Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9340
Peer-review started: March 28, 2022
First decision: May 12, 2022
Revised: May 22, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 16, 2022
Processing time: 157 Days and 15.1 Hours
Pulmonary sequestration-both intralobar and extralobar-is a rare congenital developmental malformation. Extralobar pulmonary sequestrations (EPS) have their own pleura but are separated from the bronchus and usually occur in the left lung. They are mainly found mainly between the lower lobe and the medias
We report the case of a 27-year-old man who was misdiagnosed with a neuro
Although EPS rarely occurs in the posterior mediastinum, its diagnosis should be considered when posterior mediastinal tumors are suspected.
Core Tip: Extralobar pulmonary sequestration (EPS) usually occurs in the left lung, mainly between the lower lobe and the diaphragm. As EPS occurrence is rare, symptoms have not been sufficiently established and clinicians lack experience in diagnosing and treating EPS; thus, the condition can be easily missed or misdiagnosed. Traditional surgery is the most appropriate approach for the management of EPS. Although interventional therapy may be used in certain cases, it cannot replace traditional surgery as a viable alternative. Indications should be considered individually for each patient before choosing the intervention. Three-dimensional imaging reconstruction may aid clinicians in diagnosing difficult cases.
- Citation: Jin HJ, Yu Y, He W, Han Y. Posterior mediastinal extralobar pulmonary sequestration misdiagnosed as a neurogenic tumor: A case report. World J Clin Cases 2022; 10(26): 9340-9347
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9340.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9340
Pulmonary sequestration is a rare congenital disease characterized by the separation of part of the pulmonary tissue from the lung during embryonic development. The sequestration develops independently, leading to the formation of a dysfunctional pulmonary cystic mass without respiratory function. The blood supply derives directly from the systemic circulation, and the bronchus of the isolated lung tissue may or may not communicate with the main bronchus[1]. Extralobar pulmonary sequestration (EPS) is an unusual subtype of pulmonary sequestration that has its pleura and is separate from the main bronchus. EPS usually occurs in the left lung, mainly between the lower lobe and the diaphragm, and rarely in the mediastinum[2]. EPS lacks classic pulmonary symptoms such as chest pain and hemoptysis, and its diagnosis is difficult to confirm with traditional radiography[3]. The only two confirmatory tests for EPS diagnosis are angiography and histopathological examination[4]. Herein, we report a case that was initially misdiagnosed as a neurogenic tumor, with the patient subjected to surgery to remove a posterior mediastinal mass. Following resection, histopathological analysis established a diagnosis of EPS.
A 27-year-old Chinese male (height: 178 cm, weight: 70 kg, occupation: skilled worker) had a routine physical checkup two months prior (July 18, 2020) that revealed a posterior mediastinal mass.
Computed tomography (CT) revealed an abnormal triangular mass in the left lower posterior mediastinum. The patient then presented to our hospital with a follow-up magnetic resonance imaging (MRI) scan revealing “a mass on the left side of the 9-10th thoracic vertebra, likely neurogenic”. Based on these findings, the patient was preliminarily diagnosed with a posterior mediastinal tumor. The patient had not received any treatment within two months of the discovery of the disease and did not show any unpleasant symptoms, such as chest and back pain, cough, sputum, fever, nausea, weight loss, or any specific symptoms that may result from mediastinal masses such as myasthenia gravis and Horner syndrome.
The patient had undergone the excision of a single angiolipoma in the leg eight years ago.
The patient’s family history was unremarkable, with no history of relevant disease.
There were no obvious positive physical signs at presentation.
Routine blood analysis revealed 40.1% neutrophils and 46.8% lymphocytes; the absolute lymphocyte count was 3.2 × 109/L. No abnormality was identified in other parameters related to blood biochemistry and coagulation function.
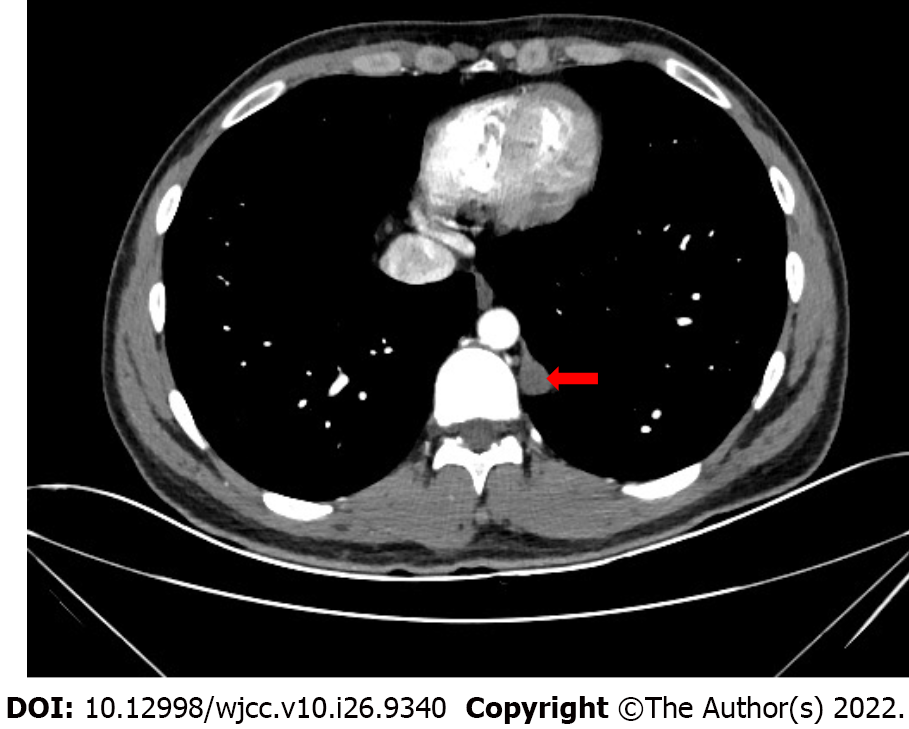
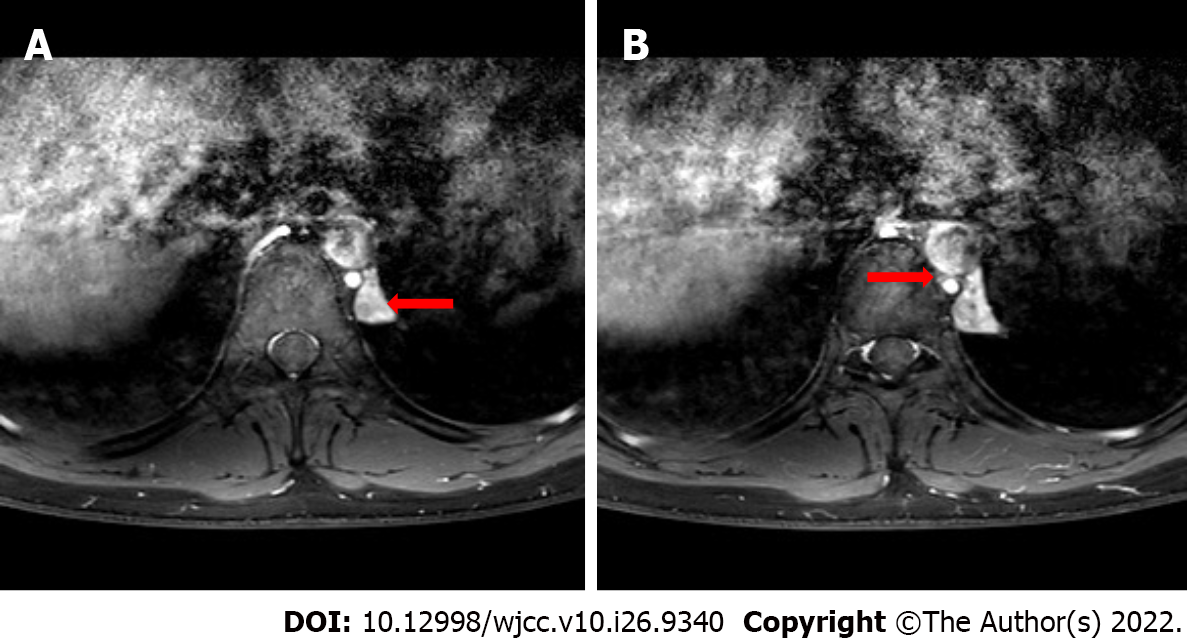
Contrast-enhanced chest CT revealed a triangular soft tissue density shadow under the pleura on the left side of the 11th thoracic vertebra, with a clear boundary and a size of about 13 mm × 16 mm × 27 mm. The enhancement scan was uneven and cystic, and mildly enhanced areas could be seen (Figure 1). Contrast-enhanced chest MRI revealed slightly longer T1 and T2 signal foci in the left posterior mediastinum next to the thoracic 9-10th vertebral body, with a size of about 1.2 cm × 1.4 cm × 3.3 cm; heterogeneous enhancement was seen after the enhanced scan (Figure 2A).
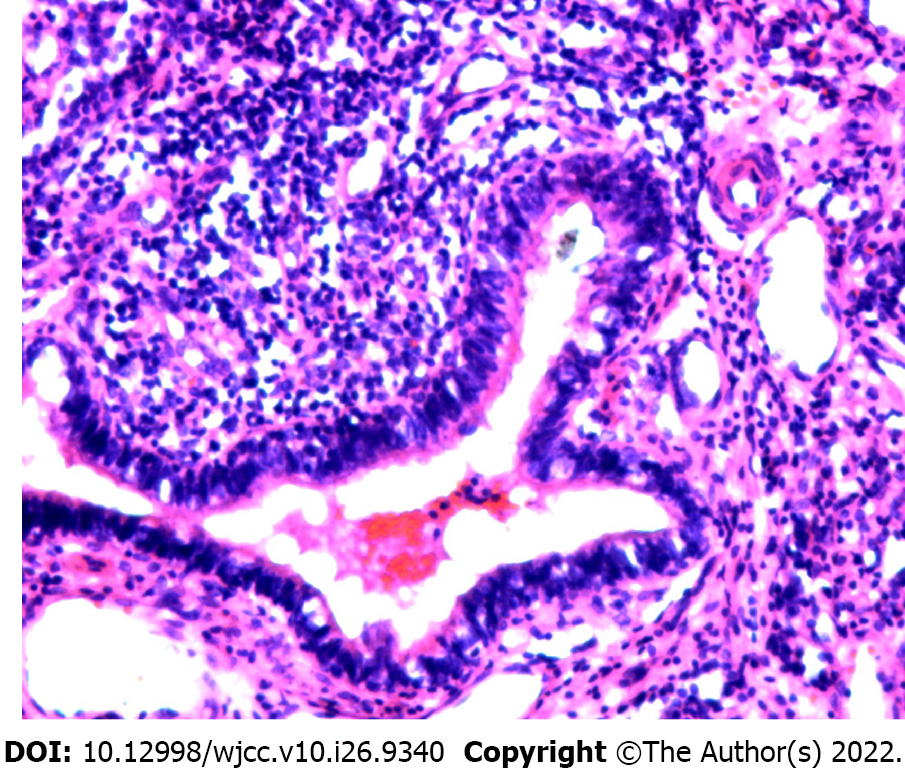
Given these postoperative histopathology findings, the patient was diagnosed with posterior mediastinal EPS.
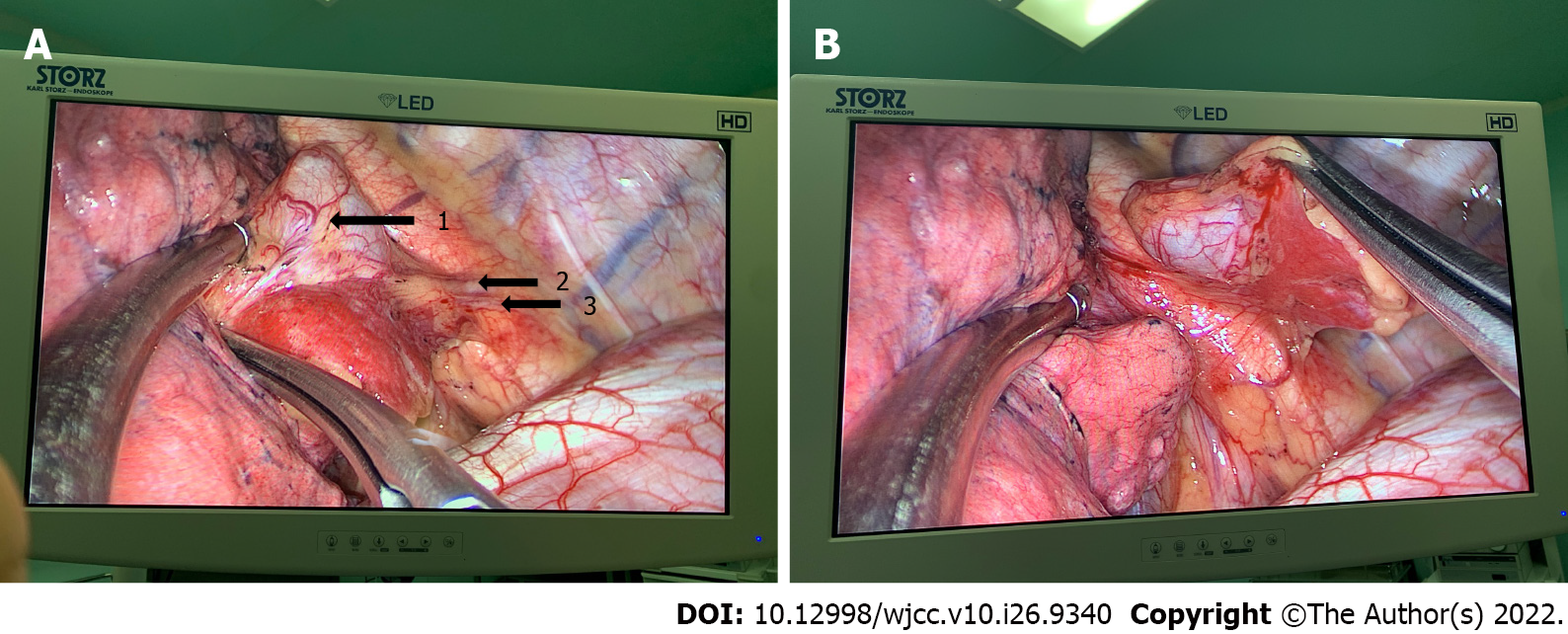
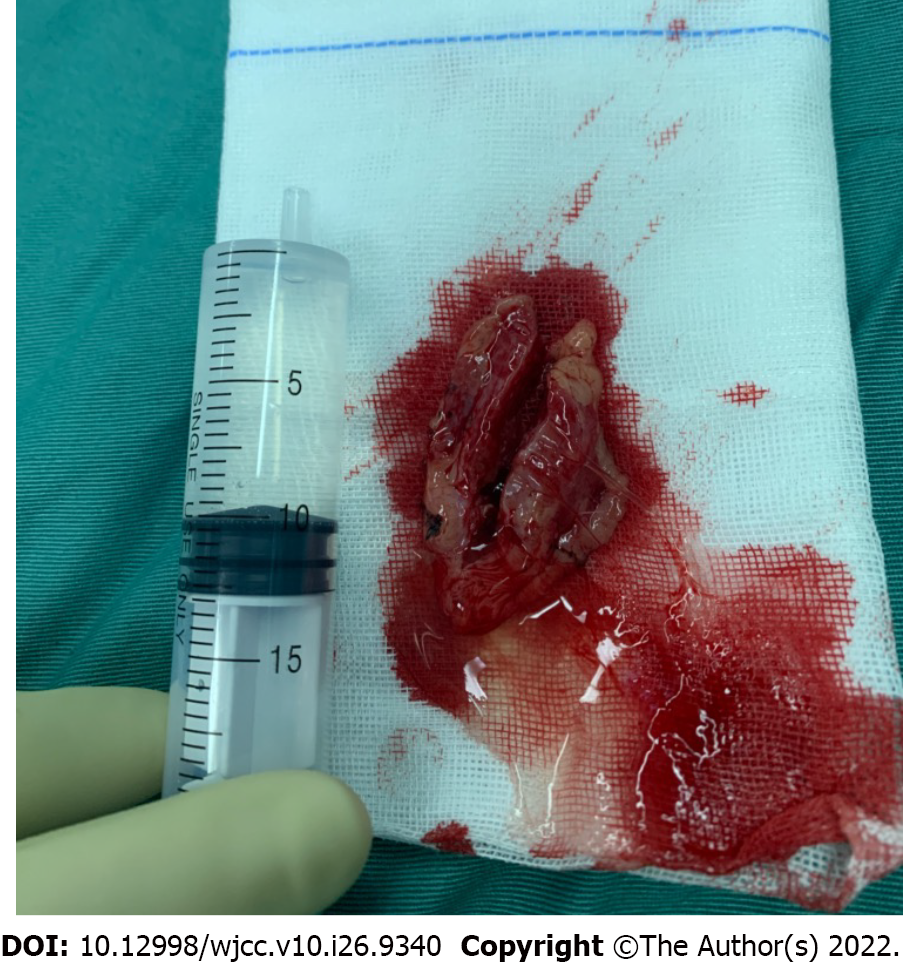
After evaluating the patient’s risks and predicted outcomes, we decided to directly excise the mass in a uniport video-assisted thoracoscopic surgery (VATS). After induction of anesthesia via single-lumen right endotracheal intubation, the patient was placed in the right lateral position. A 3 cm incision was made at the intersection of the 6th rib and the posterior axillary line. The suspected tumor was in the 11th paravertebral region and attached to the 11th thoracic vertebra behind the descending aorta in the posterior mediastinum. It was isolated from the normal lung and had its visceral pleura. A single thin arteriole and a thicker accompanying vein were seen in the pedicle of the tumor (Figure 3). MRI revealed identical findings (Figure 2B). The arteriole originated from the intercostal artery, while the vein originated directly from the hemizygous vein. The pedicle of the pyramidal tumor was incised using a harmonic scalpel. The surface of the incision was flat and was covered with gel foam to prevent hemorrhage. The mass was completely excised (Figure 4). Intraoperative histopathological analysis of rapid-frozen sections confirmed that the tumor mass was benign.
The patient made a prompt, uneventful recovery and was discharged 4 d after surgery (total stay: 10 d). The patient underwent follow-up thoracic CT 1 mo post-surgery. The CT showed no signs of pleural effusion following mediastinal surgery, and the patient reported no feelings of discomfort since discharge.
Pulmonary sequestration is a rare form of congenital pulmonary hypoplasia, accounting for 0.15%-6.4% of all congenital pulmonary malformations[5,6]. It is characterized by the absence of communication between the abnormal lung tissue and the bronchial tree, as well as the absence of normal lung function. There are various hypotheses on the mechanisms underlying the onset of lung isolation, but most studies support Pryce’s traction theory[7,8]. Compared to intralobar pulmonary sequestration (IPS), EPS is a rare type of pulmonary sequestration that usually occurs in the left lung, mainly between the left lower lobe and the diaphragm, although it may also occur within or under the diaphragm and in the mediastinum[3]. In this case, the tumor was located in the posterior mediastinum, a site in which EPS rarely occurs. Therefore, when this well-defined pyramid-like tumor was initially found by imaging, EPS was not considered as a diagnosis. As the most common tumor in the posterior mediastinum, a neurogenic tumor was initially suspected; thus, the patient underwent contrast-enhanced MRI and contrast-enhanced CT examinations, both of which indicated a neurogenic tumor. Neurogenic tumors are mostly benign, and of these, neurofibromas and schwannomas are the most common in adults. The most common age of onset is 20-30 years old, and most patients have no obvious symptoms. These diagnostic characteristics supported our diagnosis of a neurogenic tumor[9,10].
Fifty percent of EPS cases are accompanied by congenital malformations, the most common of which is a congenital diaphragmatic hernia, accounting for about 30% of cases. Other such malformations include ventricular septal defects, pericardial cysts, and pulmonary arteriovenous malformations. If the isolated lung communicates with the lower esophagus or fundus of the stomach, a diagnosis of “congenital bronchopulmonary foregut malformation” is made[11]. In this case, however, the patient did not have other congenital malformations—another reason why EPS was initially overlooked.
However, through direct observation of the tumor with thoracoscopy during the surgery, we observed the complete and independent pleura and two blood vessels of the tumor. Thus, we performed additional radiological assessments. MRI revealed vasculature with signs of a supply artery branching from the intercostal artery and a vein draining into the hemiazygos vein (Figure 5). As these vessels were minute, we attempted three-dimensional (3-D) reconstruction for verification.
3-D reconstruction is commonly used for preoperative analysis, evaluation of vascular progression, and determination of the position of the mass during sublobectomy or lobectomy. Enhanced CT images were used to generate a 3-D reconstruction of the tumor. However, due to the size of the tumor relative to the scanning plane or inappropriate placement, 3-D imaging reconstruction failed to demonstrate the vessel accurately. This technique did, however, provide us with a newer, more convenient, and cheaper diagnostic strategy than the other examination techniques.
Selective vascular digital subtraction angiography (DSA) has always been considered the gold standard for the diagnosis of pulmonary isolation[12]. However, DSA can only show vessel development and not pulmonary lesions. Moreover, it is invasive, and its high cost limits its clinical application. Compared to the DSA technique, contrast-enhanced CT and CT angiography can help determine the number, origin, and shape of the abnormal supplying arteries, in addition to lung lesions. In this case, contrast-enhanced CT scans also show lesion enhancement, which aids in distinguishing other lesions from lung tumors. Iodinated contrast agents can also differentiate veins from arteries. To an extent, this shows that contrast-enhanced CT is gradually replacing DSA in the diagnosis of pulmonary sequestration.
Pulmonary sequestration is a benign disease; however, once diagnosed, prompt treatment is needed to prevent malignant changes, abscesses, bronchiectasis, torsion, recurrent pneumonia, and other potentially serious complications[13]. Surgery is the traditional method for treating pulmonary sequestration and remains the first choice of treatment[14]. Lobectomy is performed for IPS, and isolated pneumonectomy is performed for EPS. Such procedures are associated with severe trauma, slow recovery, and several potentially life-threatening complications such as massive hemorrhage. Compared with traditional open thoracic surgery, VATS provides a better surgical field, increases the accuracy of the procedure, reduces surgical trauma, and is associated with lesser postoperative pain. In addition to these advantages, patient recovery times are much shorter. VATS is currently the first choice for pulmonary sequestration surgery[15]. Given the small size of the tumor, we selected uniport VATS, although a two- or three-port VATS is conventionally used.
Managing abnormal blood vessels during surgery is critical. Regardless of the subtype of pulmonary sequestration, accurate identification, careful dissection, and complete ligation of the anomalous vessels are crucial. Abnormal vessels often have degraded wall elasticity, making it difficult to separate them and increasing the risk of bleeding[16]. Patients with a serious infection can also have severe pleural adhesion.
Advancements in interventional technology and instrumentation have led to a gradual increase in the use of endovascular embolization to treat pulmonary sequestration. These embolization procedures are associated with reduced trauma and can correct local hemodynamics and reduce the pulmonary capillary bed pressure. They address ischemic degeneration and atrophy in the isolated lung tissue and relieve symptoms of infection and fever. Embolization procedures are especially suitable in cases of repeated hemoptysis. Lee et al[17] successfully applied interventional embolization to treat asym
For patients with bronchopulmonary sequestration and massive hemoptysis who fail to respond to conservative medical treatment without any indication for emergency surgery, embolization can be regarded as an effective treatment method[18]. The effect of interventional therapy is questionable in patients with pulmonary isolation with long-term or repeated infection, and it may easily induce or aggravate infection in the lungs. In addition to this, incomplete postoperative embolization may lead to hemoptysis, infection, and fever. Therefore, indications for each patient should be considered individually when applying interventional therapies. Interventional therapy did not apply to this case.
Because EPS is rarely seen in clinical practice, it lacks the specificity of symptoms, and clinicians lack experience in its diagnosis and treatment. Thus, it is easy to neglect or misdiagnose EPS, especially when it occurs in an atypical location. In our case, preoperative CT and MRI findings suggested a neurogenic tumor, primarily because of the location and radiological characteristics of the lesion.
Although the condition was misdiagnosed, the mass was completely resected during surgery, and the patient had an uneventful recovery. This case suggests that EPS should be considered when diagnosing tumors at this location. Thoracic surgeons should learn about the diagnosis and treatment of EPS, raise awareness of the disease, and avoid misdiagnosis by performing contrast-enhanced CT, MRI, and 3-D reconstruction techniques when encountering similar diseases. The contrast-enhanced CT and MRI examinations are also reliable for diagnosing other thoracic tumors. The 3-D reconstruction technique should also be used in the diagnosis of thoracic diseases.
In the present report, we discussed a rare case of EPS in the posterior mediastinum. Preoperatively, the mass under investigation was diagnosed as a neurogenic tumor based on contrast-enhanced CT and MRI. However, postoperative histopathology confirmed the diagnosis of EPS, which is rarely seen in the posterior mediastinum. In our case, complete resection was possible during surgery due to the small size of the suspected tumor and its associated vessels. This ensured adequate treatment and an optimal patient outcome.
Although a precise diagnosis of EPS is difficult to achieve with standard preoperative radiography and clinical examination-especially in the posterior mediastinum where neurogenic tumors are common-3-D reconstruction of the tumor and surrounding tissue, based on contrast-enhanced CT and MRI, may aid clinicians in making an accurate diagnosis. Given the continued improvements in CT and MRI technology, increased resolution may also allow for the visualization of minute vessels. Combined with the 3-D reconstruction technique, this may not only help to diagnose EPS accurately-even in unsuspected locations-but also play a major role in the diagnosis of IPS and many more thoracic diseases.
We appreciate all the technical assistance from the Department of Thoracic Surgery of Shengjing Hospital, China Medical University.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Darbari A, India; Salimi M, Iran S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Estes MEZ. Bronchopulmonary sequestration: Improving practice by evaluating for a missed diagnosis. Nurse Pract. 2017;42:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Corbett HJ, Humphrey GM. Pulmonary sequestration. Paediatr Respir Rev. 2004;5:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Lupinski RW, Agasthian T, Lim CH, Chua YL. Extralobar pulmonary sequestration simulates posterior neurogenic tumor. Ann Thorac Surg. 2004;77:2203-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Prasad R, Garg R, Verma SK. Intralobar sequestration of lung. Lung India. 2009;26:159-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Petersen G, Martin U, Singhal A, Criner GJ. Intralobar sequestration in the middle-aged and elderly adult: recognition and radiographic evaluation. J Thorac Cardiovasc Surg. 2003;126:2086-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax. 1979;34:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 433] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Qi W, Zhao J, Shi G, Yang F. Intralobar pulmonary sequestration displayed as localized emphysema on computed tomography image. J Cardiothorac Surg. 2017;12:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Mendeloff EN. Sequestrations, congenital cystic adenomatoid malformations, and congenital lobar emphysema. Semin Thorac Cardiovasc Surg. 2004;16:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Nakazono T, White CS, Yamasaki F, Yamaguchi K, Egashira R, Irie H, Kudo S. MRI findings of mediastinal neurogenic tumors. AJR Am J Roentgenol. 2011;197:W643-W652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Rodriguez EF, Jones R, Miller D, Rodriguez FJ. Neurogenic Tumors of the Mediastinum. Semin Diagn Pathol. 2020;37:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Tashtoush B, Memarpour R, Gonzalez J, Gleason JB, Hadeh A. Pulmonary Sequestration: A 29 Patient Case Series and Review. J Clin Diagn Res. 2015;9:AC05-AC08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Dresler S. Massive pleural effusion and hypoplasia of the lung accompanying extralobar pulmonary sequestration. Hum Pathol. 1981;12:862-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Sun X, Xiao Y. Pulmonary sequestration in adult patients: a retrospective study. Eur J Cardiothorac Surg. 2015;48:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Costa Júnior Ada S, Perfeito JA, Forte V. Surgical treatment of 60 patients with pulmonary malformations: what have we learned? J Bras Pneumol. 2008;34:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Li Q, Xie D, Sihoe A, Dai J, Wang H, Gonzalez-Rivas D, Zhu Y, Jiang G. Video-assisted thoracic surgery is associated with better short-term outcomes than open thoracotomy in adult patients with intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg. 2018;26:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Konecna J, Karenovics W, Veronesi G, Triponez F. Robot-assisted segmental resection for intralobar pulmonary sequestration. Int J Surg Case Rep. 2016;22:83-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Lee KH, Sung KB, Yoon HK, Ko GY, Yoon CH, Goo HW, Kim EA, Kim KS, Pi SY. Transcatheter arterial embolization of pulmonary sequestration in neonates: long-term follow-up results. J Vasc Interv Radiol. 2003;14:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Zhang SX, Wang HD, Yang K, Cheng W, Wu W. Retrospective review of the diagnosis and treatment of pulmonary sequestration in 28 patients: surgery or endovascular techniques? J Thorac Dis. 2017;9:5153-5160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |