Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9303
Peer-review started: February 13, 2022
First decision: June 7, 2022
Revised: June 15, 2022
Accepted: July 27, 2022
Article in press: July 27, 2022
Published online: September 16, 2022
Processing time: 200 Days and 13.3 Hours
Swelling after apical microsurgery is a postoperative reaction and may reduce quality of life during healing.
To evaluate periapical swelling after apical microsurgery and determine potential risk factors.
Ninety-eight apical microsurgery patients were selected for this study. Before surgery, bone shadow volume and density of pathological tissue were measured by cone beam computed tomography. The other variables (age, gender, operative teeth number, fistula, preoperative swelling, drug use and preoperative root canal treatments) were assessed during examination. Swelling degree was confirmed by questionnaires for patients on postoperative days 1, 7, 14 and 21. Statistical analyses were performed to identify predictors for swelling.
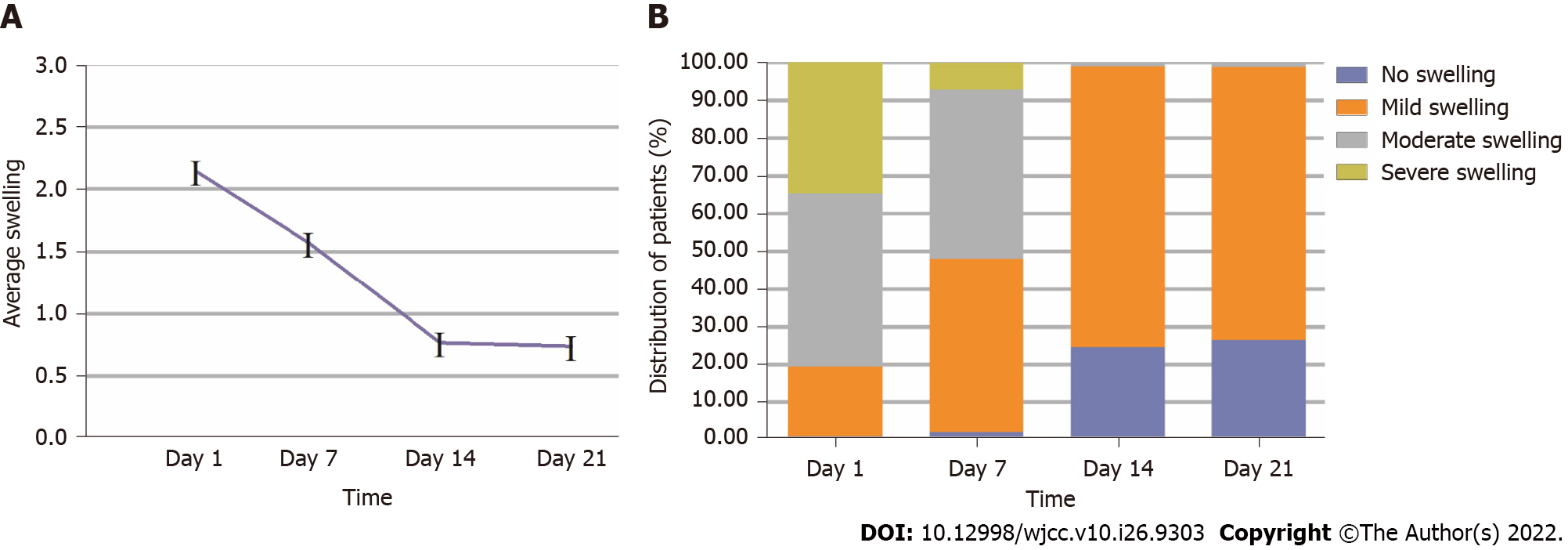
Majority of patients reported moderate (45.9%) or severe (34.7%) swelling on day 1, and moderate (44.9%) or mild (45.9%) on postoperative day 7. Ninety-nine percent of patients had no or mild swelling on postoperative day 14. The average swelling level peaked on day 1 postoperatively and gradually decreased. Of statistical significance, age, bone shadow volume and density of pathological tissue acted as predictors of swelling (P < 0.05). However, there was no significant difference in gender, tooth number, fistula, preoperative swelling, drug use, or preoperative root canal treatments (P > 0.05).
Younger patients with larger shadow volume and density were significantly more likely to develop swelling after apical microsurgery.
Core Tip: The impact of postapical swelling on daily life was viewed from the patients’ perspective. The conclusions demonstrate that postoperative swelling is more severe in those with larger volume and density of apical lesions, so the importance of preoperative cone beam computed tomography examination is emphasized. Younger patients with high postoperative prognostic requirements may have more severe postoperative swelling than older patients and should be given more clinical attention.
- Citation: Bi C, Xia SQ, Zhu YC, Lian XZ, Hu LJ, Rao CX, Jin HB, Shang XD, Jin FF, Li JY, Zheng P, Wang SH. Incidence and risk factor analysis for swelling after apical microsurgery. World J Clin Cases 2022; 10(26): 9303-9309
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9303.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9303
Apical microsurgery is a newly developed procedure that comprises periapical curettage, apicoectomy, retropreparation and retrofilling of a root canal under dental microscopy[1]. It holds a high success rate of controlling periapical infection, so as to promote periapical healing and tissue regeneration[2]. This may be due to its accuracy, minimally invasive nature, and high curative effect through the use of microscopic magnification, illumination, ultrasonic instrument tips and root-end filling materials[3-5].
Apical microsurgery cannot avoid a certain degree of swelling, which is one of the most common postoperative complications[6]. Kvist and Reit[7] stated that almost all patients developed swelling and pain, with these complications mostly peaking on the first day after surgery. Additionally, swelling of the mucoperiosteal flap is also reported after tooth extraction surgery, where the triangular flap has a greater increase in swelling than the envelope flap within a week[8]. A low probability of swelling is reported after root canal treatment, especially after root canal filling[9]. However, single or multiple root canal treatments have no significant effect on the degree of swelling. Most short- and long-term complications (pain and swelling) show a similar frequency. However, people who make a single clinical visit are more likely to experience pain and to take painkillers[10].
To date, many studies have focused on swelling after tooth extraction surgery and root canal treatments. There has also been some research that illustrates predictors of pain after apical microsurgery[11]. However, few studies have clarified predictors of swelling after apical microsurgery. The aim of this study, therefore, was to evaluate the characteristics and degree of periapical swelling after apical microsurgery and to determine the potential risk factors of periapical swelling.
This study was conducted according to the Declaration of Helsinki principles. The study protocol was approved by the Medical Ethics Committee of Hangzhou Dental Hospital (June 9, 2021). Written informed consent was obtained from each subject before participating in the study. Patients who need apical microsurgery were recruited at Microscopic Dentistry Center of Dentistry, Hangzhou Dental Hospital, from 2018 to 2020.
Basic patient information, preoperative and postoperative physical examination notes, clinical symptoms and other relevant patient records were considered in patient selection. Imaging data from preoperative and postoperative follow-up visits were included. Eligible patients were identified according to the following inclusion criteria: patients graded as Classification Ⅰ or Grade A by the American Society of Anesthesiologists; patients between 18 and 65 years of age who meet the indications for apical microsurgery surgery; patients who had a preoperative cone beam computed tomography scan taken that was of good diagnostic quality and with teeth treated by unified and standardized apical microsurgery; patients with a follow-up period > 6 mo. Ninety-eight patients’ records met the inclusion criteria and were included in this study for further analysis. All apical microsurgeries were performed by a single endodontic specialist at the VIP Center in Hangzhou Dental Hospital.
First, for mandibular posterior teeth, 2 mL 2% lidocaine with 1:100000 adrenaline was administered by inferior alveolar nerve block, and 3.4 mL 4% articaine hydrochloride with 1:100000 adrenaline was administered by infiltration to the maxillary and mandibular anterior teeth. A single vertical incision was made distant to the periapical lesion and sulcular incisions in the corresponding region, and the mucoperiosteal envelope flap was elevated. Secondly, from this stage on, all surgical procedures were performed under a dental operating microscope (OMS2350; ZUMAX, Jiangsu, China) with coaxial illumination and a zoom magnification changer. Magnifications between 3.4 and 20.4 were used. The periapical region was curetted, and the granulation tissue removed. A following apicoectomy was performed with a fissure bur 3 mm coronal to the apex. Thirdly, subsequent smoothing with a piriform bur applied rectangularly to the axis of the root resulted in a bevel of approximately 10. The resulting surface was stained with 50 mg/5 mL methylthioninium chloride and inspected by using a micromirror (JIMM2; B&L Biotech, Ansan-si, South Korea) under high magnification to detect individual canal anatomy, in particular, any additional canals, isthmuses or root fractures. After cleaning with 30% hydrogen peroxide and 96% alcohol, and drying, the prepared cavity was filled with Intermediate Restorative Material (Dentsply Caulk, Milford, DE, Untied States). Finally, the filling and the apical surface were smoothed with a piriform stainless steel bur. The flap was closed with single interrupted sutures, and a postoperative digital radio graph was taken. Patients were instructed to take 20 mg serrapeptase enteric-coated tablets or 1.5 mg dexamethasone acetate tablets after meals t.i.d., and 60 mg loxoprofen sodium tablets as needed.
On postoperative days 1, 7, 14 and 21, patients were given questionnaires with a visual analog scale to evaluate the degree of swelling: 0 (no swelling): the patient felt like usual; 1-3 (mild swelling): the patient could tolerate symptoms, the appearance did not affect his/her life and work, and no drug treatment was needed; 4-6 (moderate swelling): the patient was anxious due to changed appearance caused by swelling, which affected his/her work and life, and drug intervention was needed, indicating that the swelling was hard to tolerate; 7-10 (severe swelling): the patient felt severe anxiety and unhappiness due to changed appearance caused by swelling, which seriously affected his/her work and life, and an extended period of time was needed for drug intervention to alleviate symptoms, indicating that the swelling was severe or intolerable. All patients’ degree of swelling was collected and gathered into electronic records, which indicated their degree of swelling at different points in time.
On postoperative day 1, severe tissue damage in the surgical area led to severe swelling, which affected the judgment of predictors. Thus, swelling conditions on postoperative day 7 were chosen for data comparison in order to speculate on risk factors. The patients were finally divided into two groups for comparison. No swelling and mild swelling were classified as one group, while moderate swelling and severe swelling were the other group. Independent data analysis was performed for the following predictors: age, gender, tooth number, fistula, preoperative swelling, drug use, preoperative root canal treatment, bone shadow volume, and density of pathological tissue.
The Mann-Whitney U test and χ2 test were used to analyze and compare the basic characteristics of patients included in each group. SPSS version 26.0 (IBM Corp, Armonk, NY, Untied States) was used for all analyses. The mean ± SD (numerical range) was used for statistical description of the data and the difference was considered statistically significant if P was < 0.05.
Table 1 shows the statistical comparison of different predictors between the no or mild swelling group and the moderate or severe swelling group on postoperative day 7. In the analysis of 98 patients, those with moderate or severe swelling were significantly younger than those with no or mild swelling (P < 0.05). Additionally, larger preoperative bone shadow volume demonstrated severe swelling (P < 0.05). The density of pathological tissue was significantly different between the groups (P < 0.05), with the deeper density being more swollen. However, there was no significant difference in gender, tooth number, tooth position, fistula, preoperative swelling, drug use or preoperative root canal treatment (P > 0.05).
| No/mild swelling group (n = 47) | Moderate/severe swelling group (n = 51) | P value | |
| Age (mean ± SD) | 39.51 ± 11.08 | 33.16 ± 10.84 | 0.002 |
| Sex | |||
| Male | 15 (31.9) | 12 (23.5) | |
| Female | 32 (68.1) | 39 (76.5) | 0.353 |
| Teeth | |||
| Single | 37 (78.7) | 40 (78.4) | |
| Multiple | 10 (21.3) | 11 (21.6) | 0.972 |
| Shadow volume (mean ± SD) | 113.94 ± 235.52 | 220.02 ± 197.24 | 0 |
| Fistula | |||
| No | 36 (76.6) | 40 (78.4) | |
| Yes | 11 (23.4) | 11 (21.6) | 0.828 |
| Bone density (mean ± SD) | 1521.15 ± 384.62 | 1664.39 ± 277.03 | 0.039 |
| Swelling (preoperative) | |||
| No | 27 (57.4) | 30 (58.8) | |
| Yes | 20 (42.6) | 21 (41.2) | 0.89 |
| Medication (postoperative) | |||
| No | 32 (68.1) | 33 (64.7) | |
| Yes | 15 (31.9) | 18 (35.3) | 0.724 |
| Endodontic surgery (preoperative) | |||
| No | 7 (14.9) | 12 (23.5) | |
| Yes | 40 (85.1) | 39 (76.5) | 0.28 |
Figure 1 shows the trends after surgery. Severe swelling was most dominant on postoperative day 1 (34.7%), and gradually decreased until postoperative day 14. Most patients reported moderate (45.9%) and severe (34.7%) swelling on postoperative day 1, while moderate (44.9%) and mild (45.9%) swelling on postoperative day 7 were most reported. Ninety-nine percent of patients had no or mild swelling on postoperative day 14.
Swelling is one of the most common complications after apical surgery and is directly related to the degree of tissue trauma during surgery[12]. It is considered a normal, predictable physiological reaction after surgery that may reduce quality of life during healing. Symptoms of swelling must therefore be minimized[13]. In this research, the degree of postoperative swelling was defined from the perspective of patients according to the influence of swelling on their daily life. This degree of swelling is also applicable to other oral and maxillofacial surgery procedures, such as oral and maxillofacial bone grafting and orthognathic surgery.
Postoperative swelling is a pathological process of transformation. In the early stages after surgery, a series of inflammatory reactions and oxidative stress occur in tissue at the surgical site. Stitched wounds increase local tension, causing local arterial congestion and dilation, leading to blocked lymphatic reflux and retention of large amounts of fluid in the tissue[14]. After 7 d, the wound gradually contracts, basal cells proliferate, and granulation tissue and corresponding fibers reconstruct[15]. Fourteen days postoperatively, the wound heals, and the swelling is reduced. However, all the operations in this study involved 30% hydrogen peroxide and 96% alcohol as disinfectants. This method of disinfection has some limitations and recent studies suggest that chlorhexidine may be more effective[16].
Younger patients were more likely to experience moderate and severe swelling after surgery. This may be related to a decline in immunity and inflammatory response in older patients[17]. In addition, protective pulp calcification, reduced root canal attenuation and dentin tubule calcification in older people may also lead to reducing swelling after surgery[18].
Swelling is an increase in tissue volume caused by stress reaction in tissue. In apical microsurgery, the number of teeth operated on is not the main factor of swelling, but rather the severity of pathological changes prior to the operation[19]. In this study, the main indicators were bone shadow volume and density of pathological tissue. Bone destruction was one of the most salient causes of poor prognosis. Clinical and radiological investigations by Mikkonen et al[20] found that periodontal crest absorption and traumatic occlusion might impede the healing process after apical surgery. Another study found that peripheral bone destruction and marginal bone loss also affected tissue healing[21].
In this study, gender, tooth number, fistula, preoperative swelling, drug use after operation, and preoperative root canal therapy showed no significant difference. Results related to gender were different from those of Iqbal et al[22], who investigated the degree of pain after apical microsurgery. They found that gender had a significant impact on the incidence of severe pain, which may be related to the different pain thresholds between men and women. Periapical inflammation was mostly controlled after apical microsurgery. Apical microsurgery promoted the closure of fistula, but was not significant in the degree of swelling. The appropriate amount of dexamethasone was reported to both reduce swelling and improve prognosis[23].
The value of bone shadow volume and density of pathological tissue as predictors of postoperative pain and swelling was highlighted in the current study. This information can guide physicians to take appropriate measures in treatment plans to reduce the degree of swelling after apical microsurgery and improve prognosis.
Based on this clinical analysis, periapical swelling after apical microsurgery occurred frequently. Risk factors may be younger age, larger bone shadow volume and deeper density of pathological tissue. However, other related risk factors an methods to improve prognosis must still be further elaborated.
Apical microsurgery is a newly developed procedure that can achieve a high success rate in controlling periapical infection, so as to promote periapical healing and tissue regeneration. However, apical microsurgery cannot avoid some swelling, which is one of the most common postoperative complications.
Many studies have focused on swelling after tooth extraction or root canal treatments and illustrated predictors of pain after apical microsurgery. However, few studies have clarified predictors of swelling after apical microsurgery. So, our study focused on swelling after apical microsurgery and tried to clarify the predictors.
Through evaluating the degree and characteristics of periapical swelling after apical microsurgery to determine the potential risk factors of periapical swelling.
Ninety-eight apical microsurgery patients were selected for this study. Before surgery, cone beam computed tomography (CBCT) was used to measure the bone shadow volume and density of pathological tissue. The other variables (age, gender, operative teeth number, etc.) were assessed during examination and the swelling degree was confirmed by questionnaires for patients on postoperative days 1, 7, 14 and 21 after surgery. The predictors for swelling were performed by statistical analyses.
Most patients reported moderate (45.9%) or severe (34.7%) swelling on postoperative day 1, and moderate (44.9%) or mild (45.9%) on postoperative day 7. Ninety-nine percent patients had no or mild swelling on postoperative day 14. The average swelling level peaked on postoperative day 1 and gradually decreased. Age, bone shadow volume and density of pathological tissue acted as significant predictors of swelling. However, there was no significant difference in gender, tooth number, fistula, preoperative swelling, drug use, or preoperative root canal treatments.
Young patients with larger volume and density of shadow around the tooth apical in CBCT were more likely to develop swelling after apical microsurgery.
For patients who need microscopic apical surgery, preoperative CBCT is necessary as bone shadow volume and pathological tissue density are predictors of postoperative swelling. Based on these indicators, physicians can take appropriate measures in the treatment plan to reduce the swelling after microsurgery and the impact on the patients’ daily life.
Thanks are due to Chen RC for assistance with data guidance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Capparè P, Italy; Galiatsatos A, Greece; Sun C, United States S-Editor: Gao CC L-Editor: Kerr C P-Editor: Gao CC
| 1. | von Arx T, Walker WA 3rd. Microsurgical instruments for root-end cavity preparation following apicoectomy: a literature review. Endod Dent Traumatol. 2000;16:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Kim S, Kratchman S. Microsurgery in Endodontics. 4th ed. Hoboken, NJ: Wiley Blackwell, 2018: 37-38. |
| 3. | Tsesis I, Fuss Z, Lin S, Tilinger G, Peled M. Analysis of postoperative symptoms following surgical endodontic treatment. Quintessence Int. 2003;34:756-760. [PubMed] |
| 4. | Floratos S, Kim S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent Clin North Am. 2017;61:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Setzer FC, Shah SB, Kohli MR, Karabucak B, Kim S. Outcome of endodontic surgery: a meta-analysis of the literature--part 1: Comparison of traditional root-end surgery and endodontic microsurgery. J Endod. 2010;36:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Christiansen R, Kirkevang LL, Hørsted-Bindslev P, Wenzel A. Patient discomfort following periapical surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Kvist T, Reit C. Postoperative discomfort associated with surgical and nonsurgical endodontic retreatment. Endod Dent Traumatol. 2000;16:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Coulthard P, Bailey E, Esposito M, Furness S, Renton TF, Worthington HV. Surgical techniques for the removal of mandibular wisdom teeth. Cochrane Database Syst Rev. 2014;CD004345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Wang C, Xu P, Ren L, Dong G, Ye L. Comparison of post-obturation pain experience following one-visit and two-visit root canal treatment on teeth with vital pulps: a randomized controlled trial. Int Endod J. 2010;43:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Figini L, Lodi G, Gorni F, Gagliani M. Single vs multiple visits for endodontic treatment of permanent teeth: a Cochrane systematic review. J Endod. 2008;34:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Malagise CJ, Khalighinejad N, Patel YT, Jalali P, He J. Severe Pain after Endodontic Surgery: An Analysis of Incidence and Risk Factors. J Endod. 2021;47:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Zandi M, Amini P, Keshavarz A. Effectiveness of cold therapy in reducing pain, trismus, and oedema after impacted mandibular third molar surgery: a randomized, self-controlled, observer-blind, split-mouth clinical trial. Int J Oral Maxillofac Surg. 2016;45:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Mojsa IM, Pokrowiecki R, Lipczynski K, Czerwonka D, Szczeklik K, Zaleska M. Effect of submucosal dexamethasone injection on postoperative pain, oedema, and trismus following mandibular third molar surgery: a prospective, randomized, double-blind clinical trial. Int J Oral Maxillofac Surg. 2017;46:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 15. | Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth Analg. 2020;131:1693-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 16. | Calderini A, Pantaleo G, Rossi A, Gazzolo D, Polizzi E. Adjunctive effect of chlorhexidine antiseptics in mechanical periodontal treatment: first results of a preliminary case series. Int J Dent Hyg. 2013;11:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Vitlic A, Lord JM, Phillips AC. Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. Age (Dordr). 2014;36:9631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Carvalho TS, Lussi A. Age-related morphological, histological and functional changes in teeth. J Oral Rehabil. 2017;44:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Penarrocha M, Garcia B, Marti E, Balaguer J. Pain and inflammation after periapical surgery in 60 patients. J Oral Maxillofac Surg. 2006;64:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Mikkonen M, Kullaa-Mikkonen A, Kotilainen R. Clinical and radiologic re-examination of apicoectomized teeth. Oral Surg Oral Med Oral Pathol. 1983;55:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Hirsch JM, Ahlström U, Henrikson PA, Heyden G, Peterson LE. Periapical surgery. Int J Oral Surg. 1979;8:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Iqbal MK, Kratchman SI, Guess GM, Karabucak B, Kim S. Microscopic periradicular surgery: perioperative predictors for postoperative clinical outcomes and quality of life assessment. J Endod. 2007;33:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Al-Khateeb TH, Nusair Y. Effect of the proteolytic enzyme serrapeptase on swelling, pain and trismus after surgical extraction of mandibular third molars. Int J Oral Maxillofac Surg. 2008;37:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |