Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9148
Peer-review started: April 24, 2022
First decision: May 12, 2022
Revised: May 25, 2022
Accepted: July 20, 2022
Article in press: July 20, 2022
Published online: September 6, 2022
Processing time: 123 Days and 11.6 Hours
Primary ciliary dyskinesia (PCD) is an uncommon and genetically diverse condition. According to reports, most patients had more than 50 visits before being diagnosed with PCD, and the age at diagnosis was mostly in preschool, with an average age of about (10.9 ± 14.4) years old. CCNO is a pathogenic gene that regulates the cell cycle, and its mutation is linked to the uncommon human genetic disorder PCD. Although the prevalence of the CCNO mutation is regarded to be exceptionally low, new reports of this mutation have increased in com
Here, we report a case of a young Chinese woman diagnosed with PCD, who was found to carry the CCNO gene by whole exon gene sequencing. In this case, a young non-smoking Chinese female exhibiting recurrent cough and sputum at birth. Chest computed tomography (CT) showed bronchiectasis with infection, and sinus CT showed chronic sinusitis. However, the patient had no visceral transposition and no history of infertility. Under electron microscope, it was found that cilia were short and reduced in number, and no power arm of cilia was observed. Whole exon sequencing analysis of the genome of the patient showed that the patient carried CCNO pathogenic gene, exon c.303C>A nonsense mutation and c.248_252dup frameshift mutation. Her clinical symptoms and CT images were improved after two months of treatment with aerosol inhalation and oral azithromycin.
The results showed that CCNO is an important cause of PCD. More mutant genes that may contribute to genetically diverse disorders like PCD have been discovered as sequencing technology has advanced. Furthermore, the increase of genetic information makes it easier to diagnose uncommon diseases in clinical practice.
Core Tip: Primary ciliary dyskinesia (PCD) is a disease that is genetically diverse. Despite the discovery of more than 40 pathogenic genes, there are still insufficient case reports to help clinical diagnosis and treatment. We describe a case of primary ciliary immobility dysfunction caused by mutations in the CCNO (encoding cyclin O) gene. The patient lacked the classic PCD triad and was readily overlooked. We require further genetic research and particular case reports.
- Citation: Zhang YY, Lou Y, Yan H, Tang H. CCNO mutation as a cause of primary ciliary dyskinesia: A case report. World J Clin Cases 2022; 10(25): 9148-9155
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9148.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9148
Primary ciliary dyskinesia (PCD) is an uncommon and genetically diverse condition. According to reports, most patients had more than 50 visits before being diagnosed with PCD, and the age at diagnosis was mostly in preschool[1,2], with an average age of about (10.9 ± 14.4) years old[3]. More than 40 PCD pathogenic genes have been discovered to date[4,5], and the genetic cause may be found in around 70% of individuals with primary ciliary motility disorder[6]. CCNO is a pathogenic gene that regulates the cell cycle, and its mutation is linked to the uncommon human genetic disorder PCD. Although the prevalence of the CCNO mutation is regarded to be exceptionally low, new reports of this mutation have increased in comparison to prior ones[7]. PCD patients with CCNO are rare, and the incidence rate is no more than 2% in whole PCD patients[8].
PCD is a genetic condition that mainly affects the upper and lower respiratory tract. In 1904, Siewert was the first to identify the association of varus, chronic sinusitis, and bronchiectasis. In 1933, Kartagener[8] defined this clinical trio as a distinct congenital disease. Recessive CCNO mutations have been detected in individuals with chronic destructive lung disease due to poor airway clearance, owing to the advantage of gene sequencing technology[9,10]. In this paper, we presented a case of a young Chinese woman with CCNO who was diagnosed with PCD without visceral transposition.
A 22-year-old unmarried female patient was hospitalized due to recurrent cough and expectoration for more than 20 years.
The presented case was a young non-smoking Chinese female with recurrent cough and expectoration at birth and occasional dyspnea, which continued into adulthood. Repeated use of antibiotics, antivirals and small doses of hormone anti-inflammatory could not alleviate the symptoms.
The patient had no previous medical history.
Her uncle had a history of recurrent cough and expectoration at birth, but he did not seek medical assistance or formal medical treatment.
The patient’s vital signs were stable on admission. Bilateral lung percussion was clear, and auscultation reduces respiratory sounds in both lungs.
Laboratory markers were white blood cell 13.4 × 109/L and neutrophil ratio 78.4%, inflammatory indicators were normal, fungal culture and bacterial culture were negative, and acid-fast staining and T-SPOT were negative. Hepatitis B, syphilis, and HIV testing were all negative.
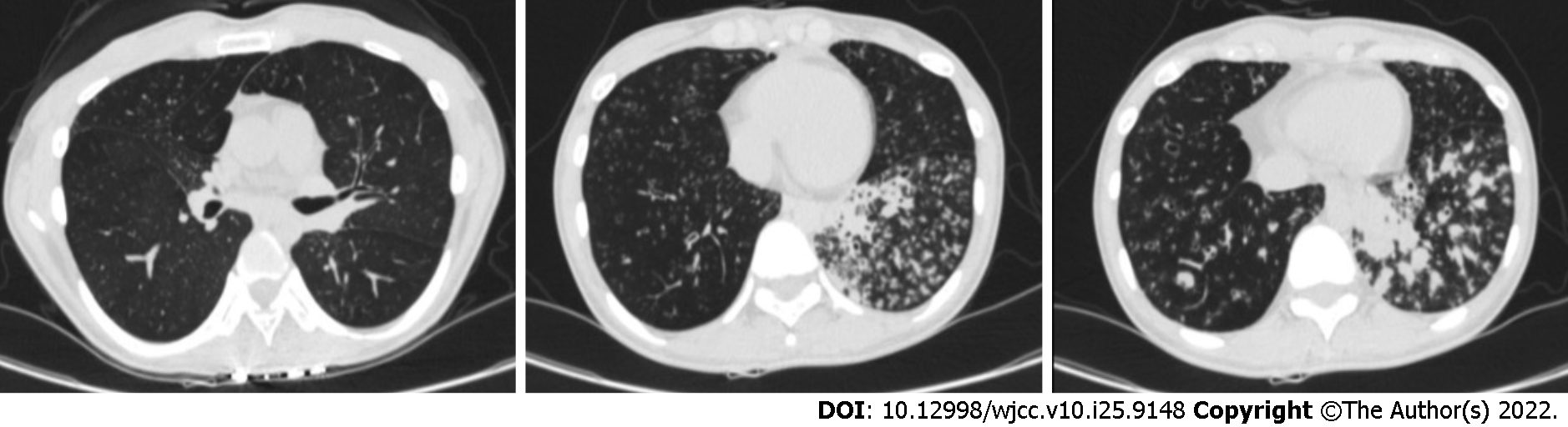
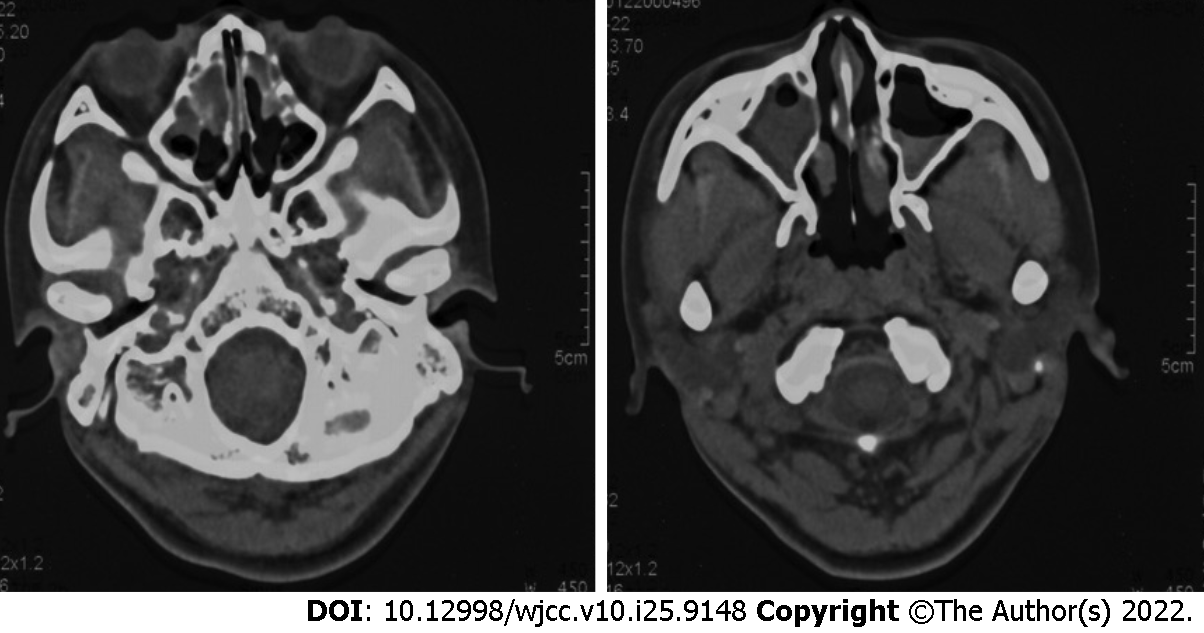
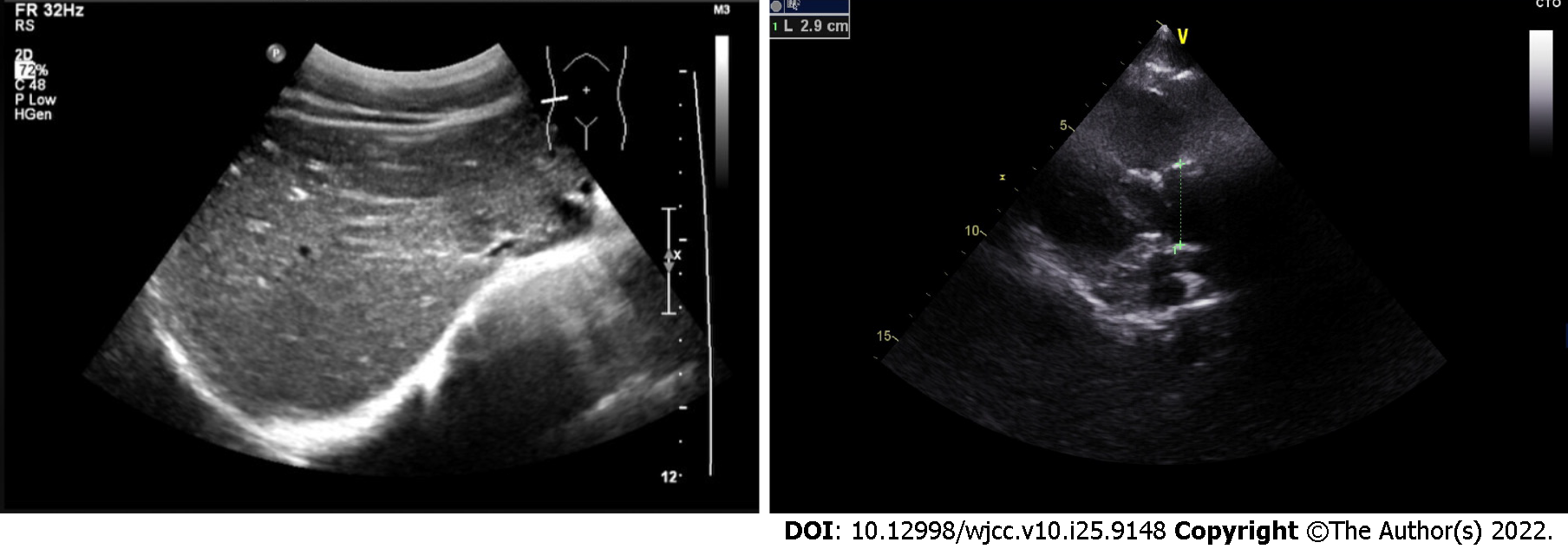
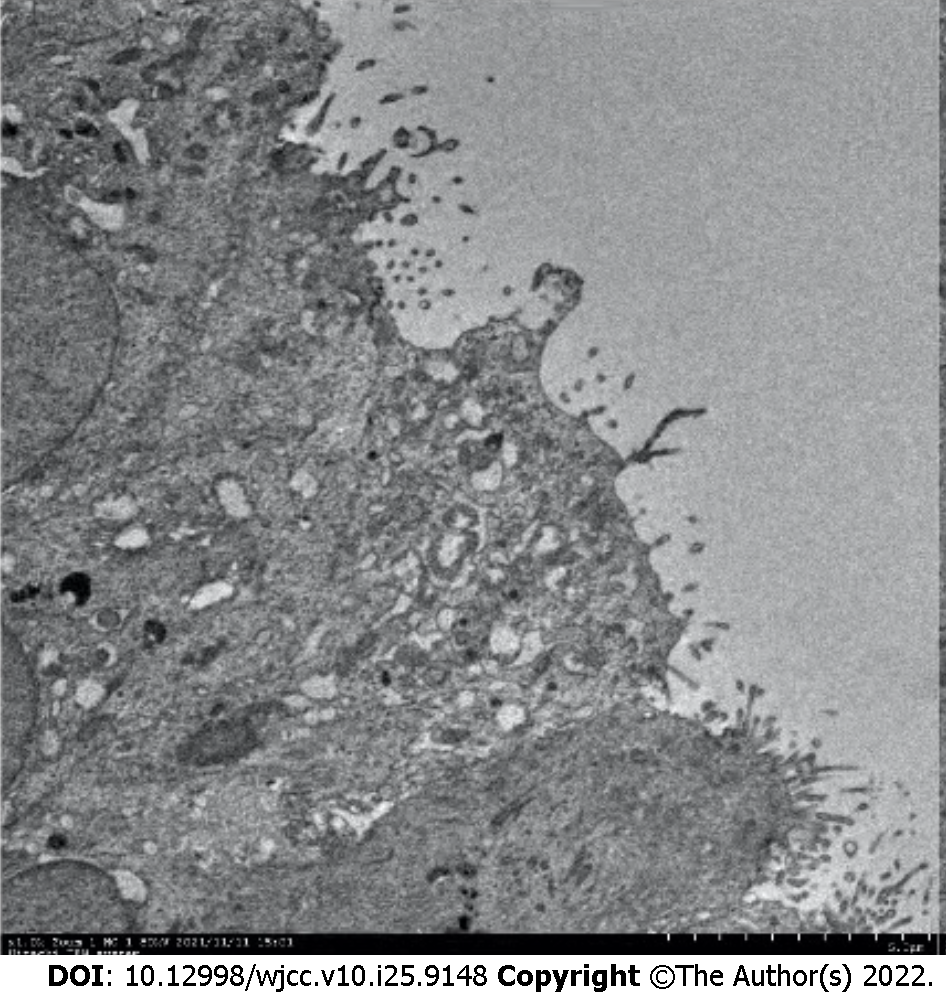
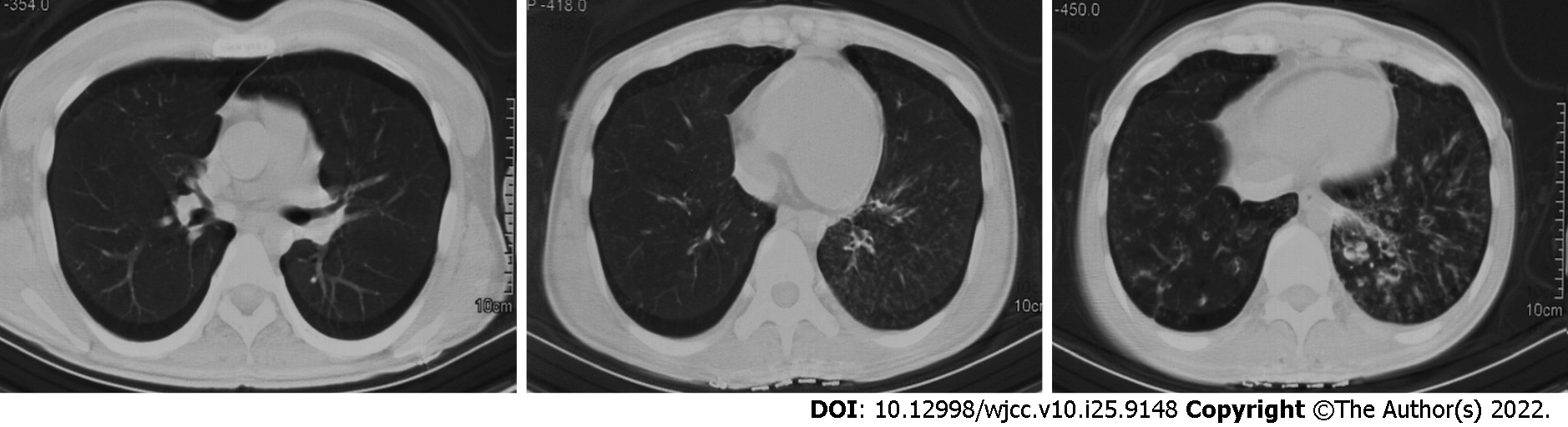
Chest computed tomography (CT) showed marked bronchiectasis with infection, typical "tree bud sign", and miliary nodules in the lower left lung (Figure 1). Sinus CT scans showed bilateral maxillary and ethmoid sinusitis (Figure 2). Abdominal ultrasound and cardiac ultrasound did not suggest visceral transposition (Figure 3). We collected a tissue biopsy from Carina with flexible fiberoptic bronchoscopy to confirm the disease, and electron microscopy revealed that some epithelial cells had few cilia, with missing and short cilia. There was no ciliated power arm after 1000 magnification (Figure 4).
The patient was diagnosed with PCD based on clinical characteristics, imaging, electron microscopic.
The patient was given aerosol sputum elimination for respiratory symptoms, followed by oral azithromycin for two months.
After two months of oral azithromycin, the lung lesion in the lower left was less than before, according to a reexamination of the chest CT scan (Figure 5).
PCD is an autosomal recessive genetic illness due to X-linked double-allele mutation, which causes aberrant ciliary movement and clearance function caused by defective ciliary ultrastructure and function[11]. In severe circumstances, at least 50% of patients may experience unexplained newborn respiratory distress, necessitating the use of a ventilator. The most prevalent symptoms are respiratory bronchiectasis, cardiovascular visceral transposition, infertility in men due to sperm motility abnormalities, and ectopic pregnancy or infertility in women due to fallopian tube cilium malfunction. This clinical trio was first identified as a unique congenital syndrome by Kartagener[8]. However, because PCD is a rare and underdiagnosed disease, reported series are typically small, with the pediatric cohort accounting for the majority of cases[12-14], and adult PCD cases are scarce. The reported prevalence rate of PCD in the general population ranges from 1/2000 to 1/40000[15]. However, the incidence of PCD in Chinese population is unknown. Furthermore, we discovered that a small percentage of PCD patients did not exhibit visceral transposition or infertility but only had respiratory symptoms. As a result, clinicians frequently missed diagnosis of PCD in these patients. In this study, the young female patient has been suffering from recurring respiratory tract infection symptoms from childhood. Clinical consequences include chronic sinusitis, bronchiectasis, and bronchiolitis. Furthermore, short and absent cilia was found under electron microscopy of tracheoscopic biopsy tissue. All of these fulfilled the PCD diagnostic criteria. However, the patient was not diagnosed as PCD in the last two decades, because she had no indications of visceral transposition and no oppotunity of infertility.
Cilia is an important accessory structure of cells, and the core structure of cilia is ciliary axoneme. Normal cilia are composed of a pair of central microtubules, surrounded by a set of peripheral microtubules which form a conventional (9 + 2) structure, and the connection between the central microtubules and the peripheral microtubules is completed by radial bonds. Among them, the direction of cilia is controlled by the change of the moving angle of the radiation arm which is responsible for the connection between the inner power arm and the central microtubule[16,17]. Under normal circumstances, hundreds of millions of cilia in respiratory mucosa act as "scavengers". The normal oscillation of cilia causes dust and bacteria sucked into bronchi to be discharged together with cell fragments and mucus secreted by respiratory tract. However, once the cilia beat is damaged, it will lead to chronic airway infection. In pathology, the most common phenomenon is the loss or shortening of the external and/or internal dynamin arms. Ultrastructural defect, decrease of cilia or ciliary movement disorder are that root cause of PCD. When mucus is unusually viscous and ciliary movement decreases, it will lead to mucociliary clearance disorder, resulting in primary ciliary movement disorder (PCD)[2,5,18].
PCD is a genetically heterogeneous illness caused by mutations in a variety of genes[19-22]. Primary ciliary dyskinesia is caused by mutations in more than 40 genes, and with breakthroughs in detection techniques, more genes may be identified in the future[4]. One of the pathogenic genes is CCNO[23]. CCNO is made up of three exons on chromosome 5q11 that code for a 1053 BP cDNA and a 350 amino acid protein. CCNO is a cyclin-like protein with two cyclin-box folds and protein-binding domains that are regarded to be involved in cell cycle and transcriptional regulation. Recessive CCNO (encoding cyclin O) mutations have been detected in individuals with chronic destructive lung disease due to poor airway clearance, thanks to rapid development of sequencing technology[9,10,23]. CCNO mutations was linked to chronic respiratory disorders and progressive loss of respiratory function[18], with all affected people experiencing recurrent upper and lower respiratory tract infections as well as bronchiectasis. This is due to the fact that CCNO mutants have a significantly reduced number of cilia, and their MMC is insufficient to clear cell debris, inhaled particles, and germs from the upper and lower respiratory tracts, resulting in recurring respiratory tract infections. According to the literature, PCD cases with CCNO mutation had early onset respiratory symptoms as the first symptom[4,11], resulting in recurring respiratory symptoms in patients. In the complete genome, heterozygous variation of the CCNO gene c.303C>A and heterozygous variation of c.248_252dup were discovered, which were also found in the female case described in this study. According to ACMG pathogenicity evidence, the heterozygous mutation was nonfunctional (LOF) variation, which could result in translation-produced proteins being truncated or degraded[24].
The most prevalent clinical sign of PCD is recurrent respiratory tract infection. PCD lung disease can progress slowly if not managed effectively. Early intervention is critical for better symptom control. According to earlier findings, Haemophilus influenzae, Pseudomonas aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus, and non-tuberculous mycobacteria are the most prevalent PCD infection microorganisms[25]. Macrolides can relieve respiratory symptoms of PCD patients and improve their prognosis to some extent, while the specific mechanism is yet unknown. Macrolides were utilized in several studies in Japan for PCD patients, and the immunomodulatory impact of macrolides was partly responsible for the relief of respiratory symptoms in PCD patients[26]. Sputum symptoms and chest CT results improved dramatically after 2 mo of azithromycin treatment, which was also found in the female patient in this study.
In conclusion, we reported a young female patient with repeated respiratory symptoms, who was diagnosed as PCD after the whole exon gene detection of CCNO. CCNO carrier is a relatively rare condition, and it is difficult to differentiate it from respiratory diseases. We believed that the diagnosis of primary ciliary dyskinesia still depended on the combination of various tests. In the past five years, certain genes have been reported to be associated with mild respiratory diseases[9,10]. The development of gene sequencing technology promotes the improvement of ciliary gene knowledge, which promotes the progress of clinical phenotype analysis. In addition, despite recent breakthroughs in understanding the underlying genetics and disease mechanisms, there is still a lack of evidence to treat this rare and under-diagnosed disease, which requires further research in the future.
We are grateful to the patient and her parents for their cooperation and willingness to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aldiansyah D, Indonesia; Ullah K, Pakistan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Popatia R, Haver K, Casey A. Primary Ciliary Dyskinesia: An Update on New Diagnostic Modalities and Review of the Literature. Pediatr Allergy Immunol Pulmonol. 2014;27:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Kurkowiak M, Ziętkiewicz E, Witt M. Recent advances in primary ciliary dyskinesia genetics. J Med Genet. 2015;52:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Sommer JU, Schäfer K, Omran H, Olbrich H, Wallmeier J, Blum A, Hörmann K, Stuck BA. ENT manifestations in patients with primary ciliary dyskinesia: prevalence and significance of otorhinolaryngologic co-morbidities. Eur Arch Otorhinolaryngol. 2011;268:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Henriques AR, Constant C, Descalço A, Pinto A, Moura Nunes J, Sampaio P, Lopes SS, Pereira L, Bandeira T. Primary ciliary dyskinesia due to CCNO mutations-A genotype-phenotype correlation contribution. Pediatr Pulmonol. 2021;56:2776-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Guan Y, Yang H, Yao X, Xu H, Liu H, Tang X, Hao C, Zhang X, Zhao S, Ge W, Ni X. Clinical and Genetic Spectrum of Children With Primary Ciliary Dyskinesia in China. Chest. 2021;159:1768-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Lucas JS, Davis SD, Omran H, Shoemark A. Primary ciliary dyskinesia in the genomics age. Lancet Respir Med. 2020;8:202-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 7. | Zariwala MA, Knowles MR, Leigh MW. Primary Ciliary Dyskinesia. Gene reviews. In: Adam MP, Ardinger HH, Pagon RA. GeneReviews: University of Washington, 2007: 1-20. |
| 8. | Kartagener M. Zur pathogenese der bronchiectasien. I Mitteilung: bronchiectasien bei situs viscerum inversus. Betr Klin Tuberk. 1933;83:498-501. |
| 9. | Wallmeier J, Al-Mutairi DA, Chen CT, Loges NT, Pennekamp P, Menchen T, Ma L, Shamseldin HE, Olbrich H, Dougherty GW, Werner C, Alsabah BH, Köhler G, Jaspers M, Boon M, Griese M, Schmitt-Grohé S, Zimmermann T, Koerner-Rettberg C, Horak E, Kintner C, Alkuraya FS, Omran H. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014;46:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 10. | Amirav I, Wallmeier J, Loges NT, Menchen T, Pennekamp P, Mussaffi H, Abitbul R, Avital A, Bentur L, Dougherty GW, Nael E, Lavie M, Olbrich H, Werner C, Kintner C, Omran H; Israeli PCD Consortium Investigators. Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum Mutat. 2016;37:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Honoré I, Burgel PR. Primary ciliary dyskinesia in adults. Rev Mal Respir. 2016;33:165-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Strippoli MP, Frischer T, Barbato A, Snijders D, Maurer E, Lucas JS, Eber E, Karadag B, Pohunek P, Zivkovic Z, Escribano A, O'Callaghan C, Bush A, Kuehni CE; ERS Task Force onPrimary Ciliary Dyskinesia in Children. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur Respir J. 2012;39:1482-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, Hesselmar B, Hogg C, Jorissen M, Lucas J, Nielsen KG, O'Callaghan C, Omran H, Pohunek P, Strippoli MP, Bush A. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, Dell S, Eber E, Escudier E, Hirst RA, Hogg C, Jorissen M, Latzin P, Legendre M, Leigh MW, Midulla F, Nielsen KG, Omran H, Papon JF, Pohunek P, Redfern B, Rigau D, Rindlisbacher B, Santamaria F, Shoemark A, Snijders D, Tonia T, Titieni A, Walker WT, Werner C, Bush A, Kuehni CE. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 444] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 15. | Goutaki M, Meier AB, Halbeisen FS, Lucas JS, Dell SD, Maurer E, Casaulta C, Jurca M, Spycher BD, Kuehni CE. Clinical manifestations in primary ciliary dyskinesia: systematic review and meta-analysis. Eur Respir J. 2016;48:1081-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, Lavange LM, Horton BJ, Qaqish B, Carson JL, Davis SD, Dell SD, Ferkol TW, Atkinson JJ, Olivier KN, Sagel SD, Rosenfeld M, Milla C, Lee HS, Krischer J, Zariwala MA, Knowles MR. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Moore A, Amselem S, Duriez B, Escudier E. Bases molécu- laires des dyskinésies ciliaires primitives. Rev Mal Respir. 21:521-526. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Werner C, Onnebrink JG, Omran H. Diagnosis and management of primary ciliary dyskinesia. Cilia. 2015;4:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Chen W, Shao C, Song Y, Bai C. Primary ciliary dyskinesia complicated with diffuse panbronchiolitis: a case report and literature review. Clin Respir J. 2014;8:425-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Geremek M, Witt M. Primary ciliary dyskinesia: genes, candidate genes and chromosomal regions. J Appl Genet. 2004;45:347-361. [PubMed] |
| 21. | Chodhari R, Mitchison HM, Meeks M. Cilia, primary ciliary dyskinesia and molecular genetics. Paediatr Respir Rev. 2004;5:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Blouin JL, Meeks M, Radhakrishna U, Sainsbury A, Gehring C, Saïl GD, Bartoloni L, Dombi V, O'Rawe A, Walne A, Chung E, Afzelius BA, Armengot M, Jorissen M, Schidlow DV, van Maldergem L, Walt H, Gardiner RM, Probst D, Guerne PA, Delozier-Blanchet CD, Antonarakis SE. Primary ciliary dyskinesia: a genome-wide linkage analysis reveals extensive locus heterogeneity. Eur J Hum Genet. 2000;8:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Funk MC, Bera AN, Menchen T, Kuales G, Thriene K, Lienkamp SS, Dengjel J, Omran H, Frank M, Arnold SJ. Cyclin O (Ccno) functions during deuterosome-mediated centriole amplification of multiciliated cells. EMBO J. 2015;34:1078-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Dahlmann J, Sahabian A, Drick N, Haase A, Göhring G, Lachmann N, Ringshausen FC, Welte T, Martin U, Olmer R. Generation of two hiPSC lines (MHHi016-A, MHHi016-B) from a primary ciliary dyskinesia patient carrying a homozygous 5 bp duplication (c.248_252dup (p.Gly85Cysfs*11)) in exon 1 of the CCNO gene. Stem Cell Res. 2020;46:101850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Bush A, Chodhari R, Collins N, Copeland F, Hall P, Harcourt J, Hariri M, Hogg C, Lucas J, Mitchison HM, O'Callaghan C, Phillips G. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92:1136-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Kido T, Yatera K, Yamasaki K, Nagata S, Choujin Y, Yamaga C, Hara K, Ishimoto H, Hisaoka M, Mukae H. Two cases of primary ciliary dyskinesia with different responses to macrolide treatment. Intern Med. 2012;51:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |