Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9354
Peer-review started: April 1, 2022
First decision: June 16, 2022
Revised: June 29, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: September 16, 2022
Processing time: 153 Days and 11.9 Hours
Epithelioid trophoblastic tumor (ETT) is a special type of gestational trophoblastic tumor. However, its pathogenesis has been incompletely elucidated. ETT rarely occurs in the ovaries and fallopian tubes, unlike placental site trophoblastic tumor, requiring a histopathological biopsy and immunohistochemistry for further diagnosis.
A 29-year-old woman with irregular vaginal bleeding and elevated serum chorionic gonadotropin (β-hCG) levels presented similar symptoms to ectopic pregnancy. Transvaginal ultrasound revealed abnormal echoes of the left adnexa. Postoperatively, the pathology of the left ovary and fallopian tube was reported as ETT. The patient was followed up with regular hCG measurements and ultr
For women of childbearing age with elevated serum β-hCG levels, practitioners should consider ETT and be alert to the poor prognosis of the disease. After surgery, the patient's condition should be closely observed to prevent recurrence and metastasis. Postoperative chemotherapy is only helpful for treating the disease to a certain extent.
Core Tip: Epithelioid trophoblastic tumor (ETT) is a rare type of intermediate trophoblastic tumor. Clinical manifestations include irregular vaginal bleeding and changing serum chorionic gonadotropin levels. This case highlights the ultimate importance of histopathological biopsy and immunohistochemistry. Survival outcomes from this disease are high but the tumor can easily relapse and metastasize. Therefore, long-term follow-up is important for the future diagnosis and treatment of ETT.
- Citation: Wang YN, Dong Y, Wang L, Chen YH, Hu HY, Guo J, Sun L. Special epithelioid trophoblastic tumor: A case report. World J Clin Cases 2022; 10(26): 9354-9360
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9354.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9354
Epithelioid trophoblastic tumor (ETT) is a rare type of intermediate trophoblastic tumor found mainly in women of childbearing age. It rarely occurs in the ovary and fallopian tubes and differs from placental site trophoblastic tumor (PSTT). Clinical manifestations include irregular vaginal bleeding and serum chorionic gonadotropin (hCG) level changes which can easily be misdiagnosed as an ectopic pregnancy or choriocarcinoma. The diagnosis should be confirmed by histopathological biopsy and immunohistochemistry. We present herein a case study of an epithelioid trophoblastic tumor.
A woman of childbearing age was found to have vaginal bleeding for a week and serum hCG level change.
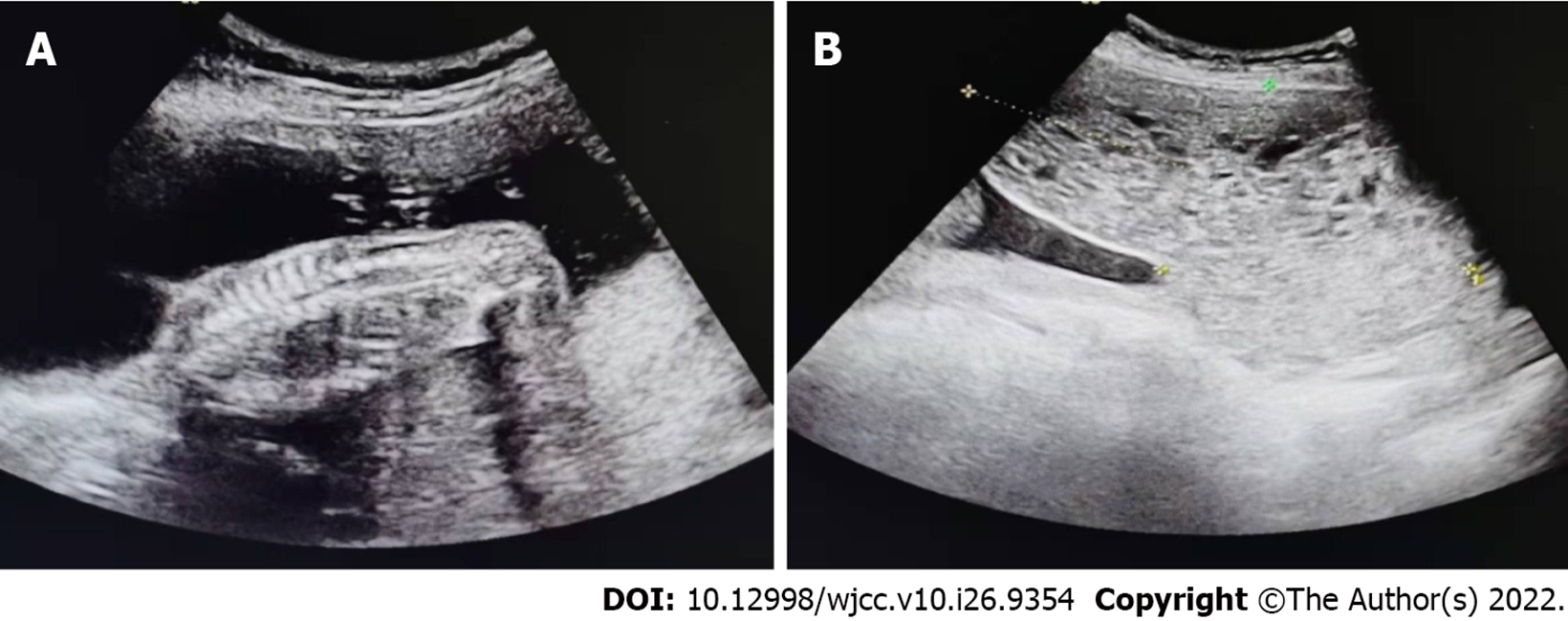
On September 12, 2020, a 29-year-old woman was found to have 46 d of amenorrhea and vaginal bleeding for a week. Her last menstruation was on July 29, 2020. On September 11, 2020, an ultrasound scan outside the hospital revealed a heterogeneous echo of the uterine cavity.
The patient had a full-term vaginal delivery of one daughter in 2011. Due to a partial hydatidiform mole, a cesarean section was performed at 19 + 2 wk of pregnancy on August 4, 2015. Postoperative pathological results revealed placental villi, decidua and umbilical cord tissues, chorionic edema with central cistern formation, trophoblast cell proliferation and a compound vesicular mole (Figure 1). After the operation, a B-ultrasound showed an "abnormal echo in the uterine cavity with pelvic effusion." Blood β-hCG levels decreased slowly and were found to be 19.11 mIU/mL on November 18, 2015. Hysteroscopic hysterectomy plus laparoscopic exploration was performed in our hospital on November 19, 2015. During the operation, yellow nodules were found on the surface of the left ovary and in the space between the tissues of the uterine cavity. Nodules on the left ovary were obtained as clinical samples. Pathology of the uterine cavity revealed proliferative endometrium with bleeding and fibroid necrosis; some of them showed polypoid changes while the left ovary exhibited degenerative decidual nodules with a small amount of trophoblastic epithelial cells. After the operation, serum β-hCG levels gradually decreased and reached 5.71 mIU/mL on February 26, 2016. On January 26, 2019, a son was delivered by cesarean section in our hospital.
The patient denied any family history of malignant tumors.
The patient had regular menstrual cycles; the last menstrual period was July 29, 2020. The patient had a positive urine pregnancy test 34 d after amenorrhea; bloody vaginal discharge occurred 7 d prior with the same menstrual flow and symptoms of backache, abdominal distension and discomfort, dizziness and fatigue. Gynecological examination was not performed to prevent mass rupture. Clinical considerations were first an ectopic pregnancy and second a trophoblastic tumor.
On September 11, 2020, the serum hCG level was 8529.8 mIU/mL and on September 12, 2020, the serum hCG level was 12878 mIU/mL in our hospital.
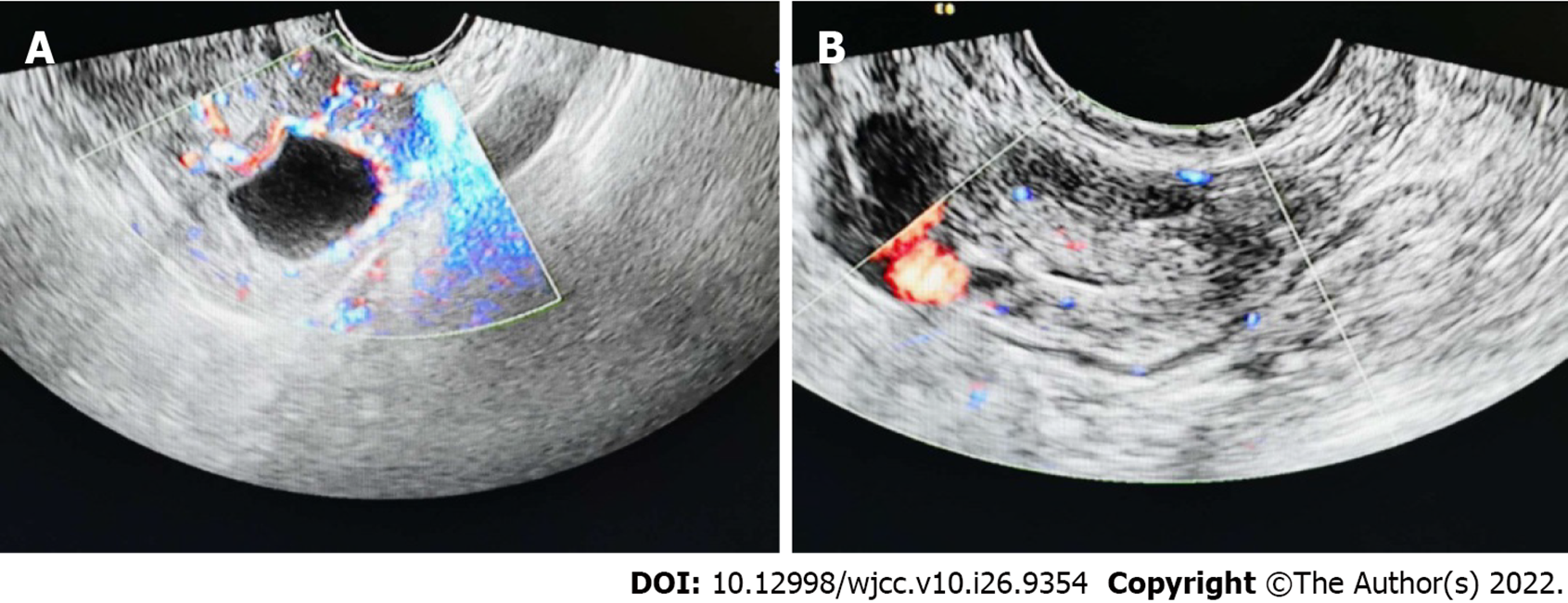
On September 11, 2020, an ultrasound scan of the patient outside the hospital revealed a heterogeneous echo of the uterine cavity. On September 12, 2020, a transvaginal ultrasound examination in our hospital showed a cystic echo in the left adnexal region: with luteal possibility, hypoechoic left adnexal area and a diameter of approximately 3 cm (Figure 2).
The final diagnosis of the presented case is ETT located in the ovaries and fallopian tubes.
The patient underwent laparoscopic oophorectomy (left), salpingectomy and diagnostic curettage. After the operation, the patient was instructed to take mifepristone tablets for 7 d. The patient received chemotherapy at Peking Union Medical College Hospital because of the rising trend in blood hCG value.
After improving the relevant examination, there were no abnormalities on chest computed tomography (CT) scan. On September 9, 2015, the serum hCG levels were decreased to 3454.00 mIU/mL. The patient was instructed to take mifepristone tablets for 7 d after which the blood hCG value was 35.46 mIU/mL. On October 15, 2020, blood β-hCG levels were 4.02 mIU/mL; on October 24, 2020, they reached < 5 mIU/mL. A magnetic resonance imaging (MRI) scan of the pelvis on October 28, 2020, did not reveal any abnormality. On November 6, 2020, serum β-hCG levels were found to be 0.59 mIU/mL. As the prognostic outcomes of the disease are unclear, there was continuous monitoring of the blood β-hCG levels. On February 17, 2021, the blood hCG value was 25.78 mIU/mL. After three consecutive observations, the blood hCG value showed an upward trend. The patient received chemotherapy at Peking Union Medical College Hospital. The patient has currently returned to normal with no abnormal blood hCG values.
ETT originates from villous intermediate epithelial trophoblasts and is rarely found in ovaries and fallopian tubes[1,2].This case is a recurrent ETT with high preoperative β-hCG values. ETT is observed in women during the reproductive period, mostly secondary to full-term pregnancy, hydatidiform mole and ectopic pregnancy. Its clinical manifestations include irregular vaginal bleeding and changes in blood β-hCG levels which can easily be misdiagnosed as ectopic pregnancy or choriocarcinoma. The diagnosis requires histopathological biopsy and immunohistochemistry. The disease has a high survival rate and is prone to metastasis; hence, corresponding examinations must be improved.
Xing et al[3] first reported that ETT has a low probability of occurring in the ovary and fallopian tube, simultaneously. The composition of the fallopian tube tumor in our case study is the same as that on the ovary and it may be the metastasis of ovarian ETT, common in women of childbearing age after various pregnancies[4,5]. The patient was 29-years-old in 2015 and due to partial hydatidiform mole surgery, the blood β-hCG value decreased slowly for 3 mo and the uterine cavity presented an abnormal echo. The patient underwent hysteroscopy and laparoscopic surgery. During the operation, the left ovarian lesion was accidentally found and removed. The pathological results found that the left ovary had degenerated decidual nodules with a small number of epithelioid trophoblast cells, after which the blood hCG value decreased. There was a normal pregnancy in 2019 and the disease occurred 19 mo after. This time, the left ovary and fallopian tube presented ETT lesions. The fallopian tube was not ruled out as a metastatic lesion. The blood hCG did not fall to the normal line 4 d after the operation and mifepristone medication was prescribed. The recurrence and metastasis of this case are related to the pregnancy history of the patient. The time between the previous pregnancy and onset of the disease suggests that these tumor components may originate from metastasis during pregnancy, surgical implantation and the small amount of left ovary found in the history of partial hydatidiform mole. Epithelioid trophoblasts also increase the probability of ETT recurrence and metastasis[5-8]. ETT usually manifests as abnormal vaginal bleeding with serum β-hCG level < 2500 U/L; the serum β-hCG, in this case, was initially 12878 U/L, and transvaginal ultrasound indicated abnormal echoes in the appendages[7,8]. The above clinical manifestations, serum β-hCG and B-ultrasound results are similar to those of ectopic pregnancy[9,10]. Further, the pathogenesis of choriocarcinoma also presents with irregular vaginal bleeding, serum β-hCG level > 10000 U/L. In clinical work, ETT is thus easily misdiagnosed as ectopic pregnancy and choriocarcinoma[6,8,11].
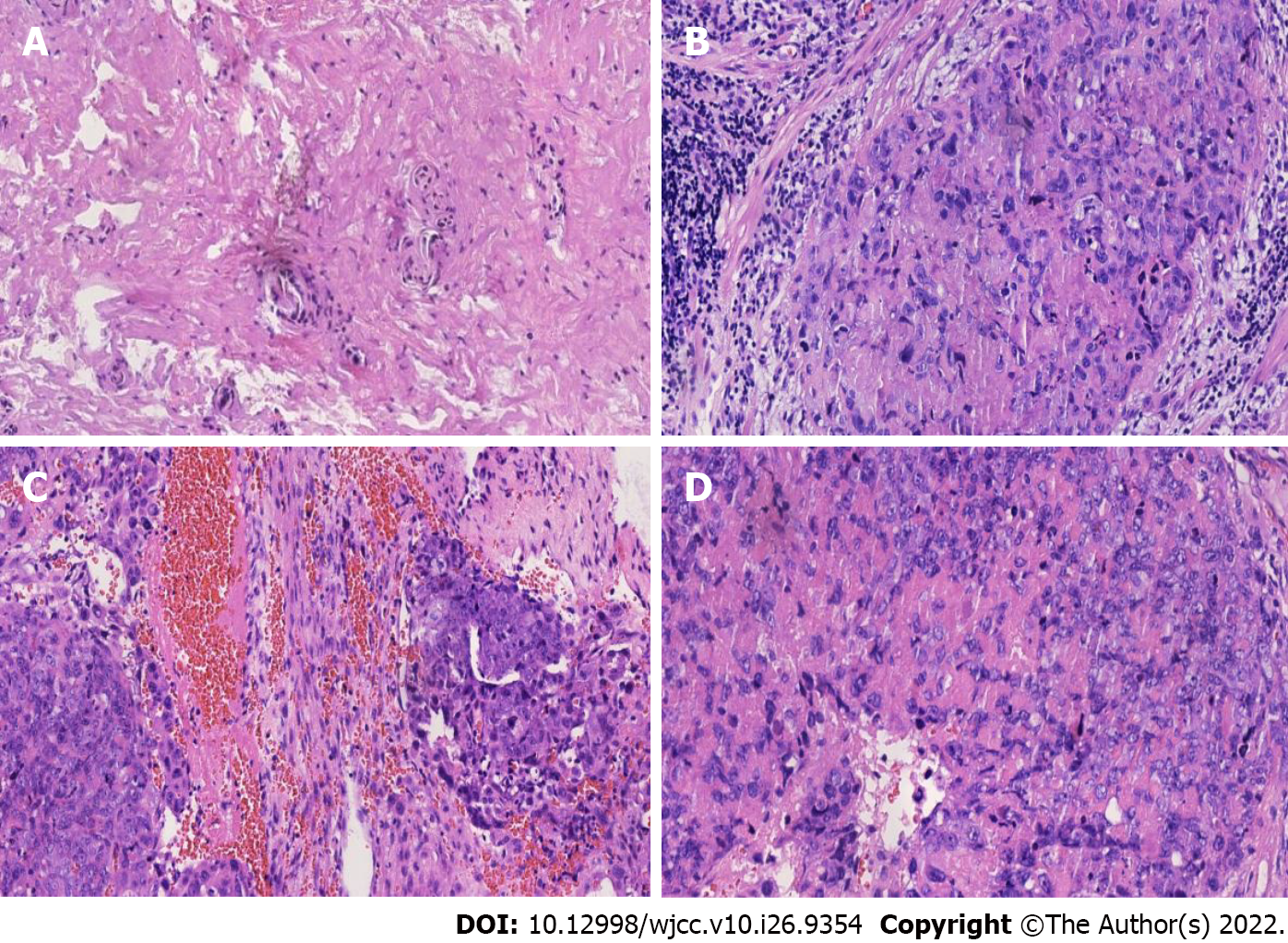
The lesions of ETT mostly occur at the bottom of the uterus in the lower segment and cervix. The postoperative pathological results of this patient was located in the left ovary. The trophoblastic tumor was accompanied by hemorrhage and necrosis, cell atypia, mitotic figures were easy to observe and tumor components were found in the fallopian tube (Figure 3), which were all differentiated from the tumor cells of the trophoblast cells of the chorionic lobe[11]. However, it must be distinguished from chorionic carcinoma, which is a biphasic growth of syncytial trophoblasts and cytotrophoblasts. Concurrently, ETT is composed of unipolar chorionic intermediate trophoblasts and the trophoblast island is surrounded by a wide necrotic region and a glassy matrix[8,11,12]. Unlike the pathological manifestations of PSTT which have no flaky growth manifestations with scattered heteromorphic nuclear cells in the tumor cells, ETT grows nodulous with smaller cells and less nuclear polymorphism[3,12,13]. In immunohistochemistry, the Ki-67 proliferation index of ETT is 10%-25% while choriocarcinoma and squamous cell carcinoma are very high (> 50%). In this case, the Ki-67 proliferation index was low, approximately + 30%. Therefore, choriocarcinoma was not considered. Associated immune expressions also help to correctly diagnose ETT. For example, P63 positive is an ideal marker for distinguishing PSTT and partial hydatidiform mole; strong expression of cytokeratin 18 in ETT can rule out the possibility of squamous cell carcinoma; hCG, hPL and CD146 in ETT may be focally positive[4,14].
ETT treatment is generally surgical and less sensitive to chemotherapy especially in patients with limited lesions, where surgical removal is the best choice. There is currently no uniform chemotherapy regimen and the main drugs are antimetabolites such as methotrexate and 5-fluorouracil. If most of the trophoblasts are in the value-add cycle, the doubling time is short, and they can have some sensitivity to chemotherapy. Frijstein et al[12] reported that 75% of ETT patients received etoposide, methotrexate and actinomycin-D with etoposide and cisplatin chemotherapy showing the role of chemotherapy in ETT treatment[7]. hCG is a specific marker of trophoblastic tumors and any level changes reflect the growth of tumors and the effects of chemotherapy. The postoperative blood β-hCG levels of the patient increased and after chemotherapy treatment, there was no recurrence, indicating that postoperative chemotherapy may be helpful for some patients. During the late stage of the disease, surgical treatment combined with multiple-drug chemotherapy, targeted therapy and immunotherapy should be considered. Laparoscopic exploratory surgery was used for this patient to remove the lesion and attention should be paid to the prognosis after the operation. The high level of serum β-hCG before treatment is related to the poor prognosis of the disease. In this case, the level of serum β-hCG before treatment was high and the serum β-hCG level was normal after 2 mo of surgical resection. Transvaginal B-ultrasound, chest CT and pelvic MRI examination found no abnormalities[11,13]. However, the serum β-hCG value increased 5 mo after the operation. ETT tends to recur and metastasize; hence, close monitoring of serum β-hCG levels has a great role in diagnosing and treating the disease. Since the recurrence and metastatic behavior of ETT are difficult to predict, patients must be followed up regularly to be alert to the occurrence of ETT.
Because this disease is rare, clinicians and pathologists without experienced knowledge may have difficulty in diagnosing ETT. Correct interpretation of histopathological features is helpful to the diagnosis and treatment of the disease. The behavior and prognosis of ETT need to be further observed. Long-term follow-up provides important data for the correct diagnosis and treatment of ETT in the future.
ETT diagnosis is difficult while its occurrence is ignored in most cases due to its low prevalence. This is the first case report of a simultaneous discovery of ETT in the ovary and fallopian tubes. While the correct diagnosis of ETT helps inform the right treatment plan, ETT prognosis should be clinically monitored. Long-term follow-up provides important data for future diagnosis and treatment of ETT.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elkady N, Egypt; Pongcharoen S, Thailand S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Shih IeM. Gestational trophoblastic neoplasia--pathogenesis and potential therapeutic targets. Lancet Oncol. 2007;8:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Allison KH, Love JE, Garcia RL. Epithelioid trophoblastic tumor: review of a rare neoplasm of the chorionic-type intermediate trophoblast. Arch Pathol Lab Med. 2006;130:1875-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Xing D, Zhong M, Ye F, O'Malley MT, Li S, Vang R, Ronnett BM. Ovarian Intermediate Trophoblastic Tumors: Genotyping Defines a Distinct Category of Nongestational Tumors of Germ Cell Type. Am J Surg Pathol. 2020;44:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Yang C, Li J, Zhang Y, Xiong H, Sheng X. Epithelioid trophoblastic tumor coexisting with choriocarcinoma around an abdominal wall cesarean scar: a case report and review of the literature. J Med Case Rep. 2020;14:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Parker A, Lee V, Dalrymple C, Valmadre S, Russell P. Epithelioid trophoblastic tumour: report of a case in the fallopian tube. Pathology. 2003;35:136-140. [PubMed] |
| 6. | Davis MR, Howitt BE, Quade BJ, Crum CP, Horowitz NS, Goldstein DP, Berkowitz RS. Epithelioid trophoblastic tumor: A single institution case series at the New England Trophoblastic Disease Center. Gynecol Oncol. 2015;137:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Zhang S, Huang W, Chen T, Yuan H, Zhang Y. Intermediate trophoblastic tumor: the clinical analysis of 62 cases and prognostic factors. Arch Gynecol Obstet. 2019;299:1353-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kong Y, Tao G, Zong L, Yang J, Wan X, Wang W, Xiang Y. Diagnosis and Management of Mixed Gestational Trophoblastic Neoplasia: A Study of 16 Cases and a Review of the Literature. Front Oncol. 2019;9:1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Thornton J. Women are at serious risk of harm from late diagnosis of ectopic pregnancy. BMJ. 2020;368:m924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kar A, Mishra C, Biswal P, Kar T, Panda S, Naik S. Differential expression of cyclin E, p63, and Ki-67 in gestational trophoblastic disease and its role in diagnosis and management: A prospective case-control study. Indian J Pathol Microbiol. 2019;62:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Froeling FEM, Ramaswami R, Papanastasopoulos P, Kaur B, Sebire NJ, Short D, Fisher RA, Sarwar N, Wells M, Singh K, Ellis L, Horsman JM, Winter MC, Tidy J, Hancock BW, Seckl MJ. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br J Cancer. 2019;120:587-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Frijstein MM, Lok CAR, van Trommel NE, Ten Kate-Booij MJ, Massuger LFAG, van Werkhoven E, Kaur B, Tidy JA, Sarwar N, Golfier F, Winter MC, Hancock BW, Seckl MJ; all the contributors to the ISSTD PSTT/ETT database. Management and prognostic factors of epithelioid trophoblastic tumors: Results from the International Society for the Study of Trophoblastic Diseases database. Gynecol Oncol. 2019;152:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Placental site trophoblastic tumor and epithelioid trophoblastic tumor: Clinical and pathological features, prognostic variables and treatment strategy. Gynecol Oncol. 2019;153:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 14. | Arafah MA, Tulbah AM, Al-Husaini H, Al-Sabban M, Al-Shankiti H, Al-Badawi IA. Extrauterine epithelioid trophoblastic tumor arising in the ovary with multiple metastases: a case report. Int J Surg Pathol. 2015;23:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |