Published online Sep 16, 2022. doi: 10.12998/wjcc.v10.i26.9264
Peer-review started: November 10, 2021
First decision: December 2, 2021
Revised: December 10, 2022
Accepted: August 5, 2022
Article in press: August 5, 2022
Published online: September 16, 2022
Processing time: 295 Days and 21 Hours
Alpha-fetoprotein (AFP) is one of the diagnostic standards for primary liver cancer (PLC); however, AFP exhibits insufficient sensitivity and specificity for diagnosing PLC.
To evaluate the effects of high-risk factors and the diagnostic value of AFP in stratified PLC.
In total, 289 PLC cases from 2013 to 2019 were selected for analysis. First, the contributions of high-risk factors in stratifying PLC were compared according to the following criteria: Child–Pugh score, clinical stage of liver cirrhosis, tumor size, and Barcelona Clinic Liver Cancer (BCLC) stage. Then, the diagnostic value of AFP was evaluated in different stratifications of PLC by receiver operating characteristic curves. For PLC cases in which AFP played little role, the diagnostic values of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), gamma-glutamyl transferase (GGT), and AFP were analyzed.
The roles of high-risk factors differed in stratified PLC. The incidence of smoking and drinking history was higher in PLC with Child–Pugh scores of C (P < 0.0167). The hepatitis B virus (HBV) infection rate in PLC with cirrhosis was more than in PLC without cirrhosis (P < 0.0167). Small tumors were more prone to cirrhosis than large tumors (P < 0.005). BCLC stage D PLC was more likely to be associated with HBV infection and cirrhosis (P < 0.0083). AFP levels were higher in PLC with cirrhosis, diffuse tumors, and BCLC stage D disease. In diagnosing PLC defined as Child–Pugh A, B, and C, massive hepatoma, diffuse hepatoma, BCLC stage B, C, and D, and AFP showed significant diagnostic value [all area under the curve (AUC) > 0.700]. However, these measures were meaningless (AUC < 0.600) in small hepatomas and BCLC A stage PLC, but could be replaced by the combined detection of CEA, CA 19-9, GGT, and AFP (AUC = 0.810 and 0.846, respectively).
Stratification of PLC was essential for precise diagnoses and benefited from evaluating AFP levels.
Core Tip: To evaluate the effects of high-risk factors and the diagnostic value of alpha-fetoprotein (AFP) in stratified primary liver cancer (PLC), 289 cases were selected for analysis. First, the contributions of high-risk factors in stratifying PLC were compared. Then, the diagnostic value of AFP was evaluated in different stratifications of PLC by receiver operating characteristic curves. For PLC cases in which AFP played little role, the diagnostic values of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9, gamma-glutamyl transferase, and AFP were analyzed. It was concluded that stratification of PLC was essential for precise diagnoses and it benefited from diagnostic values of AFP.
- Citation: Jiao HB, Wang W, Guo MN, Su YL, Pang DQ, Wang BL, Shi J, Wu JH. Evaluation of high-risk factors and the diagnostic value of alpha-fetoprotein in the stratification of primary liver cancer. World J Clin Cases 2022; 10(26): 9264-9275
- URL: https://www.wjgnet.com/2307-8960/full/v10/i26/9264.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i26.9264
Primary liver cancer (PLC) was the second leading cause of cancer-related mortality in 2014, with its death toll accounting for 51% of total global deaths[1]. Among all liver cancer patients, the 5-year overall survival rate is only 10.1%, and cirrhosis is the primary cause of death for PLC patients[2,3]. PLC incidence rates vary across clinical etiologies and conditions such as liver disease severity; even within the same clinical entity, individual PLC risk is heterogeneous across patients for unknown reasons[4]. Hence, clinically meaningful utility must be demonstrated under specific clinical scenarios for a diagnostic modality to be adopted into regular use. This was the initial purpose of stratifying the PLC cases in this study. Most previous studies have included hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection, alcohol use, non-alcoholic fatty liver, cirrhosis, gender, and age as the high-risk factors related to PLC[5]. Of these, infection by hepatoma viruses was the primary contributing factor to liver cancer in developing countries such as Asia and Africa[6,7]. HBV promotes malignant changes in liver cells by infecting the host and integrating the genome[8,9]. In contrast, non-alcoholic fatty liver disease and other metabolic diseases are the main susceptibility factors for PLC in developed countries[10,11].
Early diagnosis is vital for expanding treatment choices and improving the prognosis and quality of life of PLC patients. Alpha-fetoprotein (AFP) is a widely used serological marker that shows increased levels 8 to 11 mo before symptoms occur; thus, it is one of the diagnostic standards in the guidelines for PLC in China and Japan[12]. However, related reports have demonstrated that AFP shows deficient sensitivity and specificity for diagnosing PLC, causing missed diagnoses and misdiagnoses[13]. Therefore, this retrospective study aimed to evaluate the role of high-risk factors in diagnosing stratified PLC cases, especially the diagnostic value of AFP.
In total, 289 untreated PLC patients who were initially diagnosed at the North China University of Science and Technology Affiliated Hospital according to the guidelines for the diagnosis and treatment of PLC in China (2017 Edition)[14] were selected as the observation group. The control group consisted of 217 untreated cases with chronic hepatitis B and 279 cases with cirrhosis. There were no differences between the two groups in terms of age (χ2 = 0.536, P = 0.765) or gender (F = 2.869, P = 0.057).
Clinical criteria of the observation group were determined as follows: (1) PLC tumor size classifications were based on the Expert consensus on pathological diagnosis of PLC[15]; and (2) Child–Pugh liver function scores (similar to assessments in the Japan Society of Hepatology guidelines) and Barcelona Clinic Liver Cancer (BCLC) staging standards were derived from guidelines for the diagnosis and treatment of PLC in China (2017 Edition).
The exclusion criteria for this study were: (1) Metastatic cancer; (2) prior treatment for PLC; (3) the size and number of PLC and/or metastatic lesions were unclear from imaging examinations such as magnetic resonance imaging (MRI) or computed tomography (CT); and (4) the serological tumor markers AFP, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and gamma-glutamyl transferase (GGT) were not detected.
Clinical criteria in the control group were determined as follows: (1) Chronic hepatitis B cases were defined as chronic inflammatory diseases of the liver caused by HBV that had lasted for > 6 mo and were diagnosed according to the Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2019 Version)[16]; and (2) cirrhosis was judged by the Chinese Liver Disease Diagnosis and Treatment Management Standards[17] and confirmed by imaging and/or pathological examinations.
General data collection: Relevant patient information (gender, age, smoking and drinking history, and other basic information) were collected from the electronic medical records system. Patients with a history of smoking were defined as those who had smoked more than 1 cigarette per day for more than one year in a row. Patients with a history of drinking alcohol were defined as those who consumed more than 100 mL per day for more than 1 year in a row.
The main symptoms and signs of PLC included systemic symptoms (fatigue, loss of appetite, edema, and liver disease face), digestive symptoms (bloating, diarrhea, and abdominal pain), and bleeding symptoms ranging from a bleeding tendency to anemia. For patients with PLC with cirrhosis, neuropsychiatric performance metrics were also collected including personality, communication, behavior, calculation ability, intelligence, consciousness, orientation, and whether there were flapping tremors, increased muscle tone, tendon hyperreflexia, ankle clonus or Babinski signs, and other abnormal nervous system parameters, in addition to whether ascites were present.
Imaging data collection: Imaging data were collected from the examination results of PLC patients’ abdominal ultrasound, abdominal MRI enhancement, or abdominal CT enhancement scans, including the size and number of PLC and metastases, and the involvement of blood vessels such as the portal vein.
Laboratory-related data collection: Venous blood samples were collected after fasting (> 12 h) to detect serum markers including HBV antigen (HBsAg), HCV-Ag, albumin, total bilirubin, direct bilirubin, prothrombin time (PT), AFP, CEA, CA 19-9, and GGT. Among them, the liver function items were detected using a Beckman Coulter AU5800 or AU5821 automatic biochemical analyzer (Beckman Coulter, Brea, CA, United States); serum tumor markers were detected using a Roche 602 electrochemiluminescence immunoassay (Roche, Basel, Switzerland); PT was detected using a STAGO STAR Max instrument (Stago, Inc., Parsippany, NJ, United States); HBsAg and HCV-Ag were detected using a Mindray CL6000i (Mindray, Shenzhen, China). The cut-off level for AFP was 400 ng/mL in accordance with guidelines for the diagnosis and treatment of PLC in China (2017 Edition).
All data were analyzed using SPSS 19.0 software (IBM, Armonk, NY, United States). Counting data are presented as rates, and differences among groups were compared with the χ2 test. For pairwise comparisons between multiple sets of rates, the test standard adjustment method was used. Measurement data were first tested with the Kolmogorov–Smirnov test to check whether the measurement data of each group were normally distributed. Normally distributed data are presented as mean ± standard deviation, and differences between two groups were analyzed with the T test and differences among multiple groups were analyzed by ANOVA. Non-normally distributed measurement data are presented as median (interquartile range), and differences between two groups were analyzed with the Mann–Whitney U test, differences among independent samples were analyzed with the Kruskal–Wallis H test, and differences between two sets of measurement data were analyzed with the Bonferroni test. The method of adjusting test standards took α” = α/N as the test standard. In the formula N = k (k−1) ÷ 2, k is the number of sample rates. The other methods used α = 0.05 as the test standard.
Statement: Statistical review of the study was performed by a biomedical statistician.
PLC cases were stratified according to liver function Child–Pugh scores, clinical stage of liver cirrhosis, classification of liver cancer size, and BCLC stage. Subsequently, the influences of high-risk factors (age, gender, HBV and/or HCV infection, smoking, alcohol use, non-alcoholic fatty liver, and cirrhosis) on the stratification of PLC were compared to provide more reference. Tables 1-4 show the general data for each item with cases and rates. The main results are presented below.
| Factors | Criterion: Child-Pugh score | Statistics | P value | ||
| A | B | C | |||
| Male (%) | 40 (76.9) | 61 (77.2) | 16 (76.9) | 0.06 | 0.814 |
| Age (yr) | 59.40 ± 10.18 | 62.83 ± 9.85 | 58.50 ± 7.20 | 2.78 | 0.065 |
| History of smoking (%) | 16 (30.2) | 22 (27.8)a | 12 (60.0) | 7.74 | 0.021 |
| History of drinking (%) | 13 (24.5)a | 26 (32.9) | 11 (55.0) | 6.11 | 0.047 |
| HBsAg (+) (%) | 38 (71.7) | 56 (70.9) | 14 (70.0) | 0.02 | 0.989 |
| HCV-Ag (+) (%) | 3 (5.7) | 5 (6.3) | 0 (0.0) | 1.31 | 0.520 |
| Non-alcoholic fatty liver (%) | 9 (17.0) | 18 (22.8) | 1 (5.0) | 3.47 | 0.176 |
| Factors | Criterion: Clinical stage of cirrhosis | Statistics | P value | ||
| No cirrhosis | Compensated | Decompensated | |||
| Male (%) | 99 (72.3) | 33 (78.6) | 84 (77.1) | 1.08 | 0.582 |
| Age (yr) | 62.93 ± 11.85 | 61.21 ± 1.58 | 60.93 ± 9.04 | 1.14 | 0.322 |
| History of smoking (%) | 47 (34.3) | 13 (30.2) | 37 (33.9) | 0.26 | 0.880 |
| History of drinking (%) | 50 (36.5) | 11 (26.2) | 39 (35.8) | 1.59 | 0.451 |
| HBsAg (+) (%) | 46 (33.6) | 32 (76.2) a | 76 (69.1) a | 41.27 | < 0.001 |
| HCV-Ag (+) (%) | 9 (6.6) | 2 (4.8) | 6 (5.5) | 0.25 | 0.883 |
| Non-alcoholic fatty liver (%) | 39 (28.5) | 9 (21.4) | 19 (17.3) | 4.38 | 0.112 |
| Factors | Criterion: Pathological classification of tumor size | Statistics | P value | ||||
| Small | Medium | Large | Massive | Diffuse | |||
| Male (%) | 30 (68.2) | 17 (70.8) | 30 (66.7) | 22 (75.9) | 117 (80.1) | 5.05 | 0.283 |
| Age (yr) | 62.86 ± 9.03 | 59.45 ± 10.43 | 61.86 ± 8.86 | 57.79 ± 11.69 | 63.03 ± 11.75 | 1.75 | 0.139 |
| History of smoking (%) | 9 (20.5) | 9 (37.5) | 12 (26.7) | 12 (41.4) | 55 (37.4) | 6.29 | 0.179 |
| History of drinking (%) | 10 (22.7) | 8 (33.3) | 14 (31.1) | 11 (37.9) | 57 (38.8) | 4.28 | 0.370 |
| HBsAg (+) (%) | 20 (45.5) | 14 (58.3) | 23 (51.1) | 14 (48.3) | 83 (56.5) | 2.30 | 0.680 |
| HCV-Ag (+) (%) | 3 (6.8) | 1 (4.2) | 4 (8.9) | 2 (6.9) | 7 (4.8) | 1.32 | 0.858 |
| Cirrhosis (%) | 31 (70.5) | 17 (70.0) | 20 (44.4) | 10 (34.5)a | 74 (50.3) | 14.15 | 0.007 |
| Non-alcoholic fatty liver (%) | 10 (22.7) | 7 (29.2) | 7 (15.6) | 7 (24.1) | 36 (24.5) | 2.11 | 0.715 |
| Factors | Criterion: Barcelona stage | Statistics | P value | |||
| A stage | B stage | C stage | D stage | |||
| Male (%) | 36 (67.9) | 42 (76.4) | 51 (75.0) | 87 (77.7) | 1.90 | 0.594 |
| Age (yr) | 60.25 ± 11.57 | 65.15 ± 12.40 | 62.50 ± 11.28 | 60.80 ± 9.05 | 2.53 | 0.057 |
| History of smoking (%) | 14 (26.4) | 20 (36.4) | 23 (33.8) | 40 (35.4) | 1.58 | 0.664 |
| History of drinking (%) | 15 (28.3) | 16 (29.1) | 28 (41.2) | 41 (36.3) | 3.11 | 0.375 |
| HBsAg (+) (%) | 21 (39.6)a | 24 (43.6a | 31 (45.6)a | 78 (69.0) | 18.90 | < 0.001 |
| HCV-Ag (+) (%) | 4 (7.5) | 4 (7.3) | 3 (4.4) | 6 (5.3) | 0.79 | 0.852 |
| Cirrhosis (%) | 11 (20.8)a | 13 (23.6)a | 15 (22.1)a | 113 (100.0) | 167.33 | < 0.001 |
| Non-alcoholic fatty liver (%) | 14 (26.4) | 18 (32.7) | 15 (22.1) | 20 (17.7) | 5.08 | 0.166 |
When PLC cases were stratified by Child–Pugh scores, the rate of smoking and drinking history played the greatest roles in distinguishing class C (P = 0.013 and P = 0.007, respectively, both < 0.0167). When PLC cases were stratified by the clinical stage of cirrhosis, HBsAg showed the biggest prognostic difference. To be precise, compared with PLC cases without liver cirrhosis, PLC with cirrhosis had higher rates of HBV infection, which was not associated with the severity of cirrhosis (P < 0.001). Accordingly, when PLC cases were stratified by tumor size, the data demonstrated that liver cirrhosis occurred more frequently in patients with smaller lesions compared with patients with massive liver tumors (P = 0.002, < 0.005). Finally, when the PLC cases were stratified by BCLC stage, both HBsAg and liver cirrhosis changed at different stages. In particular, the rate of HBV infection in BCLC stage D cases was substantially increased compared with stages A, B, and C. There was likely marked cirrhosis in stage D PLC cases. In summary, the different high-risk factors weighted distinctively when PLC was stratified by each criterion, meaning it is necessary to evaluate them in certain stratifications of PLC. Otherwise the mixed factors may confuse our judgement as to the condition of individual PLC patients.
The diagnostic value of serum AFP levels in PLC, chronic hepatitis, and cirrhosis: Table 5 shows that AFP levels in the PLC group were significantly increased compared with those in the chronic hepatitis B group (P < 0.001) and the cirrhosis group (P < 0.001), demonstrating its diagnostic value (P < 0.001). As a confirmed diagnostic biomarker, AFP levels in PLC were higher than those in the other two groups. Noticeably, the median AFP level in PLC was 63.69 ng/mL, much lower than the 400 ng/mL listed in the guidelines, which was in accordance with clinical situations. Actually, AFP-negative PLC is not uncommon in clinical practice, which diminishes the diagnostic value of APF. Although, the rate of AFP-positive PLC cases is not 100%, theoretically owing to various reasons including testing methods and the period of PLC, these data still surprised us. Our data showed that AFP levels were commonly below conceivable diagnostic expectations (only 35.6% of these cases were > 400 ng/mL), which is in accordance with other studies[18].
AFP levels and diagnostic value in PLC stratified by different criteria: Tables 6 and 7 show AFP levels and its related diagnostic efficiency in each PLC stratification. AFP levels varied in different stratifications (Table 6). Furthermore, serum AFP levels in PLC with either decompensated or compensated liver cirrhosis were dramatically increased compared with PLC without cirrhosis (P = 0.004 and P = 0.005, respectively). Additionally, AFP levels in PLC with diffuse liver cancer were strikingly increased compared with cases of small liver cancers (P = 0.007, < 0.05). BCLC stage D PLC patients had significantly increased AFP levels compared with BCLC stage A PLC patients (P = 0.009, < 0.05).
| Layering standard | Group | AFP (ng / mL) | F | P value |
| Cirrhosis clinical stage | 10.03 | 0.007 | ||
| No cirrhosis | 7.32 (2.28, 7.32) | |||
| Compensated cirrhosis | 267.40 (12.56, 1210.00)a | 0.004 | ||
| Decompensated cirrhosis | 130.60 (6.69, 1210.00)a | 0.005 | ||
| Child-Pugh classification | 0.26 | 0.774 | ||
| A grade | 461.16 ± 546.16 | |||
| B grade | 516.74 ± 534.18 | |||
| C grade | 549.64 ± 561.80 | |||
| Classification of tumor size | 21.70 | < 0.001 | ||
| Small liver cancer | 4.54 (1.78, 99.19) | |||
| Medium liver cancer | 12.56 (2.66, 521.65) | |||
| Large liver cancer | 17.58 (2.10, 782.25) | |||
| Massive liver cancer | 249.90 (4.14, 1210.00) | |||
| Diffuse liver cancer | 267.40 (3.69, 1210.00)b | 0.007 | ||
| Barcelona stage | 8.56 | 0.036 | ||
| Phase A | 3.76 (1.78, 478.95) | |||
| Phase B | 103.90 (3.17, 1149.50) | |||
| Phase C | 111.90 (3.18, 1210.00) | |||
| Phase D | 116.25 (6.59, 1210.00)c | 0.009 |
| Layering standard | Group | AUC | Std.Error | P value | 95%CI | Sensitivity, % | Specificity, % |
| Child-Pugh classification | A grade | 0.759 | 0.0424 | < 0.0001 | (0.721, 0.794) | 62.26 | 84.68 |
| B grade | 0.847 | 0.0279 | < 0.0001 | (0.815, 0.875) | 77.22 | 81.45 | |
| C grade | 0.821 | 0.0575 | < 0.0001 | (0.785, 0.853) | 75.00 | 81.25 | |
| Cirrhosis clinical stage | No | 0.647 | 0.0303 | < 0.0001 | (0.608, 0.684) | 38.69 | 94.56 |
| Compensated cirrhosis | 0.846 | 0.0369 | < 0.0001 | (0.813, 0.875) | 78.57 | 81.05 | |
| Decompensated cirrhosis | 0.800 | 0.0276 | < 0.0001 | (0.766, 0.8310) | 69.09 | 83.47 | |
| Classification of tumor size | Small | 0.595 | 0.0514 | 0.0642 | (0.552, 0.637) | 38.64 | 84.68 |
| Medium | 0.684 | 0.0636 | 0.0038 | (0.642, 0.724) | 66.67 | 73.39 | |
| Large | 0.675 | 0.0520 | 0.0008 | (0.634, 0.714) | 66.67 | 71.98 | |
| Massive | 0.805 | 0.0411 | < 0.0001 | (0.769, 0.838) | 51.72 | 96.17 | |
| Diffuse | 0.785 | 0.0257 | < 0.0001 | (0.751, 0.816) | 62.59 | 90.93 | |
| Barcelona stage | Phase A | 0.592 | 0.0481 | 0.0547 | (0.550, 0.634) | 30.19 | 96.17 |
| Phase B | 0.741 | 0.0415 | < 0.0001 | (0.703, 0.778) | 52.73 | 90.93 | |
| Phase C | 0.731 | 0.0403 | < 0.0001 | (0.692, 0.767) | 52.94 | 93.35 | |
| Phase D | 0.799 | 0.0271 | < 0.0001 | (0.765, 0.830) | 68.14 | 83.47 |
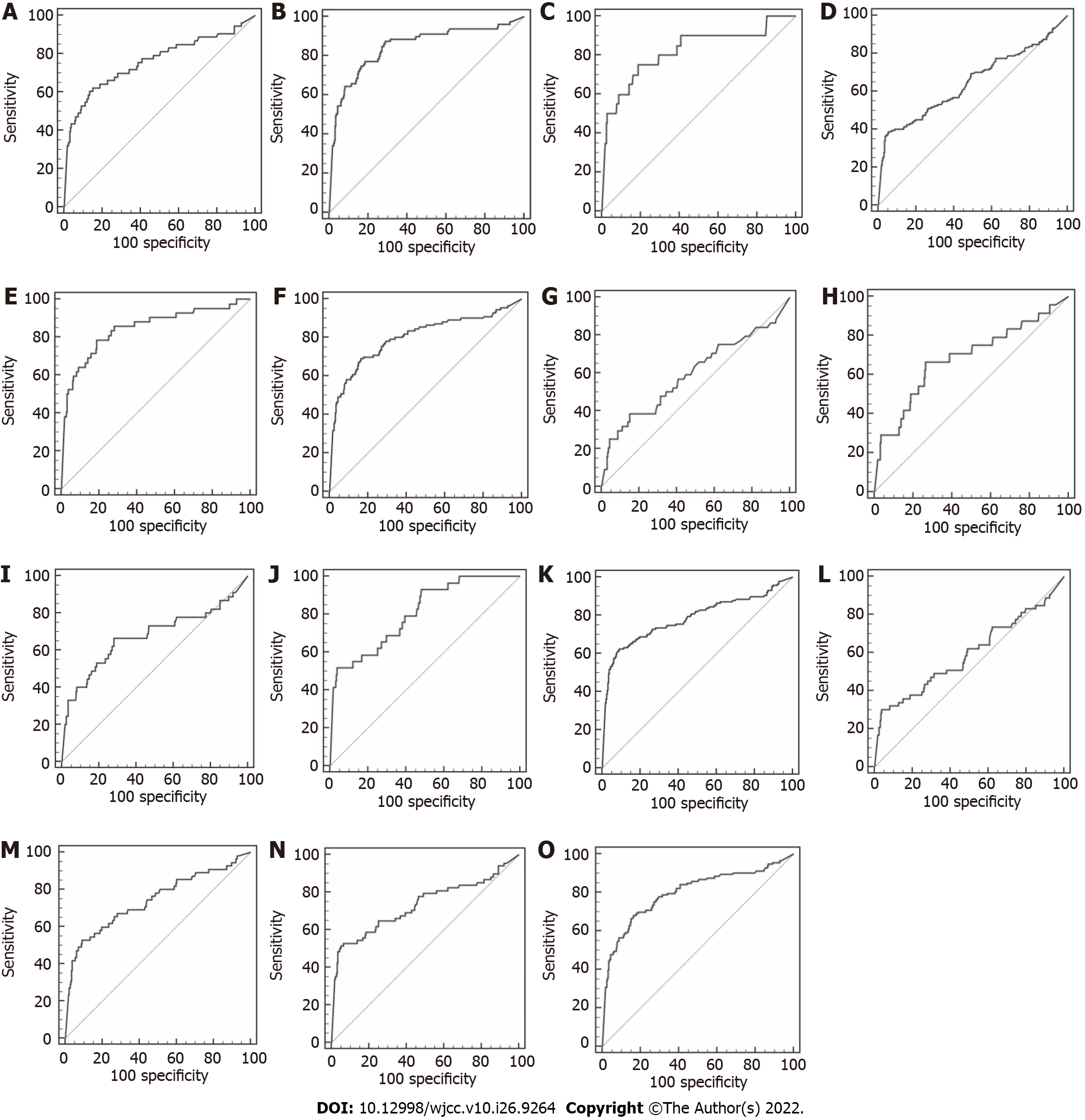
Next, the diagnostic values of AFP for stratified PLC cases were analyzed by receiver operating characteristic curves (Table 7 and Figure 1). When ranked in terms of diagnostic value, AFP had the greatest impact on PLC featuring Child–Pugh grade B, then compensated cirrhosis, Child–Pugh grade C, large tumors, and decompensated cirrhosis; moreover, area under the curve (AUC) values for AFP in these criteria were all > 0.800 (0.847, 0.846, 0.821, 0.805, and 0.800, respectively), suggesting relatively better reliability. In contrast, AUC values for AFP were decreased in PLC featuring BCLC stage D, diffuse tumors, Child–Pugh grade A, and BCLC stages B and C (0.799, 0.785, 0.759, 0.741, and 0.731, respectively, all < 0.800). Although the P values were all < 0.05, the diagnostic efficiency of serum AFP for small liver cancers and BCLC stage A liver cancer was low and meaningless (AUC = 0.595 and 0.592, respectively).
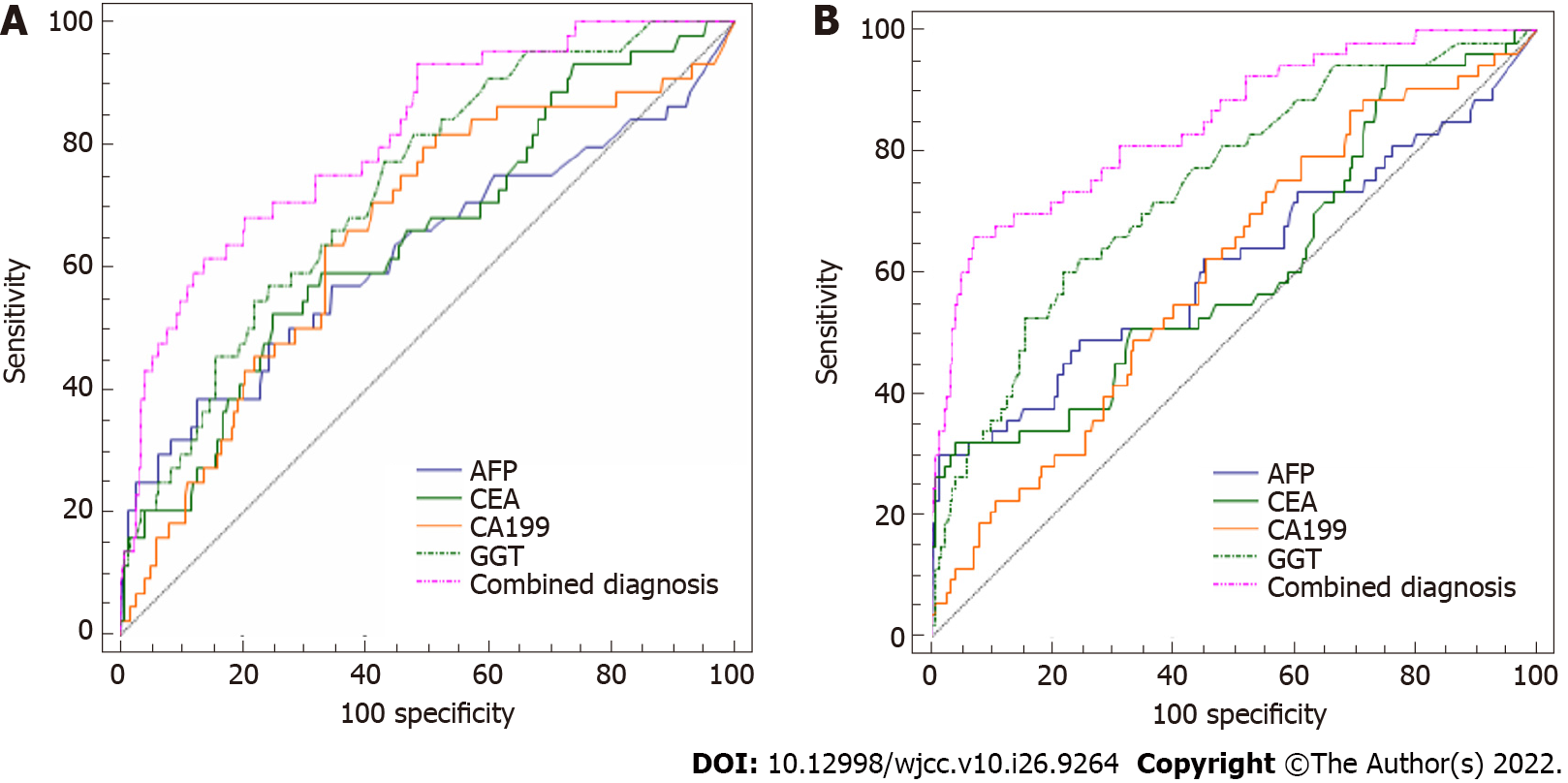
The diagnostic value of combining CEA, CA 19-9, GGT, and AFP in PLC with small tumors and BCLC stage A cases: The above findings demonstrated that AFP had little diagnostic value in PLC with small tumors and BCLC stage A cases. Thus, we next examined whether combined detection of CEA, CA 19-9, GGT, and AFP would have better results in this population. Considering that all four biomarkers are not only markers of gastrointestinal tumors, but also have intensive connections with liver diseases, the study was performed to supplement the disadvantages of AFP alone.
Combining CEA, CA 19-9, and GGT improved the diagnostic performance of AFP (all AUC values > 0.600 and greater than AFP alone) (Tables 8 and 9, Figure 2). In particular, the diagnostic value of the combination had significantly increased values for small tumors and BCLC stage A (AUC = 0.810 and 0.846, respectively, P < 0.0001). Therefore, the combination of CEA, CA 19-9, GGT and AFP is worth using to diagnose PLC with small tumors or at BCLC stage A.
| Diagnostic indicators | AUC | Sensitivity, % | Specificity, % | Std.Error | 95%CI | Z | P value |
| AFP | 0.595 | 38.64 | 84.68 | 0.0519 | (0.552, 0.632) | 1.93 | 0.067 |
| CEA | 0.649 | 52.27 | 75.22 | 0.0461 | (0.589, 0.706) | 3.23 | 0.0013 |
| CA-199 | 0.719 | 81.82 | 66.18 | 0.0467 | (0.671, 0.763) | 4.69 | < 0.0001 |
| GGT | 0.728 | 77.27 | 56.89 | 0.0390 | (0.671, 0.780) | 5.84 | < 0.0001 |
| Combined diagnosis | 0.810 | 68.18 | 79.56 | 0.0349 | (0.758, 0.855) | 8.90 | < 0.0001 |
| Diagnostic indicators | AUC | Sensitivity, % | Specificity, % | Std.Error | 95%CI | Z | P value |
| AFP | 0.593 | 30.19 | 96.17 | 0.0481 | (0.550, 0.634) | 1.93 | 0.0538 |
| CEA | 0.611 | 32.08 | 96.00 | 0.0462 | (0.551, 0.669) | 1.197 | 0.2313 |
| CA-199 | 0.608 | 75.47 | 42.86 | 0.0429 | (0.548, 0.666) | 2.511 | 0.0121 |
| GGT | 0.743 | 60.38 | 78.22 | 0.0380 | (0.688, 0.794) | 6.409 | < 0.0001 |
| Combined diagnosis | 0.843 | 66.04 | 91.96 | 0.0319 | (0.794, 0.883) | 10.738 | < 0.0001 |
Early diagnosis is crucial to decreasing the recurrence rate of PLC after surgery. Considering that current treatments tend to be more accurate and individualized, stratifying cases might play a role in making more precise PLC diagnoses. Previous studies have pointed out that chronic HBV exposure, fatty liver, cigarette and alcohol use, and liver cirrhosis could help tumor cells escape immune surveillance and promote tumor proliferation and metastasis[19,20,11]. Additionally, the prognosis of PLC is impacted by tumor size, number, the presence of vascular invasion, and lymph node metastasis[21]. In clinical practice, these high-risk factors are mixed in with the different criteria we analyzed; however, there is no good model featuring the different phases of PLC, meaning it is essential to treat the factors distinctively.
Our results demonstrate that certain factors have different diagnostic roles when PLC cases are stratified. Currently, there are various PLC scoring systems that use a combination of routine clinical features[22]. We first found when PLC was stratified by Child–Pugh score, the rates of smoking and drinking history played the biggest diagnostic roles. Generally, smoking and drinking induce direct or indirect toxic effects that increase the risk of developing PLC among chronic liver disease patients[23]. Second, when we stratified cases by clinical stage of cirrhosis, HBsAg was the most discriminatory factor. The literature suggests that HBV proteins are involved in hepatocarcinogenesis[24]. On the basis of our results, patients with HBV infection are advised to be on alert for liver cirrhosis to reduce the incidence of PLC. Subsequently, PLC was stratified by tumor size, and the data demonstrated that liver cirrhosis occurred more frequently in patients with small liver tumors than in those with massive liver tumors. It has been reported that the presence of cirrhosis or advanced liver fibrosis is a distinct predisposing factor for liver cancer, predominantly hepatocellular carcinoma[25]. Briefly, caution should be taken when treating patients with small tumors that are inclined to cirrhosis, surgery should be advised for these patients as it could improve their prognosis. Finally, when our data were stratified by BCLC stage, both HBsAg and liver cirrhosis changed at different stages. The BCLC staging system classifies cases based on the patient's life expectancy, meaning more emphasis is placed on both HBV infection and cirrhosis when evaluating prognosis.
As mentioned above, stratification might make a difference when assessing PLC. Thus, we next examined the trend of biomarkers in each phase. Toader et al[26] found that AFP was still a reliable diagnostic and prognostic tool for PLC patients, especially in developing countries. In our study, the diagnostic value of AFP alone for PLC was not as good as expected, and there was even a small proportion of AFP-negative PLC cases[27]. A meta-analysis of studies showed that concomitant use of ultrasound and AFP improved early PLC detection compared with ultrasound alone[28]. Moreover, AFP is still regarded as a biomarker in some PLC guidelines. Thus, we thought it would be valuable to further study AFP according to patient stratification. Our analysis showed that AFP had quite different functions in different stratifications of PLC, having greater utility in patients with poor liver function, advanced, massive, and multiple tumors.
We found that AFP was not suitable for diagnosing small and early-stage liver cancers. However, there has been increasing recognition that a single biomarker may not be sufficient and that a combination of biomarkers may be needed to optimize sensitivity for small and early PLC. Currently, new serum tumor markers such as Golgi protein 73[29], glypican-3[30], and liver cancer-related miRNA[31] have been shown to be promising biomarkers that could improve the diagnostic efficiency of PLC, but most of these indicators are still not suitable for clinical use. Thus, we analyzed classic liver markers including CEA, CA 19-9[32], and GGT[33]. CEA and CA 19-9 are serum tumor markers that are primarily used for screening and diagnosis of gastrointestinal and other digestive system tumors. Both show a certain degree of expression in PLC. Our results confirmed that the combined detection of these four biomarkers contributed to increasing diagnostic performance for small and early-stage liver cancers.
Due to my limited energy, no further in-depth study was conducted. In future studies, we should study AFP in patients with liver cancer and patient prognosis, 5-year survival rate, etc.
In summary, stratified diagnosis of PLC was essential, and the high-risk factors had distinct roles in PLC classifications. AFP level functioned as a diagnostic biomarker in the stratified PLC population that included the following: poor function, advanced hepatoma, and massive and diffuse tumors. For small hepatomas and BCLC A stage PLC, combined detection of CEA, CA 19-9, GGT, and AFP is a more promising approach to diagnosing PLC compared with testing AFP alone.
Stratified diagnosis of primary liver cancer (PLC) was essential, and the high-risk factors had distinct roles in PLC classifications. Alpha-fetoprotein (AFP) level functioned as a diagnostic biomarker in the stratified PLC population that included the following: Poor function, advanced hepatoma, and massive and diffuse tumors. For small hepatomas and Barcelona Clinic Liver Cancer (BCLC) A stage PLC, combined detection of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), gamma-glutamyl transferase (GGT), and AFP is a more promising approach to diagnosing PLC compared with testing AFP alone.
Stratification of PLC was essential for precise diagnoses and benefited from evaluating AFP levels.
This study aimed to evaluate the role of high-risk factors in diagnosing stratified PLC cases, especially the diagnostic value of AFP.
First, the contributions of high-risk factors in stratifying PLC were compared. Then, the diagnostic value of AFP was evaluated in different stratifications of PLC by receiver operating characteristic curves. For PLC cases in which AFP played little role, the diagnostic values of CEA, CA 19-9, GGT, and AFP were analyzed.
The roles of high-risk factors differed in stratified PLC. AFP levels were higher in PLC with cirrhosis, diffuse tumors, and BCLC stage D disease. However, these measures were meaningless [area under the curve (AUC) < 0.600] in small hepatomas and BCLC A stage PLC, but could be replaced by the combined detection of CEA, CA 19-9, GGT, and AFP (AUC = 0.810 and 0.846, respectively).
PLC incidence rates vary across clinical etiologies and conditions such as liver disease severity; even within the same clinical entity, individual PLC risk is heterogeneous across patients for unknown reasons. Hence, clinically meaningful utility must be demonstrated under specific clinical scenarios for a diagnostic modality to be adopted into regular use.
PLC was the second leading cause of cancer-related mortality in 2014. PLC incidence rates vary across clinical etiologies and conditions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Van Den Heede K, Belgium; Yamada Y, Japan S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 941] [Article Influence: 85.5] [Reference Citation Analysis (4)] |
| 2. | Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, McGlynn KA. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 523] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 5. | Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 7. | Agaratnam N, Nagaratnam K and Cheuk G. Hepatocellular carcinoma. Geriatric Diseases. 2017;1-4. |
| 8. | Ali A, Abdel-Hafiz H, Suhail M, Al-Mars A, Zakaria MK, Fatima K, Ahmad S, Azhar E, Chaudhary A, Qadri I. Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma. World J Gastroenterol. 2014;20:10238-10248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 9. | Xie Y. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Adv Exp Med Biol. 2017;1018:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 565] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 11. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 878] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 12. | Shen JY, Li C, Wen TF, Yan LN, Li B, Wang WT, Yang JY, Xu MQ. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J Surg Res. 2017;209:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Wu M, Liu H, Liu Z, Liu C, Zhang A, Li N. Analysis of serum alpha-fetoprotein (AFP) and AFP-L3 levels by protein microarray. J Int Med Res. 2018;46:4297-4305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 443] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 15. | Cong WM. Experts consensus on pathological diagnosis of PLC. Weichangbingxue he Ganbingxue Za Zhi. 2011;20:393-395. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Jia JD, Hou JL, Wei L, Zhuang H. [Highlights of the guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Jia JD, Hou JL, Wei L, Cai ZF, Chen AM, Chen CW, Chen SJ, Chen YP, Cheng J, Wei D, Ding SF, Dou XG, Zhi D, Jia F, Fan XG, Gao YQ, Gao ZL, Guo QY, Tao H, Ying H, Hu YR, Huang ZH, Ning L, Li CZ, Li YG, Lin MH, Liu JF, Lu LG, Xin M, Qing M, Mao YM, Liao XH, Wu N, Qin N, Niu JQ, Hong R, Jia S, Shao JG, Shen XZ, Hong S, Sun WL, Hong T, Tang XP, Wang GQ, Wang JF, Wang XP, Lai W, Wong XH, Qing X, Min X, Xiong LZ, Yang YF, Yin ZB, Hong Y, Yuan KJ, Zeng YL, Zhang JL, Zhang WH, Zhang YL, Zhang YX, Zhong HZ, Zhou BP, Hui Z. White Paper on "Regulations on Management of Liver Diseases in China" (excerpt). Linchuang Gandanbing Za Zhi. 2014;30:197-209. [DOI] [Full Text] |
| 18. | Sanai FM, Sobki S, Bzeizi KI, Shaikh SA, Alswat K, Al-Hamoudi W, Almadi M, Al Saif F, Abdo AA. Assessment of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma in Middle Eastern patients. Dig Dis Sci. 2010;55:3568-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Chang PE, Wong GW, Li JW, Lui HF, Chow WC, Tan CK. Epidemiology and Clinical Evolution of Liver Cirrhosis in Singapore. Ann Acad Med Singap. 2015;44:218-225. [PubMed] |
| 20. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3087] [Article Influence: 220.5] [Reference Citation Analysis (0)] |
| 21. | Gao T, Xia Q, Qiu DK, Feng YY, Chi JC, Wang SY, Xi ZF, Zhang JJ, Xu N, Chen SY, Qiu YL, Shen LW, Zhou TT, Dong XJ, Li QG, Li H. Comparison of survival and tumor recurrence rates in patients undergoing liver transplantation for hepatitis B-related hepatocellular carcinoma using Milan, Shanghai Fudan and Hangzhou criteria. J Dig Dis. 2013;14:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S, Loglio A, Siakavellas S, Keskın O, Gatselis N, Hansen BE, Lehretz M, de la Revilla J, Savvidou S, Kourikou A, Vlachogiannakos I, Galanis K, Yurdaydin C, Berg T, Colombo M, Esteban R, Janssen HLA, Lampertico P. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | Mehta G, Macdonald S, Cronberg A, Rosselli M, Khera-Butler T, Sumpter C, Al-Khatib S, Jain A, Maurice J, Charalambous C, Gander A, Ju C, Hakan T, Sherwood R, Nair D, Jalan R, Moore KP. Short-term abstinence from alcohol and changes in cardiovascular risk factors, liver function tests and cancer-related growth factors: a prospective observational study. BMJ Open. 2018;8:e020673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 25. | Xu X, Qu K, Wan Y, Song S, Huang Z, Wang Z, Liu C. Tumor existence and tumor size as prognostic factors in hepatitis B virus-related cirrhosis patients who underwent liver transplantation. Transplant Proc. 2014;46:1389-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Toader E, Bancu A, Mitrică DE, Constantinescu G, Ştefănescu G, Bălan GG. Interrelations between elevated alpha-fetoprotein levels and tumor morphology of patients with hepatocellular carcinoma. Rom J Morphol Embryol. 2019;60:181-187. [PubMed] |
| 27. | Marrero JA, El-Serag HB. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology. 2011;53:1060-1; author reply 1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 804] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 29. | Zhao S, Long M, Zhang X, Lei S, Dou W, Hu J, Du X, Liu L. The diagnostic value of the combination of Golgi protein 73, glypican-3 and alpha-fetoprotein in hepatocellular carcinoma: a diagnostic meta-analysis. Ann Transl Med. 2020;8:536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Xu D, Su C, Sun L, Gao Y, Li Y. Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis. Ann Hepatol. 2019;18:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Lyra-González I, Flores-Fong LE, González-García I, Medina-Preciado D, Armendáriz-Borunda J. MicroRNAs dysregulation in hepatocellular carcinoma: Insights in genomic medicine. World J Hepatol. 2015;7:1530-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Edoo MIA, Chutturghoon VK, Wusu-Ansah GK, Zhu H, Zhen TY, Xie HY, Zheng SS. Serum Biomarkers AFP, CEA and CA19-9 Combined Detection for Early Diagnosis of Hepatocellular Carcinoma. Iran J Public Health. 2019;48:314-322. [PubMed] |
| 33. | Zhang JB, Chen Y, Zhang B, Xie X, Zhang L, Ge N, Ren Z, Ye SL. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |