastroenterol. 2000;95:302-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Forouhar-Graff H, Dennis-Yawingu K, Parke A. Insidious onset of massive painless ascites as initial manifestation of systemic lupus erythematosus. Lupus. 2011;20:754-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Gao F, Xu Y, Yang G. Pseudo-pseudo Meigs' syndrome presenting with a combination of polyserositis, elevated serum CA 125 in systemic lupus erythematosus: A case report. Medicine (Baltimore). 2019;98:e15393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Cheah CK, Ramanujam S, Mohd Noor N, Gandhi C, D Souza BA, Gun SC. A case of mixed connective tissue disease with pseudo-pseudo Meigs' syndrome (PPMS)-like features. Lupus. 2016;25:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Hung JC, Gadient KR, Mahoney DW, Murray JA. In-house preparation of technetium 99m-labeled human serum albumin for evaluation of protein-losing gastroenteropathy. J Am Pharm Assoc (Wash). 2002;42:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Aziza Bawazier L. Asymptomatic Lupus Cystitis with Bilateral Hydronephrosis. Case Rep Nephrol Dial. 2018;8:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Liberski S, Marczak D, Mazur E, Miętkiewicz K, Leis K, Gałązka P. Systemic lupus erythematosus of the urinary tract: focus on lupus cystitis. Reumatologia. 2018;56:255-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yuan S, Ye Y, Chen D, Qiu Q, Zhan Z, Lian F, Li H, Liang L, Xu H, Yang X. Lupus mesenteric vasculitis: clinical features and associated factors for the recurrence and prognosis of disease. Semin Arthritis Rheum. 2014;43:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Li H, Lin S, Yang S, Chen L, Zheng X. Diagnostic value of serum complement C3 and C4 Levels in Chinese patients with systemic lupus erythematosus. Clin Rheumatol. 2015;34:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Tjalma WA. Ascites, pleural effusion, and CA 125 elevation in an SLE patient, either a Tjalma syndrome or, due to the migrated Filshie clips, a pseudo-Meigs syndrome. Gynecol Oncol. 2005;97:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Ural UM, Kiliç A, Güngör T, Ozdal B, Mollamahmutoğlu L. Tjalma's or pseudo-pseudo-Meigs' syndrome: a case report. Clin Exp Dermatol. 2008;33:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Bes C, Soy M. Pseudo-pseudo Meigs syndrome developed under the leflunomide therapy. Rheumatol Int. 2011;31:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Bes C, Dağlı Ü, Memedoğlu P, Soy M. A rare form of SLE: pseudo-pseudo meigs syndrome and hydrocephalus. Rheumatol Int. 2013;33:2175-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Lee SY, Lee SW, Chung WT. Severe inflammation may be caused by hyperferritinemia of pseudo-pseudo Meigs' syndrome in lupus patients: two cases reports and a literature review. Clin Rheumatol. 2013;32:1823-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | McVorran S, Song J, Pochineni V, Abrudescu-Opran A. Systemic Lupus Erythematosus Presenting with Massive Ascites: A Case of Pseudo-Pseudo Meigs Syndrome. Case Rep Rheumatol. 2016;2016:8701763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Torres Jiménez AR, Solís-Vallejo E, Céspedes-Cruz AI, Zeferino Cruz M, Rojas-Curiel EZ, Sánchez-Jara B. Tjalma syndrome (pseudo-pseudo Meigs') as initial manifestation of juvenile-onset systemic lupus erythematosus. Reumatol Clin (Engl Ed). 2019;15:e41-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Zampeli E, Skopouli FN, Moutsopoulos HM. Polyserositis in a Patient with Active Systemic Lupus Erythematosus: A Case of Pseudo-pseudo Meigs Syndrome. J Rheumatol. 2018;45:877-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Awad A, Essam M, Ezzat A, El Menyawi M. Systemic Lupus Erythematosus With Lupus Nephritis Presented With Recurrent Massive Ascites: A Case of Pseudo-Pseudo Meigs Syndrome. Arch Rheumatol. 2019;34:243-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tansir G, Kumar P, Pius A, Sunny SK, Soneja M. Pseudo-pseudo Meigs' syndrome: a rare presentation of systemic lupus erythematosus. Reumatismo. 2019;71:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Li T, Xie QB. A case report of pseudo-pseudo Meigs' syndrome. Chin Med J (Engl). 2019;132:1497-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ahmed O, Malley T, Kitchen J. A case of pseudo-pseudo Meigs' syndrome. Oxf Med Case Reports. 2019;2019:omy136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Quintero-Muñoz E, Gómez Pineda MA, Araque Parra C, Vallejo Castillo CA, Ortega Marrugo V, Bonilla Jassir J, Polo Nieto JF, Parra-Medina R, Rojas-Villarraga A. Is there any relationship between massive ascites and elevated CA-125 in systemic lupus erythematosus? Mod Rheumatol Case Rep. 2021;5:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Meena DS, Kumar B, Gopalakrishnan M, Kachhwaha A, Kumar S, Sureka B, Gupta S, Bohra GK, Garg MK. Pseudo-pseudo Meigs' syndrome (PPMS) in chronic lupus peritonitis: a case report with review of literature. Mod Rheumatol Case Rep. 2021;5:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Karadeniz O, Bahat PY, Koyan Karadeniz GN, Yaman İ, Palalıoglu RM. Pseudo-pseudo Meig's syndrome presenting as an acute surgical abdomen: A rare entity and review of the literature. J Obstet Gynaecol Res. 2022;48:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |