Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9112
Peer-review started: April 11, 2022
First decision: June 7, 2022
Revised: June 20, 2022
Accepted: July 25, 2022
Article in press: July 25, 2022
Published online: September 6, 2022
Processing time: 137 Days and 0.5 Hours
Liver cysts in infants are uncommon. With modern diagnostic imaging, we can achieve an early diagnosis of congenital hepatic cysts. Our purpose was to investigate the clinical features, surgical treatment methods and prognosis of infants with congenital hepatic cysts. Herein, we report a case series of congenital hepatic cysts.
Eleven infants with hepatic cysts were retrospectively analysed. Ten of them had simple hepatic cysts, and a girl with a large hepatic mass was diagnosed with a solitary intrahepatic biliary cyst accompanied by a choledochal cyst. Among the ten simple hepatic cysts, eight were solitary and two were multiple. A total of 87.5% (7 of 8) of infants with solitary hepatic cysts were detected before delivery, and 86% (6 of 7) of those cysts were located in the right lobe of the liver. Surgical intervention was required for symptomatic hepatic cysts. Cyst resection or unroofing with fulguration of the cyst bed was employed. No recurrence of cysts was observed in these infants.
Congenital hepatic cyst is a condition with a narrow differential diagnosis. Accurate diagnosis is essential for appropriate management. Unroofing is the favoured treatment in infants with symptomatic cysts. Most infants with con
Core Tip: Liver cysts in infants often pose significant diagnostic challenges, and paediatric surgeons should be aware of the characteristics of liver cysts to ensure appropriate treatment. Here, we report our experience with congenital hepatic cysts in infants, including a case of solitary intrahepatic biliary cyst, and review the recent literature.
- Citation: Du CX, Lu CG, Li W, Tang WB. Congenital hepatic cyst: Eleven case reports. World J Clin Cases 2022; 10(25): 9112-9120
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9112.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9112
Congenital hepatic cysts are comparatively rare, but with the routine use of prenatal ultrasound, liver cysts are being diagnosed earlier and more often. Liver cysts in newborns often pose significant diagnostic challenges. Solitary liver cysts can be further subdivided as simple liver cysts (SLCs) and solitary intrahepatic biliary cysts (SIBCs), depending on the biliary connection[1,2]. Some SLCs are symptomatic in childhood, even in newborns[3], while they are commonly found in adults incidentally. It is imperative to be aware of the characteristics of liver cysts in infants to ensure that any intervention is appropriate.
Some paediatric congenital hepatic cysts have been reported, but most descriptions have been case reports. Here, we report a series of eleven infant patients diagnosed with hepatic cysts at our department whose clinical features and surgical treatments are described in detail. We discuss the possible aetiological basis and review the common manifestations and surgical options for congenital hepatic cysts in infants.
Case 1: A 1-mo-old girl presented with a prenatal cystic abdominal mass at 34 wk of gestation; Case 2: A 1-mo-old boy presented with a prenatal cystic abdominal mass at 26 wk of gestation; Case 3: A 13-h-old boy presented with a prenatal cystic abdominal mass at 38 wk of gestation; Case 4: A 4-mo-old girl presented with a prenatal cystic abdominal mass at 28 wk of gestation; Case 5: A 6-d-old boy presented with a prenatal cystic abdominal mass at 39 wk of gestation; Case 6: A 1-mo-old girl presented with a prenatal cystic abdominal mass at 24 wk of gestation; Case 7: A 1-mo-old boy presented with a prenatal cystic abdominal mass at 26 wk of gestation; Case 8: A 3-h-old boy presented with an abnormal umbilical cord for 3 h; Case 9: A 2-mo-old girl presented with a prenatal cystic abdominal mass at 26 wk of gestation; Case 10: A 1-h-old boy presented with an abnormal umbilical cord for 1 h; Case 11: A 1-mo-old girl presented with abdominal distension and an abdominal mass for 3 d.
Case 1: The patient remained asymptomatic.
Case 2: The patient suffered vomiting.
Case 3: The patient remained asymptomatic, but progressive enlargement of the cyst was observed.
Case 4: The patient remained asymptomatic.
Case 5: The patient suffered abdominal distension.
Case 6: The patient remained asymptomatic, but progressive enlargement of the cyst was observed.
Case 7: The patient suffered abdominal distension and vomiting.
Case 8: Emergency surgery for omphalocele was conducted when the patient was born.
Case 9: The patient was born at the 34 + 2nd gestational week. The patient remained asymptomatic, but progressive cyst enlargement was observed.
Case 10: The patient was born at the 33 + 4th gestational week, and emergency surgery was conducted for omphalocele.
Case 11: The patient was premature with a birthweight of 1850 g. When the girl was born, she was admitted to the neonatal intensive care unit for evaluation and management. The infant suffered from respiratory problems as a result of progressive abdominal distension.
None of the patients had a history of specific illnesses.
None of the patients had a relevant personal or family history.
Cases 1 and 3: There was slight jaundice upon admission.
Cases 2, 3, and 9: A mass was found in the abdomen.
Cases 8 and 10: A lump in the umbilical cord surrounded by a capsule was found when they were admitted.
Cases 5 and 7: Physical examination revealed a distended abdomen.
Case 11: The patient experienced abdominal distension and breathing difficulty when she was admitted, with slight jaundice.
Cases 4 and 6: No positive signs were found.
Case 1: Alanine aminotransferase, 15 U/L; aspartate aminotransferase, 28 U/L; total bilirubin, 46.67 µmol/L; and direct bilirubin, 35.94 µmol/L.
Case 3: Alanine aminotransferase, 13 U/L; aspartate aminotransferase, 43 U/L; total bilirubin, 146 µmol/L; and direct bilirubin, 132.4 µmol/L.
Case 9: Alanine aminotransferase, 8 U/L; aspartate aminotransferase, 161 U/L; total bilirubin, 42.23 µmol/L; and direct bilirubin, 27.87 µmol/L.
Case 11: White cell count, 12.99 × 109/L; C-reactive protein, 28 mg/L; total bilirubin, 35.11 µmol/L; direct bilirubin 21.99 µmol/L.
Cases 2, 4, 5, 6, 7, 8, and 10: Liver function and bilirubin showed no abnormal changes.
Case 1: Ultrasound showed a mass (41 mm × 35 mm) in the right upper abdomen when he was admitted. Computed tomography (CT) was then performed.
Case 2: Ultrasound showed a mass (75 mm × 50 mm × 63 mm) in the right upper abdomen when he was admitted. Upper gastrointestinal examination (UGI) showed that the bowel was compressed.
Case 3: Ultrasound showed a mass (64 mm × 34 mm) in the middle upper abdomen when he was admitted. Magnetic resonance imaging (MRI) showed that the abdominal organs were compressed.
Case 4: Ultrasound and MRI showed a mass (40 mm × 23 mm) in the right upper abdomen when he was admitted.
Case 5: Ultrasound showed a mass (60 mm × 50 mm × 40 mm) in the right upper abdomen when he was admitted. CT showed that the bowel was compressed.
Case 6: Ultrasound and CT showed a mass (39 mm × 25 mm) in the right upper abdomen when he was admitted.
Case 7: Ultrasound and CT showed a mass (51 mm × 35 mm) in the right upper abdomen when he was admitted.
Case 9: An ultrasound scan showed a 6.9 cm × 5.6 cm cystic mass in the right upper abdomen and an intrahepatic cyst 2.3 cm × 1.2 cm in size, with no intrahepatic biliary duct dilatation.
MRI showed a mass (3.0 cm × 2.3 cm × 2.5 cm) in the right middle cranial fossa and a mass (0.5 cm × 0.5 cm × 0.5 cm) in the sellar region. Specifically, this patient had comorbid polycystic kidneys diagnosed on the first ultrasound scan.
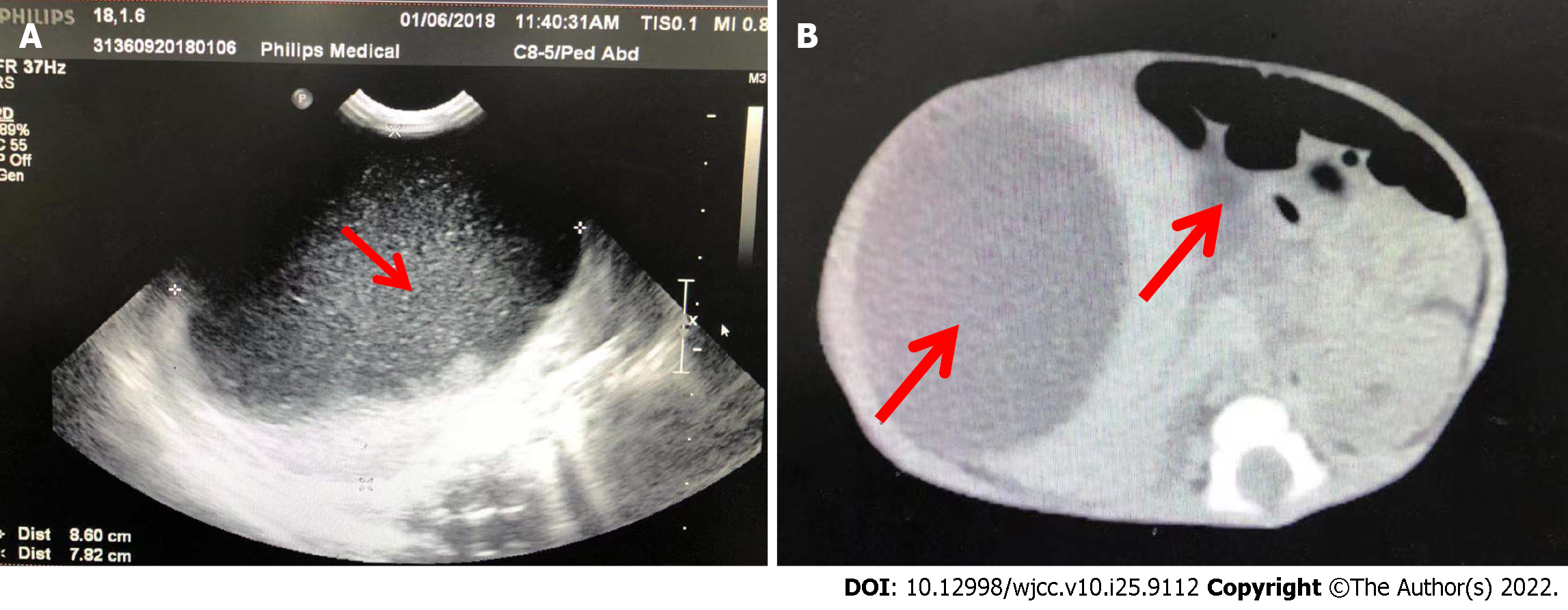
Case 11: An ultrasound scan (Figure 1A) showed a mass in the abdomen. CT scans (Figure 1B) showed two large intraabdominal cysts. The larger cyst (8.5 cm × 7.9 cm) was located in the right lobe of the liver, and the other cyst (4.3 cm × 2.0 cm) located in the porta hepatis was considered a choledochal cyst. The extrahepatic bile ducts and gallbladder were obscured by the massively enlarged cyst. No definite intrahepatic bile duct dilatation was observed. However, compression effects on other organs were noticeable.
Cases 8 and 10: Since the cysts were discovered accidentally during surgery, no imaging data were available.
Combined with the patient’s medical history, the final diagnosis was a SLC.
The final diagnosis was omphalocele accompanied by a SLC.
An uncertainly diagnosed mass accompanied by a choledochal cyst was considered before surgery. Combined with the intraoperative findings, the final diagnosis was a solitary intrahepatic biliary cyst (SIBC).
All patients underwent surgery. Total cyst excision was performed for four patients, and unroofing with fulguration of the cyst bed was performed for three patients. The cysts were operated on via a laparoscopic approach in three patients. Open abdominal excision or fenestration was conducted in four patients. The reasons for converting to open laparoscopy were uncertain diagnosis, abdominal adhesion or large size. Details of the clinical and pathological presentations of the cases are shown in Tables 1 and 2. A postoperative pathology test identified all cysts as SLCs. In regard to size, the solitary hepatic cysts averaged 5.43 cm in diameter, larger than that of multiple cysts. Six of seven solitary cysts were located in the right lobe of the liver. The simple hepatic cysts in this series were lined by cuboidal epithelium that stained positively for cytokeratin. The fluid from the simple cysts was yellow or yellowish.
| Patient | Sex | Premature | Symptoms | USG | CT/MRI/UGI | Surgery | Complications |
| 1 | F | No | Jaundice | 34 w prenatally | CT | L unroofing | None |
| 2 | M | No | Vomit | 26 w prenatally | UGI | L unroofing | None |
| 3 | M | No | Enlargement | 38 w prenatally | MRI | L-C unroofing | None |
| 4 | F | No | Confusing diagnosis | 28 w prenatally | MRI | L CE | None |
| 5 | M | No | Abdominal distension | 39 w prenatally | CT | CE | None |
| 6 | F | No | Enlargement | 24 w prenatally | CT | L-C CE | None |
| 7 | M | No | Vomit, abdominal distension | 26 w prenatally | MRI | L-C CE | None |
| 8 | M | No | Confusing diagnosis | / | / | Unroofing | Omphalocele |
| 9 | F | 34 + 2 w | Enlargement | 26 w prenatally | CT and MRI | L-C unroofing | Polycystic kidneys |
| 10 | M | 33 + 4 w | Confusing diagnosis | / | / | CE | Omphalocele |
| Patient | Type of Cyst | Size (cm) | Epithelial lining | Cytokeratin | Fluid |
| 1 | Solitary | 3 × 4 × 4 | Cuboidal | Not available | Yellowish |
| 2 | Solitary | 7 × 6 × 4 | Cuboidal | Positive | Yellowish |
| 3 | Solitary | 8 × 8 × 6 | Cuboidal | Not available | Yellowish |
| 4 | Solitary | 3 × 4 | Cuboidal | Positive | Yellowish |
| 5 | Solitary | 6 × 5 × 4 | Cuboidal | Not available | Yellow |
| 6 | Solitary | 4 × 3 × 2 | Cuboidal | Positive | Yellow |
| 7 | Solitary | 4 × 5 | Cuboidal | Positive | Yellow |
| 8 | Solitary | 2 × 2 × 1 | Not Oberserved | Not available | Not described |
| 9 | Multiple | 6 × 9 × 6 in right; 1.5 × 0.5 in left | Cuboidal | Positive | Yellowish |
| 10 | Multiple | Not described | Cuboidal | Positive | Not described |
For Case 11, an uncertainly diagnosed mass accompanied by a choledochal cyst was considered; therefore, we performed a laparoscopic examination, revealing obvious adhesion and a large cystic mass in the abdomen. The gallbladder was identified. The cyst wall was opened, and it was filled with bile-stained fluid and muddy stones, so an intracavitary catheter was placed. She was presumed to have SIBC without pathologic examination.
No recurrence of cysts was observed in any of these infants.
For case 9, the cysts in the kidneys spontaneously and completely resolved after two weeks.
For case 11, after the operation, the abdominal distension and breathing difficulty were relieved. Total bilirubin was 17.87 µmol/L with a direct bilirubin of 10.72 µmol/L on postoperative day 6. Bile drainage through the catheter stopped on the ninth day after the operation, but the patient developed fever, abdominal distension and tense abdominal skin. Blockage of the drainage tube was considered and confirmed by emergency operation. After adjusting the drainage tube, the infant had an uneventful postoperative course. The bile drainage decreased and turned clear before it eventually stopped. Ultrasonography confirmed the complete disappearance of the intrahepatic cyst. The drains were removed two months and five days after the initial operation. When the patient underwent surgery for the choledochal cysts at nine months of age, no hepatic cysts were observed. A follow-up ultrasound examination showed no recurrence when she was one year and one month old.
Congenital liver cysts are categorized as nonparasitic simple cysts or polycystic liver disease (PCLD). Simple hepatic cysts can be solitary or multiple[4]. Although multiple cysts in the liver are common in these patients, the condition is distinct from PCLD, an autosomal dominant condition associated with PKD1 and PKD2 gene mutations[5]. PCLD may be associated with congenital polycystic kidney disease or dilatation of the bile ducts (Caroli’s disease), while SLCs are usually never accompanied by other organ cysts[6]. However, in our series, our 9th patient was diagnosed with SLCs complicated with polycystic disease of the kidney. This is not consistent with a previous study. Perhaps further gene screening of this infant can provide a definitive explanation.
The pathogenesis of hepatic cysts is related to the type of cyst. It has been hypothesized that SLC might be caused by abnormal bile duct formation during embryogenesis[7]. SIBC is believed to arise from inadequate drainage of part of the intrahepatic biliary tree[3,8]. Most solitary hepatic cysts are SLCs. Pathologically, SLC has a single layer of cuboidal or columnar epithelium lining the inside of the cyst. The cystic fluid may be clear[9]. SIBC displays the same characteristics as SLC, but it contains bile[1]. We reported a case diagnosed with SIBC based on previous reports[1,8,10]. Unfortunately, retrograde cholangiography could not be performed because of a wide adhesion in the abdomen. The boy with SIBC reported by Soyer et al[8] was revealed by prenatal ultrasonography. He suffered respiratory distress, similar to the premature girl in our report. Additionally, our reported girl exhibited abdominal distension and jaundice. The cyst was not found prenatally. Moreover, the hepatic cyst was accompanied by a choledochal cyst in this report. Interestingly, the previously reported boy has an older brother who was diagnosed with a choledochal cyst. These findings may indicate an aetiological relationship between SIBC and choledochal cysts.
Solitary liver cysts are slightly more common in female patients, with a 1.5:1.0 female-to-male ratio. Previous research has shown that oestrogen may account for this sex difference[11]. In contrast to previous reports, more boys exhibited congenital hepatic cysts in this study. This might be due to the small sample size resulting in random variation, and multicentre studies with more samples will be more credible in establishing the sex ratio.
In our cases, the median gestational age at detection for prenatally detected cysts was 30 wk (24-39 wk). These infants with solitary hepatic cysts were all born at term. An ultrasound scan was postnatally performed on the infants with prenatally detected cysts. Asymptomatic hepatic cysts can be monitored by ultrasound because a complete spontaneous resolution of prenatally diagnosed hepatic cysts has been reported in a recent systematic review and meta-analysis[12-14]. Occasionally, an intraoperatively encountered cyst is found to be pedunculated and is easily excised. A simple cyst resection is recommended for these cysts due to the risk of torsion.
Nevertheless, simple cysts can become symptomatic cysts requiring surgical treatment when progressive enlargement or symptoms are present or if the imaging characteristics cannot lead to a definitive diagnosis. Large simple cysts in newborns may cause abdominal distention, pain, jaundice, feeding difficulties and respiratory distress[15-17]. If it is over 5 cm and/or the cyst has radiologic findings that are suspicious for neoplasia[11], surgery becomes necessary.
Ultrasonography-guided percutaneous aspiration may serve as a diagnostic tool, with a recurrence rate of 100%[18]. To achieve permanent ablation, instillation of a sclerosant, such as ethanol, tetracycline, minocycline or pantopaque, is conducted. The sclerosing agent induces an obvious inflammatory reaction that potentially produces obliteration of the cyst space[19,20]. Communication of the cyst with the biliary tree is an absolute contraindication to injection sclerotherapy[21].
Most authors advocate surgical treatment for symptomatic hepatic cysts, including unroofing, cyst excision or liver resection[22,23]. Total excision should be attempted for easily accessible SLCs because it eliminates the risk of recurrence[24]. In most cases, complete excision is not possible because of the close proximity of the cyst wall to nearby parenchymal and hilar structures, and a portion of the cyst wall should be excised.
Unroofing liver cysts can be undertaken as an open or laparoscopic surgery[25]. Laparoscopic unroofing has become the gold standard treatment with low recurrence rates between 0% and 13.8%[26,27]. Some centres employ fulguration of the cyst bed and omental transposition to reduce the risk of cyst recurrence[28]. However, recent data suggest that transposition of the omentum is not needed. The greater omentum flap could contribute to local recurrence by reducing the size of the parenchymal opening and inducing adhesion[29]. Recently, laparoscopic liver cyst fenestration with indocyanine green (ICG) fluorescent imaging has been believed to be particularly useful for delineating adequate margins for the procedure and for avoiding duct injuries[30-32]. In this study, unroofing, followed mainly by fulguration of the cyst bed, led to no recurrence.
Hepatic lobectomy for simple cysts has been reported in patients with complex or recurrent cystic disease[33]. Additionally, for symptomatic SIBC caused by insufficient drainage during the neonatal period, internal drainage via cystoenterostomy or Roux-en-Y hepaticojejunostomy is recommended[34]. However, partial excision of symptomatic SIBC also had a good prognosis in a previous study[8]. Recently, a case reported that endoscopic ultrasound-guided drainage could also achieve a good prognosis[35]. This is consistent with our experience. In our opinion, the excellent prognosis of our patient is potentially associated with bile duct inflammation, which induced the occlusion of the small canalicular bile ducts. A long-term follow-up is certainly necessary.
With the widespread use of prenatal sonography, aspiration of giant hepatic cysts in the foetus in utero has been reported[34,36]. Due to fairly high recurrence rates in adults, simple aspiration of the cyst should be performed only for the emergency control of symptoms.
Overall, congenital hepatic cysts have a good prognosis, and complications are rare[37]. The median follow-up of this study was 11.7 mo (range 1-36 mo). There were no reported postoperative complications or recurrences in this series of patients.
Liver cysts in infants often pose significant diagnostic challenges, and paediatric surgeons should be aware of the characteristics of liver cysts to ensure appropriate treatment. Here, we report our experience with congenital hepatic cysts in infants and review the recent literature. Additional prospective and large-scale studies are warranted.
The authors thank Mr. Xiu-Rui Lv (Nanjing Children’s Hospital Affiliated to Nanjing Medical University) for language polishing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Mijwil MM, Iraq; Suda T, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Brown DK, Kimura K, Sato Y, Pringle KC, Abu-Yousef MM, Soper RT. Solitary intrahepatic biliary cyst: diagnostic and therapeutic strategy. J Pediatr Surg. 1990;25:1248-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Klein L, Meurisse N, Honoré P, De Roover A, Detry O. [Diagnosis and management of liver cysts]. Rev Med Liege. 2021;76:661-665. [PubMed] |
| 3. | Bhosale M, Singh D. Giant congenital solitary nonparasitic cyst of the liver causing respiratory distress in a neonate. J Indian Assoc Pediatr Surg. 2016;21:72-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Garcea G, Rajesh A, Dennison AR. Surgical management of cystic lesions in the liver. ANZ J Surg. 2013;83:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kataoka H, Watanabe S, Sato M, Manabe S, Makabe S, Akihisa T, Ushio Y, Iwasa N, Yoshida R, Tsuchiya K, Nitta K, Mochizuki T. Predicting liver cyst severity by mutations in patients with autosomal-dominant polycystic kidney disease. Hepatol Int. 2021;15:791-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Temmerman F, Missiaen L, Bammens B, Laleman W, Cassiman D, Verslype C, van Pelt J, Nevens F. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment Pharmacol Ther. 2011;34:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Otani Y, Takayasu H, Ishimaru Y, Okamura K, Yamagishi J, Ikeda H. Secretion and expression of epithelial markers supports the biliary origin of solitary nonparasitic cyst of the liver in infancy. J Pediatr Surg. 2005;40:e27-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Soyer T, Karnak I, Senocak ME. Congenital solitary intrahepatic biliary cyst in a newborn: report of a case. Surg Today. 2007;37:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Sanfelippo PM, Beahrs OH, Weiland LH. Cystic disease of the liver. Ann Surg. 1974;179:922-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Viswanathan S, Kumar D. Diagnostic challenge of large congenital liver cyst in the newborn. Pediatr Int. 2014;56:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Schiff ER. Advances in hepatology: current developments in the treatment of hepatitis and hepatobiliary disease. Gastroenterol Hepatol (N Y). 2009;5:414-416. [PubMed] |
| 12. | Leombroni M, Buca D, Celentano C, Liberati M, Bascietto F, Gustapane S, Marrone L, Manzoli L, Rizzo G, D’Antonio F. Outcomes associated with fetal hepatobiliary cysts: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Serban DM, Serban C, Navolan D, Sas I. Spontaneous postnatal complete resolution of an antenatally diagnosed fetal hepatic cyst: A case report. Medicine (Baltimore). 2018;97:e11133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Sepulveda W, Sepulveda F, Gonzalez G, Arce C, Alcalde E. Congenital hepatic cyst: Prenatal and postnatal imaging findings. Ultrasound. 2021;29:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kelly K, Weber SM. Cystic diseases of the liver and bile ducts. J Gastrointest Surg. 2014;18:627-34; quiz 634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Celebi S, Kutluk G, Bestas CB, Kuzdan Ö, Sander S. Current diagnosis and management of simple hepatic cysts detected prenatally and postnatally. Pediatr Surg Int. 2014;30:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Nicolau PB, Lázaro JL, Viladomiu L, Casabiel XM, Riveiro-Barciela M. Inferior Cava Vein Syndrome and Heart Compression Due to a Giant Liver Cyst. Am J Gastroenterol. 2017;112:984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Saini S, Mueller PR, Ferrucci JT Jr, Simeone JF, Wittenberg J, Butch RJ. Percutaneous aspiration of hepatic cysts does not provide definitive therapy. AJR Am J Roentgenol. 1983;141:559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 19. | vanSonnenberg E, Wroblicka JT, D’Agostino HB, Mathieson JR, Casola G, O’Laoide R, Cooperberg PL. Symptomatic hepatic cysts: percutaneous drainage and sclerosis. Radiology. 1994;190:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Wijnands TF, Görtjes AP, Gevers TJ, Jenniskens SF, Kool LJ, Potthoff A, Ronot M, Drenth JP. Efficacy and Safety of Aspiration Sclerotherapy of Simple Hepatic Cysts: A Systematic Review. AJR Am J Roentgenol. 2017;208:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Moorthy K, Mihssin N, Houghton PW. The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl. 2001;83:409-414. [PubMed] |
| 22. | Rygl M, Snajdauf J, Petrů O, Kodet R, Kodetová D, Mixa V. Congenital solitary liver cysts. Eur J Pediatr Surg. 2006;16:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Cowles RA, Mulholland MW. Solitary hepatic cysts. J Am Coll Surg. 2000;191:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 24. | Nordin AB, Fallon SC, Carter BA, Brandt ML. Congenital hepatic cyst with antenatal diagnosis: a case report and literature review. Pediatr Surg Int. 2013;29:847-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Zacherl J, Imhof M, Függer R, Fritsch A. Laparoscopic unroofing of symptomatic congenital liver cysts. Surg Endosc. 1996;10:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Antonacci N, Ricci C, Taffurelli G, Casadei R, Minni F. Systematic review of laparoscopic versus open surgery in the treatment of non-parasitic liver cysts. Updates Surg. 2014;66:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Zhang JY, Liu Y, Liu HY, Chen L, Su DW, Wang YB. Comparison of the Recurrence Rates of Nonparasitic Hepatic Cysts Treated With Laparoscopy or With Open Fenestration: A Meta-Analysis. Surg Laparosc Endosc Percutan Tech. 2018;28:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Brozzetti S, Miccini M, Bononi M, Al Mansour M, Borghese O, Gregori M, Fraioli F, De Toma G, Tocchi A. Treatment of congenital liver cysts. A surgical technique tailored through a 35-year experience. Ann Ital Chir. 2013;84:93-98. [PubMed] |
| 29. | Wahba R, Kleinert R, Prenzel K, Bangard C, Hölscher AH, Stippel DL. Laparoscopic deroofing of nonparasitic liver cysts with or without greater omentum flap. Surg Laparosc Endosc Percutan Tech. 2011;21:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Tanioka N, Maeda H, Shimizu S, Munekage M, Uemura S, Hanazaki K. Indocyanine green fluorescence-guided laparoscopic deroofing of a liver cyst: A case report. Asian J Endosc Surg. 2022;15:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 31. | Une N, Fujio A, Mitsugashira H, Kanai N, Saitoh Y, Ohta M, Sasaki K, Miyazawa K, Kashiwadate T, Nakanishi W, Tokodai K, Miyagi S, Unno M, Kamei T. Laparoscopic liver cyst fenestration with real-time indocyanine green fluorescence-guided surgery: a case report. J Surg Case Rep. 2021;2021:rjab196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Hanaki T, Yagyu T, Uchinaka E, Morimoto M, Watanabe J, Tokuyasu N, Takano S, Sakamoto T, Honjo S, Fujiwara Y. Avoidance of bile duct injury during laparoscopic liver cyst fenestration using indocyanine green: A case report. Clin Case Rep. 2020;8:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Madariaga JR, Iwatsuki S, Starzl TE, Todo S, Selby R, Zetti G. Hepatic resection for cystic lesions of the liver. Ann Surg. 1993;218:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Berg C, Baschat AA, Geipel A, Krapp M, Germer U, Smrcek JM, Sigge W, Gembruch U. First-trimester diagnosis of fetal hepatic cyst. Ultrasound Obstet Gynecol. 2002;19:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Shionoya K, Koizumi K, Masuda S, Suno Y, Kawachi J, Kimura K, Makazu M, Kubota J, Nishino T, Sumida C, Tasaki J, Ichita C, Sasaki A, Hadano H, Kako M. Liver cyst with biliary communication treated with endoscopic ultrasound-guided drainage: A case report. Medicine (Baltimore). 2022;101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Allan M, Asimakidou M, Davenport M. Antenatally-detected liver cysts: Causes and characteristics, indications for intervention. J Pediatr Surg. 2020;55:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Macutkiewicz C, Plastow R, Chrispijn M, Filobbos R, Ammori BA, Sherlock DJ, Drenth JP, O’Reilly DA. Complications arising in simple and polycystic liver cysts. World J Hepatol. 2012;4:406-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |