Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9440
Peer-review started: April 20, 2022
First decision: June 16, 2022
Revised: July 4, 2022
Accepted: August 16, 2022
Article in press: August 16, 2022
Published online: September 16, 2022
Processing time: 135 Days and 0.8 Hours
Immunoglobin G4 (IgG4)-related hypophysitis (IgG4-RH) is a rare form of IgG4-related disease (IgG4-RD), which often manifests as a single organ disease and is easily misdiagnosed as a pituitary tumor clinically and by imaging. There are few reports of imaging findings of IgG4-RH. Therefore, we describe a case of IgG4-RH, which mimicked a pituitary macroadenoma, that was detected by computed tomography (CT) and magnetic resonance imaging (MRI), and review the previous literature in order to further the understanding of IgG4-RH.
A 47-year-old man presented with a history of blurred vision for more than 2 mo, without other symptoms. A preoperative unenhanced CT scan revealed a slightly hyperdense mass in the sellar region measuring 2.5 cm × 2.3 cm × 1.8 cm, with a CT value of 45 HU. T1-weighted imaging (T1WI) and T2-weighted imaging showed iso-hypointensity, and gadolinium contrast-enhanced T1WI showed obvious homogeneous enhancement. The MRI revealed involvement of the pituitary gland and stalk. Preoperative laboratory tests revealed abnormal pituitary hormone levels, including an increased prolactin level, and decreased levels of insulin-like growth factor, dehydroepiandrosterone, and testosterone. The lesion was surgically resected. Postoperative histopathological examination of a tissue sample and an elevated serum IgG4 level confirmed the diagnosis of IgG4-RH. The patient was treated with cortisone acetate postoperatively and made a good recovery without developing any neurological deficit.
An elevated serum IgG4 concentration is the main clue for diagnosis of IgG4-RD. Imaging combined with laboratory testing is useful for preoperative diagnosis of IgG4-RH.
Core Tip: Immunoglobin G4 (IgG4)-related hypophysitis (IgG4-RH) is a rare form of IgG4-related disease, which is easily misdiagnosed as a pituitary tumor. IgG4-RH commonly occurs in middle-aged and older men and is characterized by hypointensity on T2-weighted imaging and homogeneous and obvious enhancement on gadolinium contrast-enhanced T1-weighted imaging, accompanied by hypopituitarism. The imaging findings help to differentiate it from pituitary tumors. An elevated serum IgG4 level is the main clue to the diagnosis of IgG4-RH, and imaging, histopathology, or response to glucocorticoid therapy can be used to confirm the diagnosis.
- Citation: Lv K, Cao X, Geng DY, Zhang J. Imaging findings of immunoglobin G4-related hypophysitis: A case report. World J Clin Cases 2022; 10(26): 9440-9446
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9440.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9440
The most common immunoglobin G4 (IgG4)-related disease (IgG4-RD) is type I autoimmune pancreatitis, followed by head and neck lesions that usually involve the salivary gland, orbit, or thyroid. IgG4-RD of the central nervous system (CNS) is rare. IgG4-RD usually involves multiple systems, but can affect the CNS alone[1,2]. When IgG4-RD involves the CNS, it usually involves the pituitary gland and pituitary stalk, collectively referred to as IgG4-related hypophysitis (IgG4-RH)[3]. Although hypertrophic dura mater meningitis and hypophysitis are recognized as relatively common manifestations of involved CNS, there are few reports of imaging findings of IgG4-RH, making the noninvasive, definitive diagnosis more difficult. Although cases of intracranial IgG4-RD have been reported[4,5], reports of IgG4-RH are relatively rare, though there have been a few reports of head and neck IgG4-RD. IgG4-RH occurs predominantly in adults aged over 60 years (average age: 62 years), with a male preponderance (male:female ratio approximately 3.6:1)[6], and is easily misdiagnosed as a pituitary tumor, lymphoid tissue hyperplasia, or other types of hypophysitis (such as lymphocytic, granulomatous, and immune checkpoint inhibitor-related hypophysitis)[7]. It is important to differentiate IgG4-RH from these conditions because their treatment and prognosis are different. Imaging examination is an important method for noninvasive diagnosis, and magnetic resonance imaging (MRI) is the gold standard method for the diagnosis of pituitary diseases. Therefore, it is necessary to supplement knowledge of the imaging characteristics of IgG4-RH. We herein describe a case of IgG4-RH mimicking a pituitary macroadenoma that was detected by computed tomography (CT) and MRI, and review the previous literature.
In July 2021, a 47-year-old man presented with a history of blurred vision for more than 2 mo.
The patient had developed blurred vision more than 2 mo previously without any obvious precipitating factor, and had subsequently experienced progressive worsening of the symptoms. He did not report any dizziness, headache, nausea, vomiting, limb weakness, loss of consciousness, facial changes, or sexual dysfunction. His mood was normal and he reported a healthy appetite, normal sleeping pattern, normal bowel movements, and no weight loss.
The patient did not have any past illnesses of note.
The patient had no special personal and family history.
Physical examination revealed decreased visual acuity, without any other abnormal neurological signs.
Preoperative laboratory examination showed an increased prolactin level (49.96 ng/mL; normal range: 3.86–22.80 ng/mL); decreased levels of insulin-like growth factor: 25.3 μg/L; normal range: 94.0–284.0 μg/L), dehydroepiandrosterone (DHEA: 0.1 μmol/L; normal range: 1.91–13.40 μmol/L), and testosterone (0.09 μmol/L; normal range: 9.90–27.8 μmol/L); and levels of thyroid-stimulating hormone (TSH), growth hormone, and adrenocorticotropic hormone within the normal range. Postoperative laboratory tests revealed a decrease in the prolactin level (20.86 ng/mL) to within the normal range, and decreased levels of TSH (0.16 mLU/L; normal range: 0.550–4.780 mLU/L), DHEA (0.03 μmol/L), and testosterone (0.09 μmol/L). A serological IgG4 examination was subsequently performed. The serum IgG4 level was elevated (2.98 g/L; normal range: 0.03–2.01 g/L).
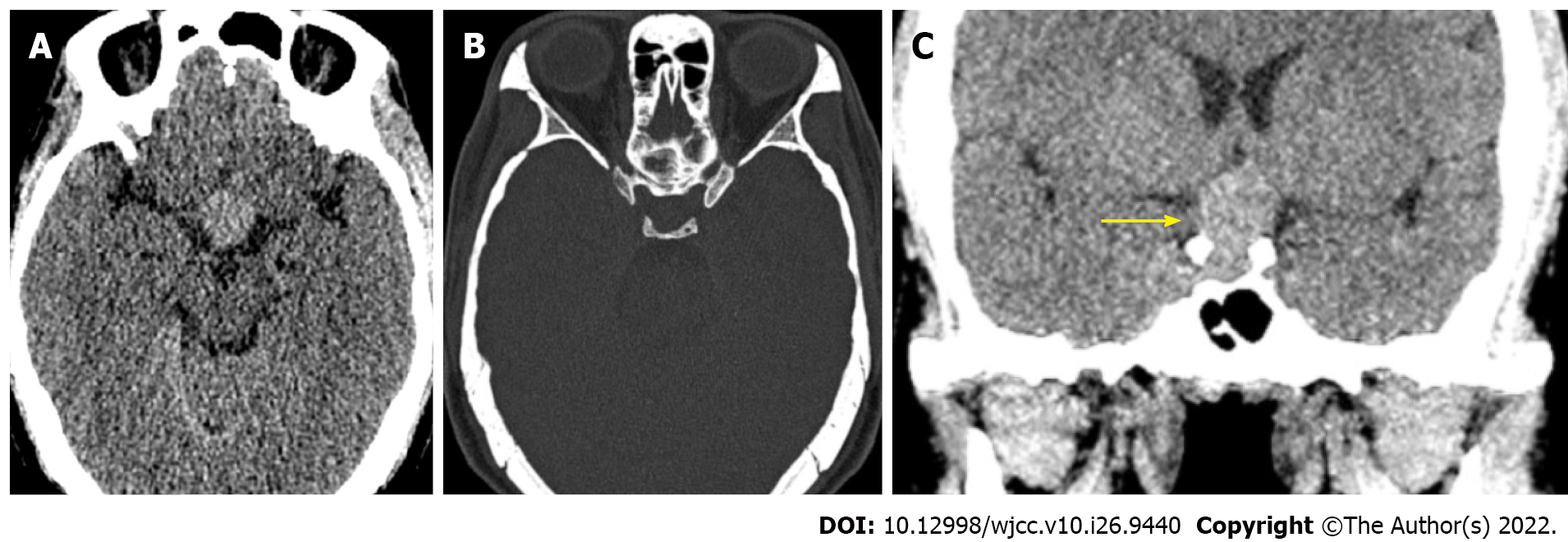
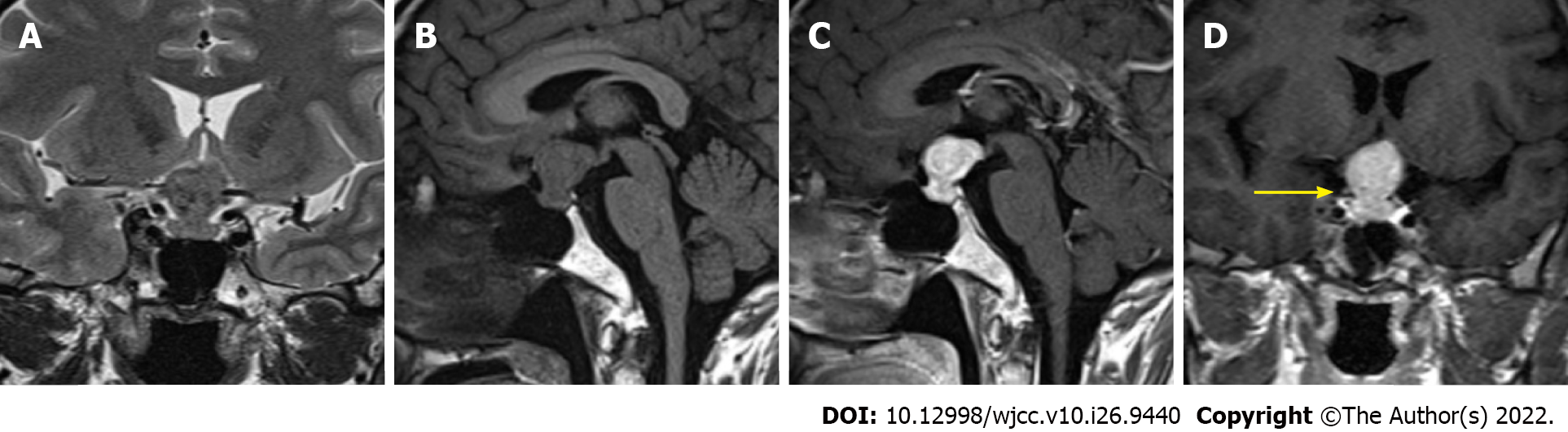
An unenhanced CT scan revealed a slightly hyperdense mass in the sellar region measuring 2.5 cm × 2.3 cm × 1.8 cm with a CT value of 45 HU (Figure 1). This was followed by an MRI scan (Figure 2). T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) revealed iso-hypointensity with an hourglass-shaped mass (“hourglass sign”), and gadolinium contrast-enhanced T1WI showed obvious homogeneous enhancement, without hyperintensity of the posterior lobe of the pituitary gland. The MRI findings indicated involvement of the pituitary gland and stalk. The mass was protruding upward and compressing the optic chiasm.
Based on the above findings, the patient was diagnosed with IgG4-RH.
The pituitary lesion was resected, and the patient was treated with cortisone acetate postoperatively.
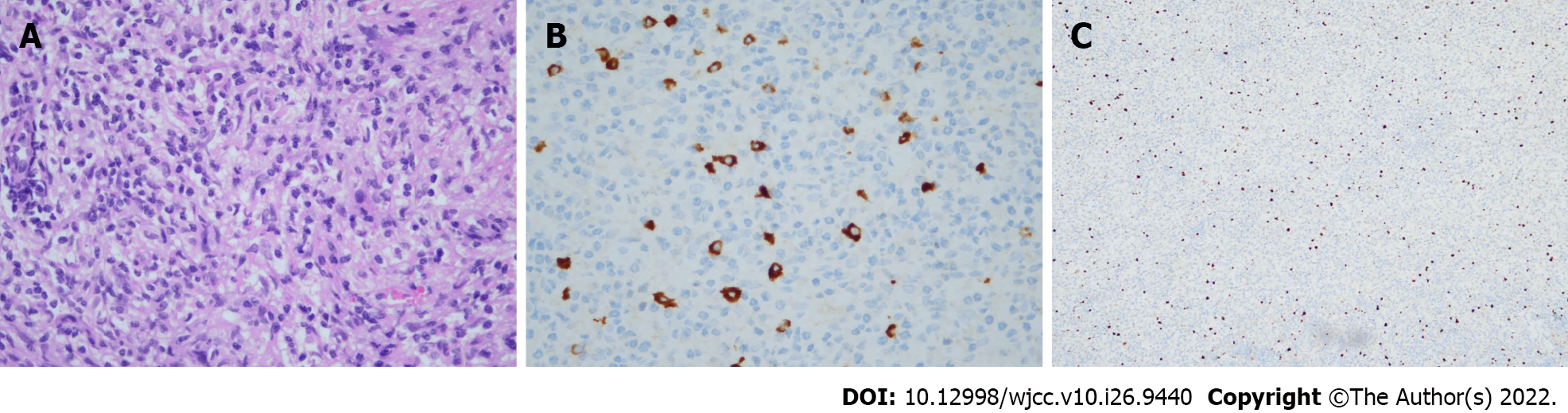
Postoperative histopathological examination (Figure 3) of the lesion showed abundant plasma cells and lymphocyte infiltration with local fibrosis. Immunohistochemistry revealed IgG4 (+)/IgG (+) > 40%, and IgG4 (+) cells > 10/high-powered field (HPF). The patient’s postoperative recovery was good, and he did not develop any neurological deficit. He had not experienced a recurrence at the time of his most recent follow-up in March 2022.
IgG4-RD is a benign, immune-mediated disease, involving multiple organs, which has features in common with several malignancies, infections, and inflammatory diseases. The histopathological findings are consistent in a wide range of organ systems[7]. The three main pathological features of IgG4-RD are lymphoplasmacytic infiltration, storiform fibrosis, and obliterative phlebitis. Eosinophil infiltration is common, but neutrophil infiltration is rare[8]. Storiform fibrosis, in which collagen fibers are arranged radially, is a typical feature of IgG4-RD, but may not be detected in biopsy tissue due to patchy distribution. In addition, there is some organ-specific variability in its manifestations, such as a lack of storiform fibrosis in the lacrimal glands and lymph nodes, and a low incidence of obliterative phlebitis in the salivary glands[8,9]. IgG4-RD usually has a subacute presentation, with symptoms and organ dysfunction existing for months or even years before diagnosis. In specific organs, the disease may progress slowly, occasionally resolves spontaneously, and may temporarily improve or remain dormant for long periods[7]. The clinical manifestations may differ according to the site. IgG4-RH is characterized by dysfunction of the anterior and posterior pituitary lobes and hormonal disturbances. When the anterior pituitary is involved, it often manifests as hyposexuality, hypogonadism, hypothyroidism, and adrenal hypofunction. When the posterior pituitary or pituitary stalk is involved, it often manifests as polyuria and polydipsia. According to previous reports, hypopituitarism and diabetes insipidus are present in 83% and 72% of cases, respectively, and both syndromes are present in 59% of cases with pituitary involvement[10,11]. It has also been reported that the disease may manifest as normal pituitary function in the early stages of onset[12]. Large lesions involving adjacent structures can cause corresponding symptoms, such as visual impairment when the optic nerve/chiasm is involved. Although biopsy is the gold standard for diagnosis in most cases, clinicopathological correlation needs to be confirmed, even if there is supporting histopathological evidence. An increased serum IgG4 level is the main clue to the diagnosis of IgG4-RH[13]. The two main diagnostic criteria for IgG4-RD are: (1) Serum IgG4 concentration > 135 mg/dL; and (2) > 40% of IgG+ plasma cells being IgG4+ and > 10 IgG4+ cells/HPF of biopsy sample in any affected organ[14]. However, the serum IgG4 level alone lacks sensitivity and specificity[7]. The serum IgG4 level is diagnostic if it is more than four times the upper limit of normal. Moreover, a normal serum IgG4 level does not exclude a diagnosis of IgG4-RD. Although imaging findings also lack specificity, imaging modalities such as CT, MRI, and positron emission tomography play an important role in the noninvasive diagnosis of IgG4-RH.
IgG4-RH is usually confined to the pituitary, rather than occurring in multiple organs as in other forms of IgG4-RD[6]. This case only reported pituitary lesions, and showed panhypophysitis. However, the presence of lesions in other body parts cannot be excluded because of a lack systemic examination. The inflammatory process of IgG4-RH can be divided into anterior lobe lesions, posterior lobe and pituitary stalk lesions, and panhypophysitis. Panhypophysitis is the most common form[10]. To our knowledge, there are few reports on the imaging findings of IgG4-RH, although there have been a few reports of the imaging findings of IgG4-RD in the head and neck[15,16]. Based on previous reports[17,18] and the imaging findings in this case, the imaging findings of IgG4-RH are enlargement of pituitary and/or pituitary stalk with a clear boundary and regular morphology. Necrosis and calcification are rare and the diagnosis should be questioned if they are present[7,16]. A cystic structure can be seen in the enlarged gland, and empty sellar syndrome can occur after glucocorticoid treatment[10]. In addition, CT shows homogeneous hypodensity to mild hyperdensity, usually without bone destruction, but compressive bone erosion or osteosclerosis may be present. Compared with the gray matter, the lesion is usually isohypointense on both T1WI and T2WI, which corresponds with an increased number of cells and degree of fibrosis in the lesion on histopathology. Hyperintensity of the posterior pituitary lobe on T1WI is usually absent, and gadolinium contrast-enhanced T1WI shows homogeneous, obvious, and gradual enhancement, which may be associated with the inflammatory process[15]. Because of the similarity in imaging findings, it is usually necessary to differentiate IgG4-RH from pituitary macroadenoma, histiocytosis, and other relatively common types of hypophysitis, such as lymphocytic, granulomatous, and immune checkpoint inhibitor-related hypophysitis. Unlike IgG4-RH, which is more common in middle-aged and older men, these diseases are more common in young and middle-aged women[6]. IgG4-RH should be distinguished from pituitary macroadenomas that require surgical intervention. Pituitary adenomas over 10 mm are called macroadenomas. The mass effect usually causes bone erosion and sellar enlargement. In an epidemiological study, 74% of macroadenomas were non-functional[19]. The intensity of a pituitary adenoma on T1WI is similar to that of the gray matter, but lower than that of the pituitary gland. Hyperintensity can be seen on T1WI if hemorrhage is present. The intensity on T2WI is variable due to hemorrhage, cyst, or necrosis. Pituitary macroadenoma usually invades the surrounding area, and the degree of enhancement after enhanced scan is usually lower than that of hypophysitis[20]. The underlying diagnosis of histiocytosis is often known, or is suggested by the presence of associated bone lesions and other intracranial mass lesions[21]. Immune checkpoint inhibitor–induced hypophysitis is characterized by geographic hypoenhancing lesions in the anterior lobe of the pituitary gland on CE-T1WI[22]. Lymphocytic hypophysitis occurs predominantly in young women, classically during pregnancy or in the early postpartum period, and usually presents as symmetrical enlargement of the pituitary gland with marked homogeneous enhancement on MRI[23]. Granulomatous hypophysitis is characterized by a sellar mass with a tongue-like suprasellar and retrosellar extension, which sometimes infiltrates the basal hypothalamus[24]. Additionally, the possibility of metastases from other sites needs to be excluded in older adults. Depending on the site of the primary tumor, pituitary metastases often manifest as intrasellar or suprasellar masses with isointensity or hypointensity on T1WI, hyperintensity on T2WI, and strong post-gadolinium enhancement[25]. Although these characteristics are helpful in the differential diagnosis, they are not always present. Therefore, the diagnosis of these diseases still needs to be considered comprehensively.
Drug therapy is the first choice for the treatment of IgG4-RD. Glucocorticoids are the first-line drug for the majority of patients and most symptoms rapidly improve following the administration of glucocorticoid treatment. The response of IgG4-RD to glucocorticoids is related to the affected organs and the degree of fibrosis. They usually respond to glucocorticoids in the inflammatory stage, but relapse and refractory cases are not uncommon[26]. Therefore, early diagnosis and treatment are important. In addition, other diagnoses should be considered if the condition does not respond to glucocorticoids. Conventional steroid-sparing agents, such as azathioprine, mycophenolate mofetil, and methotrexate, can also be used for IgG4-RD, but comparative studies with glucocorticoids have not been conducted. Patients who do not respond to glucocorticoids and conventional steroid-sparing agents may respond to rituximab, but its efficacy and side effects need further study[7,27].
The incidence of IgG4-RH is much lower than that of pituitary adenoma. IgG4-RH occurs most frequently in middle-aged and older men and is characterized by hypopituitarism, hypointensity on T2WI, and obvious homogeneous enhancement on gadolinium contrast-enhanced T1WI. These findings should alert clinicians to the possibility of IgG4-RH. Overall, a diagnosis of IgG4-RD requires a comprehensive workup, including histology, imaging, serology, search for other organ involvement, and assessing the response to glucocorticoid therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kurokawa R, Japan; Vujasinovic M, Sweden S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Bhatti RM, Stelow EB. IgG4-related disease of the head and neck. Adv Anat Pathol. 2013;20:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Baptista B, Casian A, Gunawardena H, D'Cruz D, Rice CM. Neurological Manifestations of IgG4-Related Disease. Curr Treat Options Neurol. 2017;19:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061-3067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 486] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 4. | Tang H, Ding G, Xiong J, Zhu H, Hua L, Xie Q, Gong Y. Clivus Inflammatory Pseudotumor Associated with Immunoglobulin G4-Related Disease. World Neurosurg. 2018;118:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Liu X, Wang R, Li M, Chen G. IgG4-Related Inflammatory Pseudotumor Involving the Clivus: A Case Report and Literature Review. Front Endocrinol (Lausanne). 2021;12:666791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | AbdelRazek MA, Venna N, Stone JH. IgG4-related disease of the central and peripheral nervous systems. Lancet Neurol. 2018;17:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 8. | Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol. 2010;34:1812-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 9. | Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1763] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 10. | Bando H, Iguchi G, Fukuoka H, Taniguchi M, Yamamoto M, Matsumoto R, Suda K, Nishizawa H, Takahashi M, Kohmura E, Takahashi Y. The prevalence of IgG4-related hypophysitis in 170 consecutive patients with hypopituitarism and/or central diabetes insipidus and review of the literature. Eur J Endocrinol. 2014;170:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Caputo C, Bazargan A, McKelvie PA, Sutherland T, Su CS, Inder WJ. Hypophysitis due to IgG4-related disease responding to treatment with azathioprine: an alternative to corticosteroid therapy. Pituitary. 2014;17:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Hattori Y, Tahara S, Ishii Y, Kitamura T, Inomoto C, Osamura RY, Teramoto A, Morita A. A case of IgG4-related hypophysitis without pituitary insufficiency. J Clin Endocrinol Metab. 2013;98:1808-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Shimatsu A, Oki Y, Fujisawa I, Sano T. Pituitary and stalk lesions (infundibulo-hypophysitis) associated with immunoglobulin G4-related systemic disease: an emerging clinical entity. Endocr J. 2009;56:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 625] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 15. | Toyoda K, Oba H, Kutomi K, Furui S, Oohara A, Mori H, Sakurai K, Tsuchiya K, Kan S, Numaguchi Y. MR imaging of IgG4-related disease in the head and neck and brain. AJNR Am J Neuroradiol. 2012;33:2136-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Katsura M, Mori H, Kunimatsu A, Sasaki H, Abe O, Machida T, Ohtomo K. Radiological features of IgG4-related disease in the head, neck, and brain. Neuroradiology. 2012;54:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Bhargava R, Hussein Z, Dorward NL, Grieve JP, Jaunmuktane Z, Marcus HJ, Proctor I, Baldeweg SE. IgG4-related hypophysitis: a retrospective cohort study. Acta Neurochir (Wien). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Wright K, Kim H, Hill T, Lee M, Orillac C, Mogar N, Pacione D, Agrawal N. Preoperative differentiation of hypophysitis and pituitary adenomas using a novel clinicoradiologic scoring system. Pituitary. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Agustsson TT, Baldvinsdottir T, Jonasson JG, Olafsdottir E, Steinthorsdottir V, Sigurdsson G, Thorsson AV, Carroll PV, Korbonits M, Benediktsson R. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol. 2015;173:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Zamora C, Castillo M. Sellar and Parasellar Imaging. Neurosurgery. 2017;80:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Jipa A, Jain V. Imaging of the sellar and parasellar regions. Clin Imaging. 2021;77:254-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Kurokawa R, Ota Y, Gonoi W, Hagiwara A, Kurokawa M, Mori H, Maeda E, Amemiya S, Usui Y, Sato N, Nakata Y, Moritani T, Abe O. MRI Findings of Immune Checkpoint Inhibitor-Induced Hypophysitis: Possible Association with Fibrosis. AJNR Am J Neuroradiol. 2020;41:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Yang MG, Cai HQ, Wang SS, Liu L, Wang CM. Full recovery from chronic headache and hypopituitarism caused by lymphocytic hypophysitis: A case report. World J Clin Cases. 2022;10:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 24. | Unlu E, Puyan FO, Bilgi S, Kemal Hamamcioglu M. Granulomatous hypophysitis: presentation and MRI appearance. J Clin Neurosci. 2006;13:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Caranci F, Leone G, Ponsiglione A, Muto M, Tortora F, Cirillo S, Brunese L, Cerase A. Imaging findings in hypophysitis: a review. Radiol Med. 2020;125:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1861] [Article Influence: 143.2] [Reference Citation Analysis (83)] |
| 27. | Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |