Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9332
Peer-review started: March 25, 2022
First decision: May 30, 2022
Revised: June 8, 2022
Accepted: July 21, 2022
Article in press: July 21, 2022
Published online: September 16, 2022
Processing time: 160 Days and 20.6 Hours
We explored the genotype-phenotype correlation of the novel deletion 16p13.2p12.3 in an 8-year-old child with progressive total ophthalmoplegia, cervical dyskinesia, and lower limb weakness by comparing the patient’s clinical features with previously reported data on adjacent copy number variation (CNV) regions.
Specifically, we first performed whole-exome sequencing, CNV-sequencing, and mitochondrial genome sequencing on the patient and his parents, then applied “MitoExome” (the entire mitochondrial genome and exons of nuclear genes encoding the mitochondrial proteome) analysis to screen for genetic mitoch
Taken together, these results indicated that 16p13.2p12.3 deletion causes a syndrome with the phenotype of early-onset total ophthalmoplegia. The “MitoExome” analysis is powerful for the differential diagnosis of mitochondrial diseases. We report a novel copy number variant in this case, but further confirmation is required.
Core Tip: At present, little is known about the associated phenotypes of copy number variations in the short arm of chromosome 16. The most remarkable copy number variations is 16p13.11 microdeletion/microduplication. The main clinical features of this syndrome are a series of neurological abnormalities such as mental retardation, autism, schizophrenia, epilepsy, and attention deficit hyperactivity disorder. We identified a de novo 7.23 Mb deletion, covering 16p13.2p12.3. 16p13.11 was included in the deleted region, which is the recurrent distinct region associated with neurodevelopmental disorder. However, the patient only displayed features of progressive total ophthalmoplegia, cervical dyskinesia, and weakness in the lower limbs without neurodevelopmental disorder.
- Citation: Xu M, Jiang J, He Y, Gu WY, Jin B. Early-onset ophthalmoplegia, cervical dyskinesia, and lower extremity weakness due to partial deletion of chromosome 16: A case report. World J Clin Cases 2022; 10(26): 9332-9339
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9332.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9332
At present, little is known regarding the associated phenotypes of copy number variations (CNV) in the short arm of chromosome 16. The most remarkable CNV is 16p13.11 microdeletion/microduplication. Notably, nuclear distribution gene E homolog 1 and N-terminal asparagine amidase genes located in 16p13.11 region have been associated with a series of neurological abnormalities, such as intellectual disabilities, autism, schizophrenia, epilepsy, and attention-deficit hyperactivity disorder in patients with this syndrome[1,2]. The adjacent regions of 16p13.11, chromosome 16p13.3 deletion causes a syndrome, which is characterized as failure to thrive, hypotonia, short stature, microcephaly, characteristic facial features, mild to moderate intellectual disability, organ anomalies, and vulnerability to infections[3]. Previous studies have shown that chromosome 16p11.2 locus, the other neighboring region characterized by recurrent CNVs, is the chromosomal region related to Autistic Spectrum Disorder[4,5]. It seems that 16p13 and its adjacent regions are highly suspected sites associated with a series of neuropsychiatric disorders. Primary or secondary causes of internal ophthalmoplegia usually indicate a central nervous system situation. For example, patients with mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes syndrome may display primary encephalopathy, including ptosis (external ophthalmoplegia) with internal ophthalmoplegia[6]. Notably, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes syndrome is caused by mitochondrial DNA mutations, while mitochondrial variations have been attributed to occurrence of diseases of Kearns-Sayre syndrome (KSS), which is associated with early-onset ophthalmoplegia[7,8].
The patient was an 8-year-old boy, who presented with gradually progressive unilateral ptosis at the age of 6 years, which developed to bilateral ptosis at 8 years and 4 mo.
The patient was an 8-year-old boy with bilateral ptosis.
At the end of December 2017, the patient developed a fever of unknown origin, with his body temperature found to be as high as 38.5 °C. He recovered after being given oral antipyretics. One week after the fever, the patient had a stroke of dyskinesia and kept tilting his head to the left (cervical dyskinesia), a phenomenon that was accompanied by eye movement disorders.
The patient was the first child born to non-consanguineous parents, and there are no similarly affected family members. Although he had a “history of congenital heart disease,” subsequent cardiac color Doppler ultrasound examination revealed normal results. The patient had no developmental milestone delays.
Physical examination revealed that he had bilateral upper eyelid dropping (50% pupils covered). Moreover, he had limited abduction in the right eye, neck dystonia, and slightly reduced (degree 5-) muscle strength in bilateral lower limbs, while his bilateral knee reflexes disappeared. He also exhibited right cryptorchidism and a small penis. He could neither squat nor jump on one foot.
Blood tests showed unremarkable results. Notably, he had a blood acetylcholine receptor antibody level of 0.82 nmol/L, while IgG tests for anti-MuSK, anti-Titin antibody, and human low-density lipoprotein receptor-related protein 4 were negative.
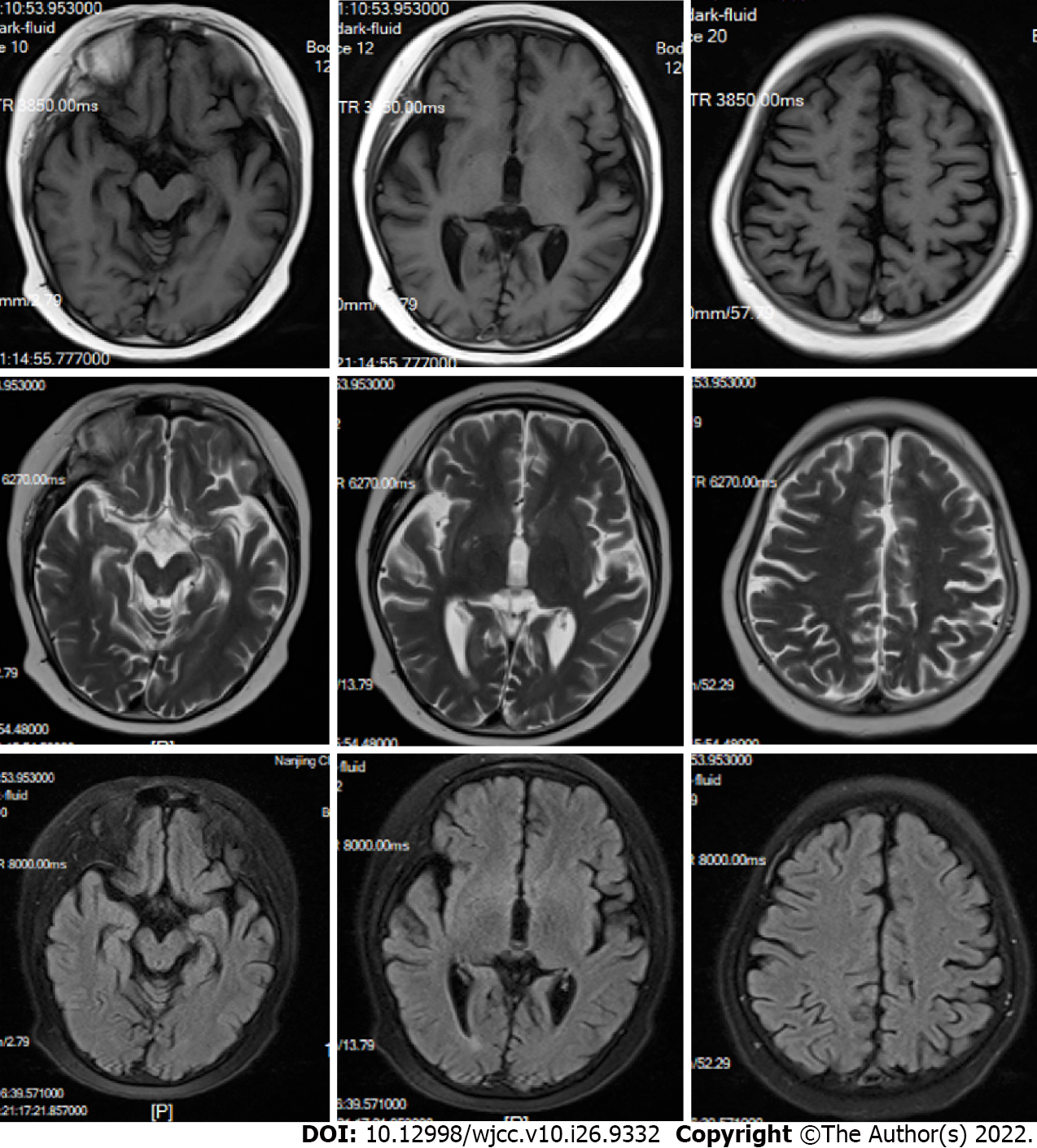
Brain magnetic resonance imaging revealed diffused signals in the occipital, parietal cortex, and basal ganglia (Figure 1). His right ptosis was better, although it did not disappear after treatment with prednisone, neostigmine, and omeprazole from January 2018 to October 2019. His eye lid dropping reappeared in March 2020 and progressed to be bilateral. In August 2019, he was hospitalized due to a recurrent headache and was unresponsive to mannitol and carbamazepine. The patient was currently in the first grade of elementary school with medium grades.
Whole exome libraries were prepared using the xGen Exome Research Panel v1.0 (IDT, Iowa, United States) and sequenced on the Novaseq 6000 platform (Illumina, San Diego, CA, United States). Raw data were cleaned using the fastp software package. Subsequently, the paired-end reads were performed using Burrows-Wheeler Aligner to the Ensemble GRCh37/hg19 reference genome. Synonymous and short indel calling were conducted using GATK software package followed by ANNOVAR annotation. Prediction was performed using the Provean, Sift, Polypen2_hdiv, Polypen2_hvar, Mutationtaster, M-Cap, and Revel software packages. The pathogenicity of all the variants was interpretated according to the guidelines of the American College of Medical Genetics and Genomics.
We performed CNV-sequencing[9], a CNV detection method based on high-throughput sequencing, in the patient. Briefly, genomic DNA was first sheared to 200-300 bp fragments via sonication, then subjected to quality control via electrophoresis. Ends of DNA fragments were patched using a DNA repair enzyme system to generate blunt ends, then a single adenine nucleotide was added to the 3’ end to form an overhanging A-tail. Subsequently, the genome was amplified by ligation-mediated PCR for 4-6 cycles. We used the same sequencing platform and data cleaning protocols to detect CNVs with a length of 100 kB and above using Chigene independently developed software packages. After that, we employed Decipher, ClinVar, OMIM, DGV, and ClinGen for annotation.
At least 2 mL peripheral blood was collected from the patient, and mitochondrial DNA was extracted using the mitochondrial DNA extraction kit. Full-length mitochondrial DNA was amplified and purified via PCR, using the high-fidelity DNA polymerase and visualized via agarose gel electrophoresis. Paired-ended 150 bp sequencing was performed on the Novaseq6000 sequencing system. The sequenced data was aligned to reference sequence of NC_012920 (human complete mitochondrial genome 16569 bp circular DNA) using the Burrows-Wheeler Aligner software. The variants were then mapped onto the MITOMAP database, while pathogenicity was performed according to the MITOtip.
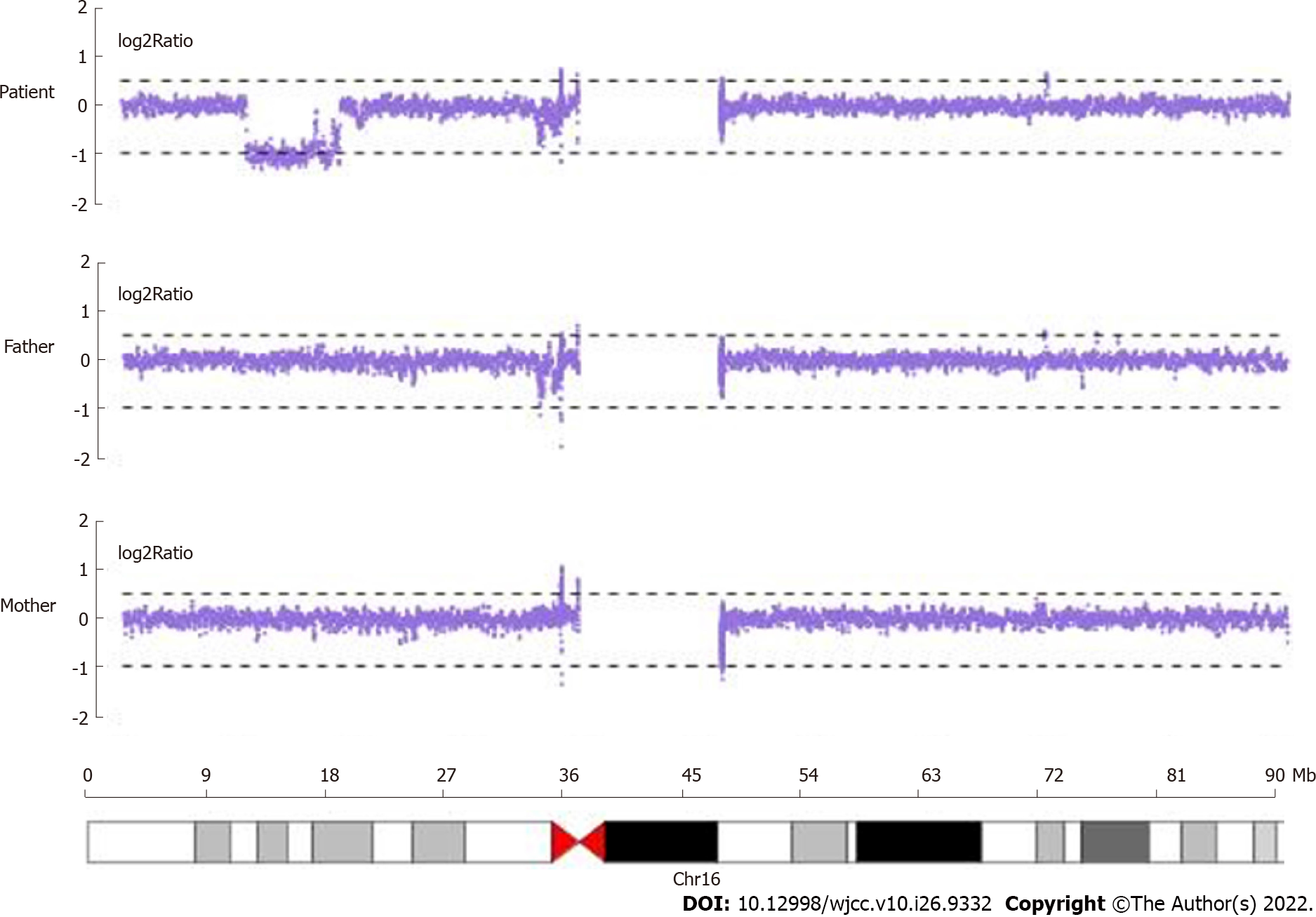
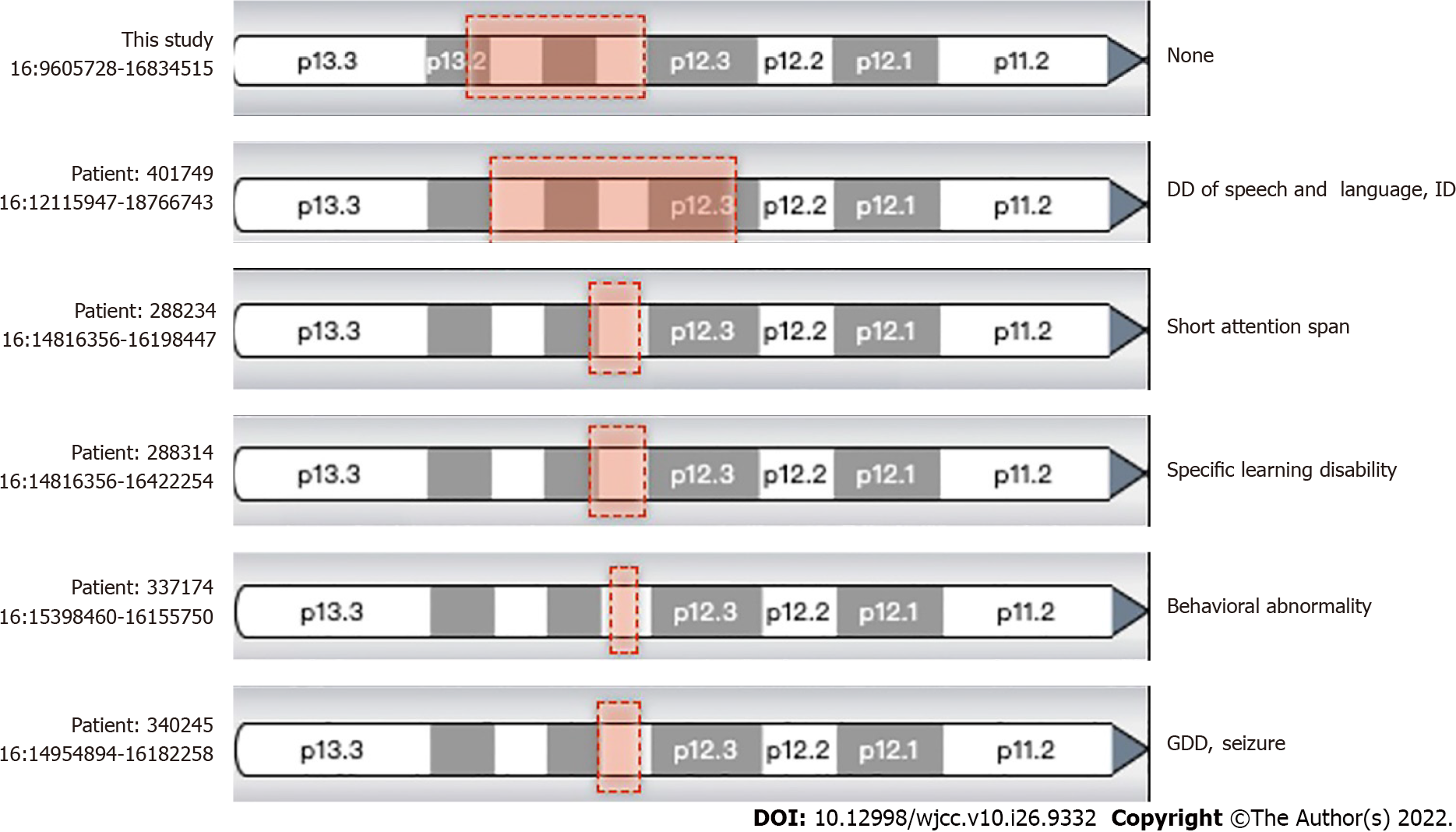
Whole-exome sequencing sequencing results revealed no suspected disease-causing variants. Next, we employed the CNV analysis method (developed by Chigene) on whole-exome sequencing data and found that two deletions of the neighboring region of Chr16:15,125,591-16326688 (approximately 1.20 Mb) and 9857005-14989502 (approximately 5.13 Mb). CNV sequencing data revealed de novo heterozygous deletion of chr16:9699585-16928372 (approximately 7.23 Mb) (Figure 2). Next, we compared this region and central nervous system phenotype with Decipher patients previously documented (Figure 3). Genes related to OMIM disorders are listed in Table 1. The mitochondrial DNA sequencing revealed negative results.
| Gene | Disorder | Inheritance |
| ABCC1 | Deafness, autosomal dominant 77 (?) | AD |
| ABCC6 | Arterial calcification, generalized, of infancy, 2 | AR |
| ABCC6 | Pseudoxanthoma elasticum | AR |
| ABCC6 | Pseudoxanthoma elasticum, forme fruste | AD |
| CIITA | Rheumatoid arthritis, susceptibility to | |
| CIITA | Bare lymphocyte syndrome, type II, complementation group A | AR |
| EMP2 | Nephrotic syndrome, type 10 | AR |
| ERCC4 | XFE progeroid syndrome | AR |
| ERCC4 | Fanconi anemia, complementation group Q | AR |
| ERCC4 | Xeroderma pigmentosum, type F/Cockayne syndrome | AR |
| GRIN2A | Epilepsy, focal, with speech disorder and with or without mental retardation | AD, ADIP |
| LITAF | Charcot-Marie-Tooth disease, type 1C | AD, H |
| MYH11 | Megacystis-microcolon-intestinal hypoperistalsis syndrome 2 | AR |
| MYH11 | Aortic aneurysm, familial thoracic 4 | AD |
| MYH11 | Visceral myopathy 2 | AD |
| NDE1 | Microhydranencephaly (?) | AR |
| NDE1 | Lissencephaly 4 (with microcephaly) | AR |
| PARN | Dyskeratosis congenita, autosomal recessive 6 | AR |
| PARN | Pulmonary fibrosis and/or bone marrow failure, telomere-related, 4 | AD, ADIP |
| TAT | Tyrosinemia, type II | AR |
| YY1AP1 | Grange syndrome | AR |
A de novo heterozygous deletion of chr16:9699585-16928372 (approximately 7.23 Mb) was identified. Due to this deletion on chromosome 16, the patient has early-onset ophthalmoplegia, cervical dyskinesia, and lower extremity weakness.
Since the patient showed elevated anti-acetylcholine receptor antibody levels, treatment with glucocorticoids and an acetylcholinesterase inhibitor were used.
The treatment effect was successful.
Ophthalmoplegia cases are characterized by central neurological, muscular, and synaptic abnormalities. In the present case, the evidence of the stroke-like onset of internal ophthalmoplegia and cervical dyskinesia and brain magnetic resonance imaging findings revealed a central nervous system disorder. Ophthalmoplegia is usually caused by damage to the midbrain. This patient’s magnetic resonance imaging revealed no abnormal signals in his midbrain and middle cranial fossa. In the patient’s anamnesis, we originally considered ophthalmoplegic migraine or recurrent ophthalmoplegic neuropathy, a rare condition manifesting as episodes of ipsilateral headache followed by ocular cranial nerves palsy, owing to his unexplained headache during the treatment. However, previous studies have shown that ophthalmoplegia typically persists for weeks to months and was reversible in patients with ophthalmoplegic migraine/recurrent ophthalmoplegic neuropathy[10,11]. Since the patient displayed elevated anti-acetylcholine receptor antibody levels and responded well to treatment using glucocorticoid and acetylcholinesterase inhibitor, myasthenia gravis (MG) was also the candidate diagnosis. However, MG failed to explain the early and urgent pattern of onset as well as the brain changes.
Mitochondrial diseases characterized by ocular symptoms include chronic progressive external ophthalmoplegia and KSS. Chronic progressive external ophthalmoplegia is a relatively mild mitochondrial disease characterized by extraocular muscle weakness and ptosis, often with weakness in the extremities[12]. Ptosis usually progresses to ophthalmoplegia over months or years. KSS was defined as chronic progressive external ophthalmoplegia initiated before age 20 with retinopathy of pigmentation and associated with at least one of the following: cardiac conduction disturbances, cerebellar ataxia, or elevated cerebrospinal fluid protein concentrations[13]. KSS, mitochondrial cytopathy due to mitochondrial CNV[14], could explain the early-onset, progressive external ophthalmoplegia, and suspected cardiomyopathy. However, analysis revealed negative results.
Our patient exhibited different clinical features from the previously reported cases of chromosome 16p CNVs. As far as we know, CNVs in chromosome 16p, including the proximal 16p11.2 deletion/duplication, intermedia 16p13.11 microdeletion/microduplication, and terminal 16p13.3 deletion, have been strongly associated with neurodevelopmental and/or neuropsychiatric disorders[1-5]. Moreover, Redaelli et al[14] identified four smallest regions of overlapping, one located in 16p13.11, one located in at 16p12.2, and two close smallest regions of overlapping occurring in 16p11.2. Based on the results of 27 CNVs in chromosome 16, the authors concluded that developmental delay in those patients was caused by recurrent non-allelic homologous recombination in chromosome 16[15]. Notably, the CNV in our patient resulted from full-length deletion of 16p13.11, while it is supposed to be associated with signs of 16p13.11 microdeletion syndrome, that is developmental delay, microcephaly, epilepsy, short stature, facial dysmorphism, and behavioral problems. However, we found none of these signs in our patient. Identification of the novel pathogenic CNV in our patient was inconclusive, according to the documented cases and OMIM genes (Table 1).
In conclusion, we identified a CNV heterozygous deletion in the 16p region in a Chinese patient with premature ophthalmoplegia, cervical spine dyskinesia, and lower extremity weakness through whole-exome sequencing, CNV sequencing, and MitoExome analysis strategies. MitoExome analysis is critical for differential diagnosis of mitochondrial diseases. Particularly, ptosis, ophthalmoplegia, and proximal muscle weakness are common occurrences in both mitochondrial diseases (MDs) and MG, which strongly indicate neuromuscular junction (NMJ) disorders. Generally, clinical grounds alone cannot effectively distinguish MDs and MG in patients with NMJ dysfunction[16,17]. Since NMJ abnormalities are common in MDs, there is need to perform MD-associated genetic tests. We recommend that “MitoExome” analysis should be applied in patients with an ambiguous diagnosis of NMJ disorders.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozden F, Turkey; Sharaf MM, Syria S-Editor: Wand DM L-Editor: Filipodia A P-Editor: Wang DM
| 1. | Ramalingam A, Zhou XG, Fiedler SD, Brawner SJ, Joyce JM, Liu HY, Yu S. 16p13.11 duplication is a risk factor for a wide spectrum of neuropsychiatric disorders. J Hum Genet. 2011;56:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Tropeano M, Andrieux J, Collier DA. Clinical utility gene card for: 16p13.11 microdeletion syndrome. Eur J Hum Genet 2014;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Bartsch O, Rasi S, Delicado A, Dyack S, Neumann LM, Seemanová E, Volleth M, Haaf T, Kalscheuer VM. Evidence for a new contiguous gene syndrome, the chromosome 16p13.3 deletion syndrome alias severe Rubinstein-Taybi syndrome. Hum Genet. 2006;120:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Taylor CM, Smith R, Lehman C, Mitchel MW, Singer K, Weaver WC, Chung W. 16p11.2 Recurrent Deletion. 2009 Sep 22. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–. [PubMed] |
| 5. | Niarchou M, Chawner SJRA, Doherty JL, Maillard AM, Jacquemont S, Chung WK, Green-Snyder L, Bernier RA, Goin-Kochel RP, Hanson E, Linden DEJ, Linden SC, Raymond FL, Skuse D, Hall J, Owen MJ, Bree MBMVD. Psychiatric disorders in children with 16p11.2 deletion and duplication. Transl Psychiatry. 2019;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Malfatti E, Laforêt P, Jardel C, Stojkovic T, Behin A, Eymard B, Lombès A, Benmalek A, Bécane HM, Berber N, Meune C, Duboc D, Wahbi K. High risk of severe cardiac adverse events in patients with mitochondrial m.3243A>G mutation. Neurology. 2013;80:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Barshop BA, Nyhan WL, Naviaux RK, McGowan KA, Friedlander M, Haas RH. Kearns-Sayre syndrome presenting as 2-oxoadipic aciduria. Mol Genet Metab. 2000;69:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Puoti G, Carrara F, Sampaolo S, De Caro M, Vincitorio CM, Invernizzi F, Zeviani M. Identical large scale rearrangement of mitochondrial DNA causes Kearns-Sayre syndrome in a mother and her son. J Med Genet. 2003;40:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Xie C, Tammi MT. CNV-seq, a new method to detect copy number variation using high-throughput sequencing. BMC Bioinformatics. 2009;10:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Gelfand AA, Gelfand JM, Prabakhar P, Goadsby PJ. Ophthalmoplegic "migraine" or recurrent ophthalmoplegic cranial neuropathy: new cases and a systematic review. J Child Neurol. 2012;27:759-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | López-Gallardo E, López-Pérez MJ, Montoya J, Ruiz-Pesini E. CPEO and KSS differ in the percentage and location of the mtDNA deletion. Mitochondrion. 2009;9:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Heidenreich JO, Klopstock T, Schirmer T, Saemann P, Mueller-Felber W, Auer DP. Chronic progressive external ophthalmoplegia: MR spectroscopy and MR diffusion studies in the brain. AJR Am J Roentgenol. 2006;187:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Remes AM, Peuhkurinen KJ, Herva R, Majamaa K, Hassinen IE. Kearns-Sayre syndrome case presenting a mitochondrial DNA deletion with unusual direct repeats and a rudimentary RNAase mitochondrial ribonucleotide processing target sequence. Genomics. 1993;16:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Redaelli S, Maitz S, Crosti F, Sala E, Villa N, Spaccini L, Selicorni A, Rigoldi M, Conconi D, Dalprà L, Roversi G, Bentivegna A. Refining the Phenotype of Recurrent Rearrangements of Chromosome 16. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Ben Yaou R, Laforêt P, Bécane HM, Jardel C, Sternberg D, Lombès A, Eymard B. [Misdiagnosis of mitochondrial myopathies: a study of 12 thymectomized patients]. Rev Neurol (Paris). 2006;162:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Whittaker RG, Schaefer AM, Taylor RW, Turnbull DM. Differential diagnosis in ptosis and ophthalmoplegia: mitochondrial disease or myasthenia? J Neurol. 2007;254:1138-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Braz LP, Ng YS, Gorman GS, Schaefer AM, McFarland R, Taylor RW, Turnbull DM, Whittaker RG. Neuromuscular Junction Abnormalities in Mitochondrial Disease: An Observational Cohort Study. Neurol Clin Pract. 2021;11:97-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |